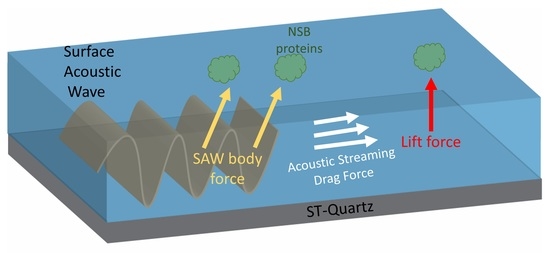
Removal of Non-Specifically Bound Proteins Using Rayleigh Waves Generated on ST-Quartz Substrates
Abstract
:1. Introduction
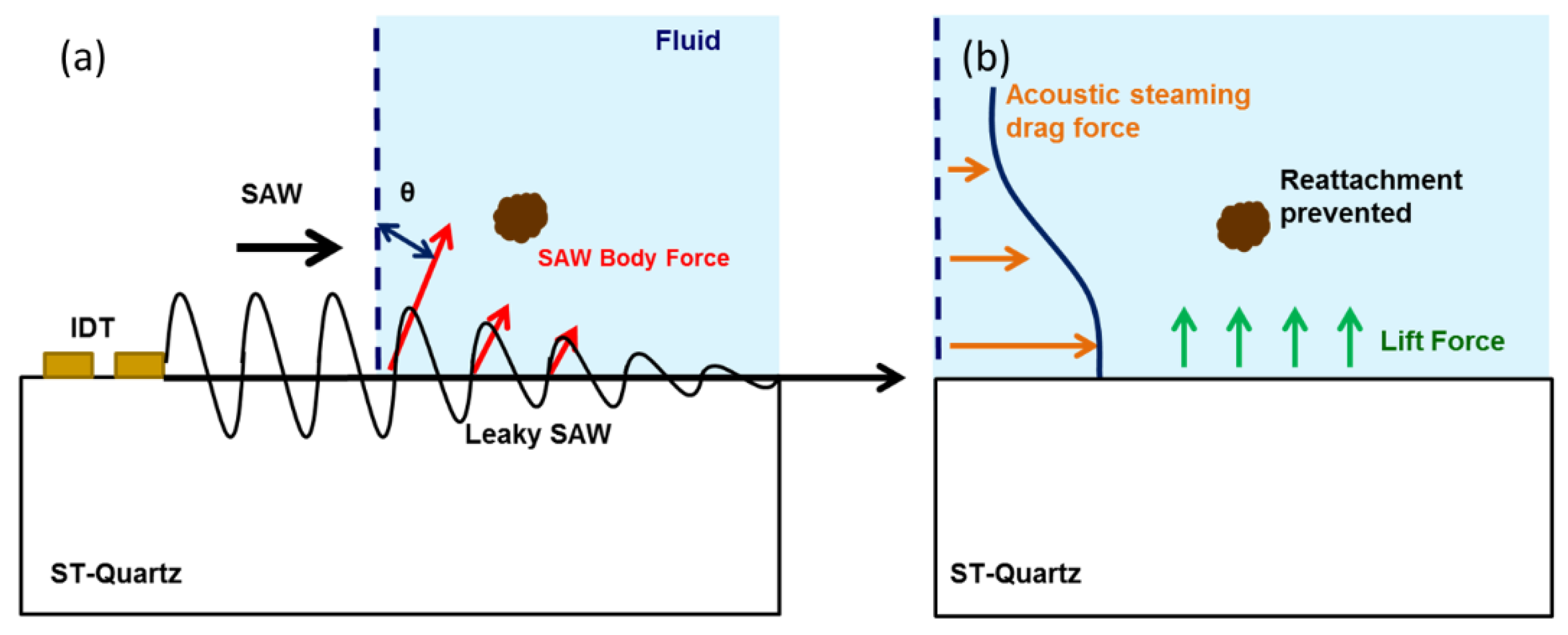
2. Mechanism of NSB Protein Removal
3. Computational Details
3.1. Model Setup
3.2. Piezoelectric Domain
3.3. Fluid Domain
3.4. Coupling from Solid Domain to Fluid Domain
3.5. Coupling from Fluid Domain to Solid Domain
4. Computational Results and Discussion
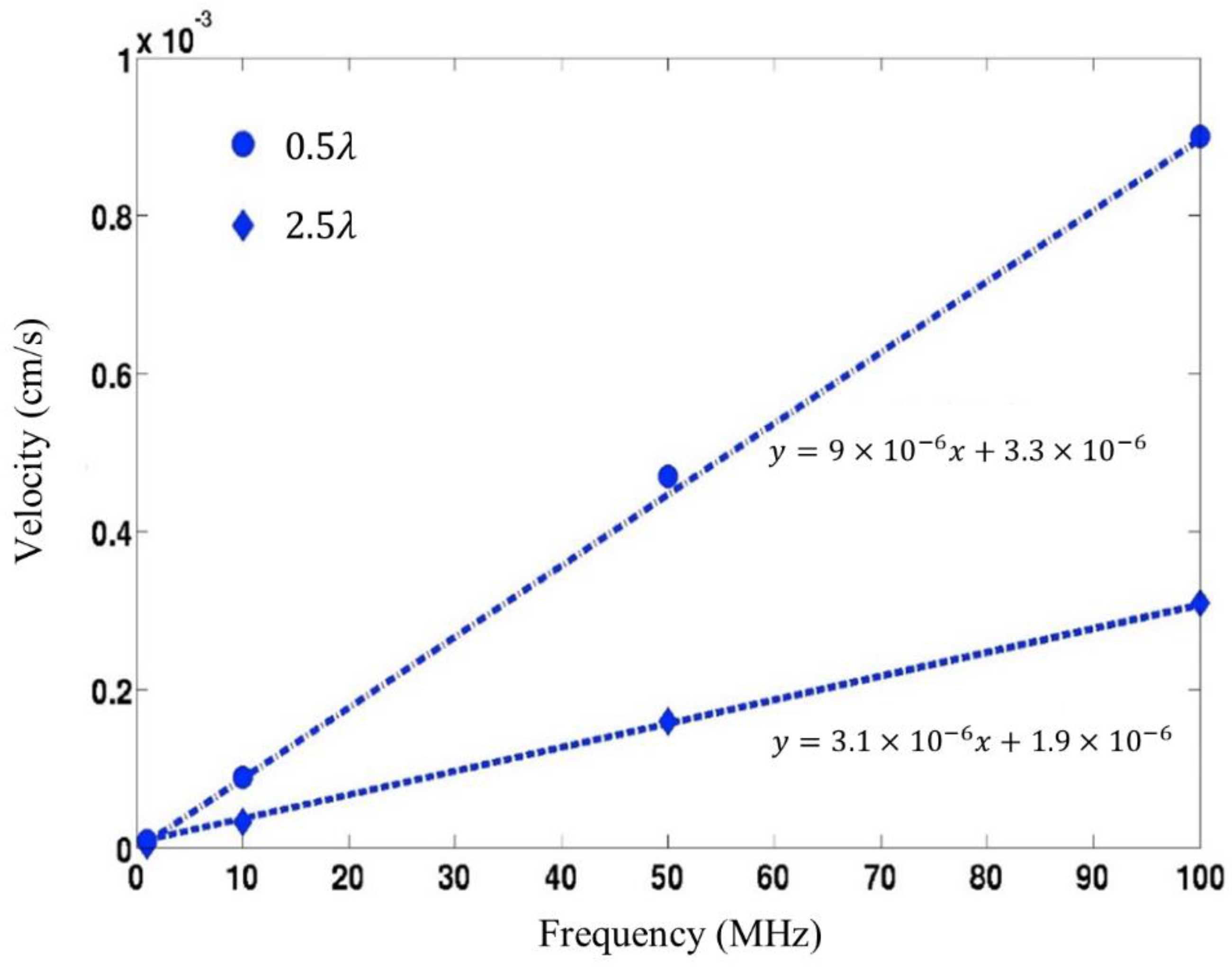
4.1. Streaming Induced Removal of Non-Specifically Bound Proteins in ST-Quartz
4.2. Comparison of the Removal Efficiency amongst the Various SAW Substrates
5. Materials and Methods
5.1. SAW Device Fabrication
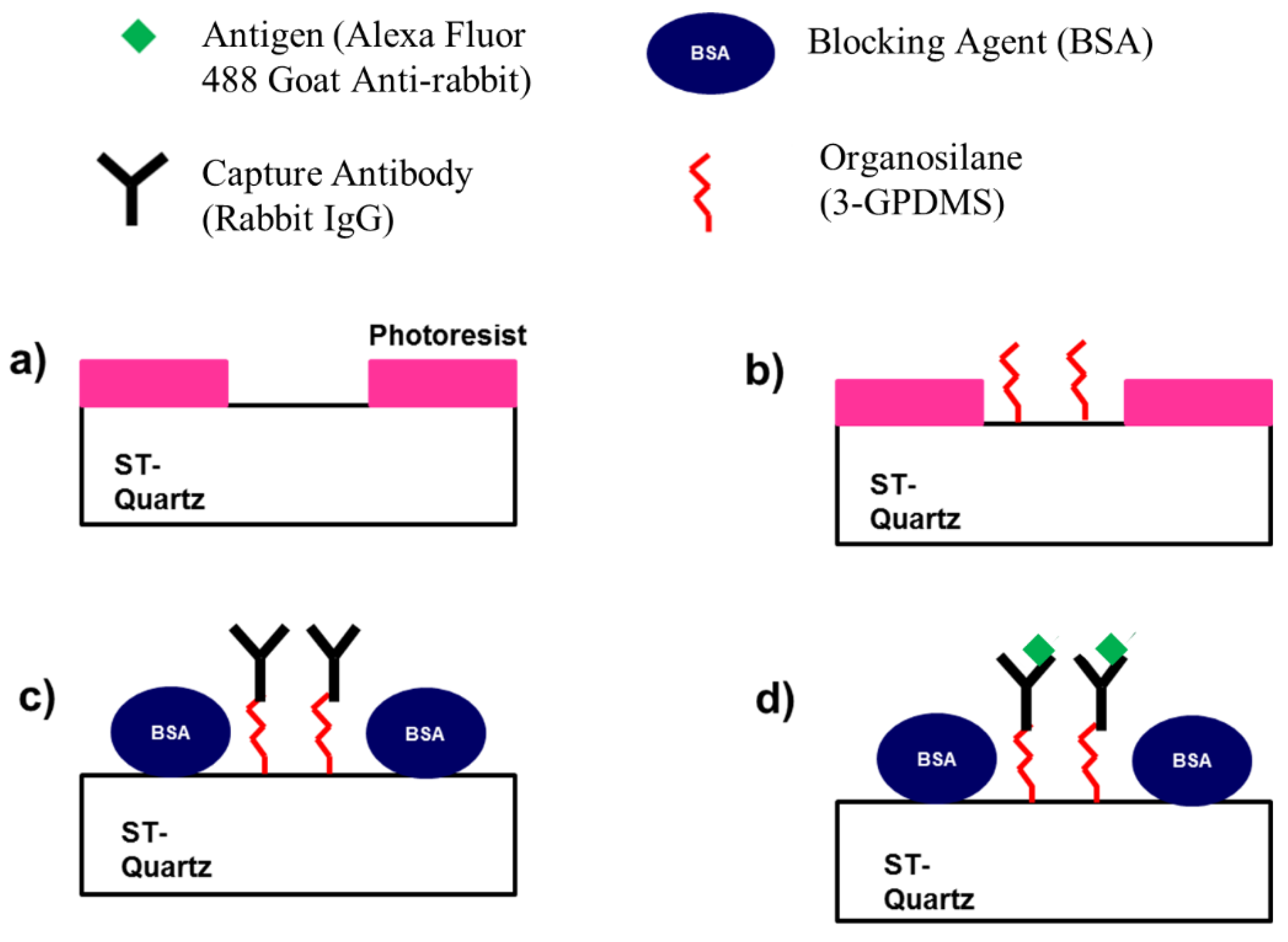
5.2. Micropatterning the SAW Delay Path
5.3. Surface Modification and Attachment of Capture Antibody
5.4. Determination of Specific and Non-Specific Binding
5.5. Fluorescence Imaging
5.6. Protein Removal with Acoustic Energy
6. Experimental Results and Discussion
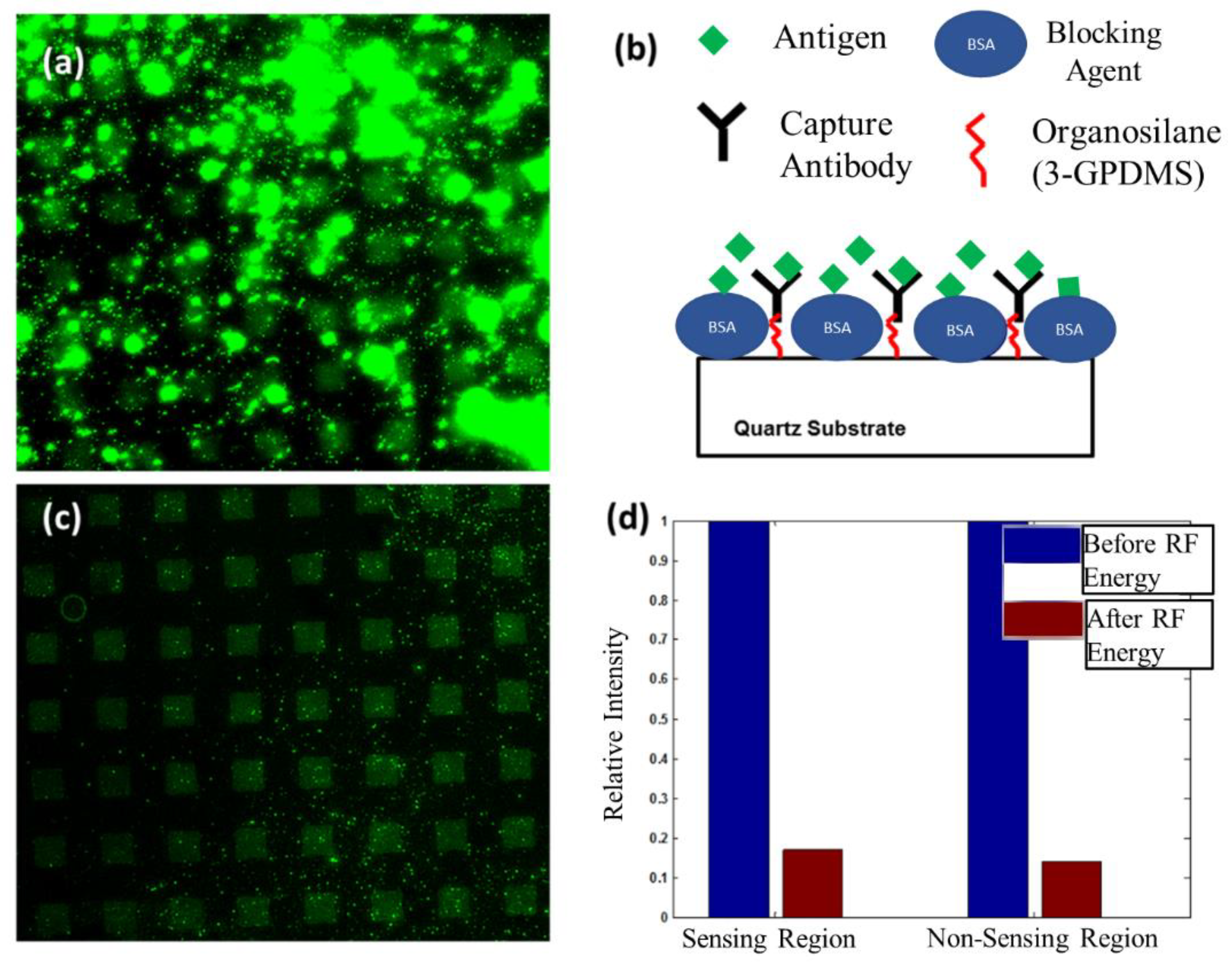
6.1. Removal of Non-Specifically Bound Antigen from Sensing and Non-Sensing Regions
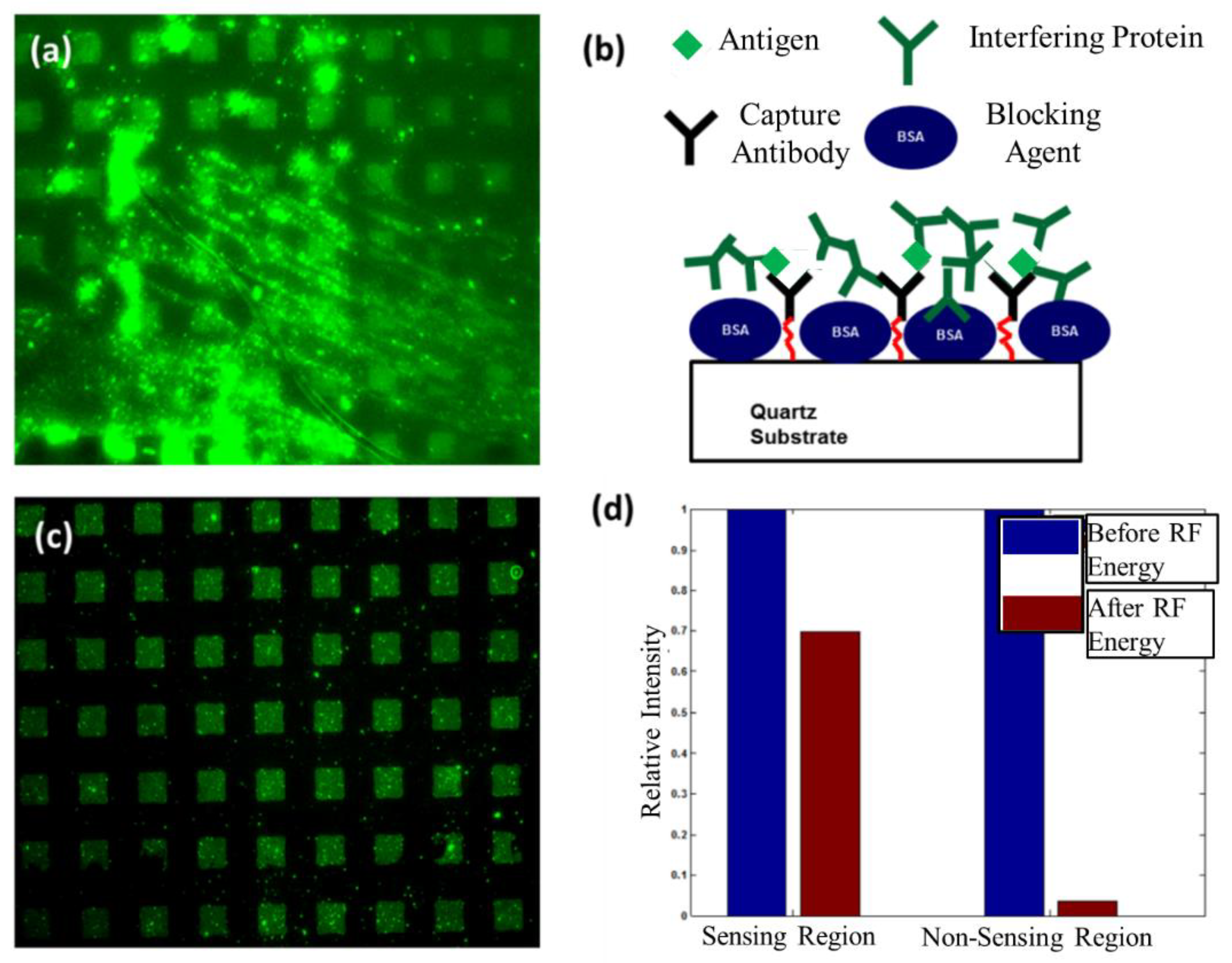
6.2. Removal of an Interfering Protein from Sensing and Non-Sensing Regions
6.3. Renewal of Sensor Surface
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kane, R.S.; Deschatelets, P.; Whitesides, G.M. Kosmotropes Form the Basis of Protein-Resistant Surfaces. Langmuir 2003, 19, 2388–2391. [Google Scholar] [CrossRef]
- Teramura, Y.; Iwata, H. Label-free immunosensing for α-fetoprotein in human plasma using surface plasmon resonance. Anal. Biochem. 2007, 365, 201–207. [Google Scholar] [CrossRef]
- Holmlin, R.E.; Chen, X.; Chapman, R.G.; Takayama, S.; Whitesides, G.M. Zwitterionic SAMs that Resist Nonspecific Adsorption of Protein from Aqueous Buffer. Langmuir 2001, 17, 2841–2850. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef]
- Fernández-Sánchez, C.; González-García, M.B.; Costa-García, A. AC voltammetric carbon paste-based enzyme immunosensors. Biosens. Bioelectron. 2000, 14, 917–924. [Google Scholar] [CrossRef]
- Welsch, W.; Klein, C.; Öksüzoglu, R.M.; von Schickfus, M.; Hunklinger, S. Immunosensing with surface acoustic wave sensors. Sens. Actuators A Phys. 1997, 62, 562–564. [Google Scholar] [CrossRef]
- Sun, C.; Miao, J.; Yan, J.; Yang, K.; Mao, C.; Ju, J.; Shen, J. Applications of antibiofouling PEG-coating in electrochemical biosensors for determination of glucose in whole blood. Electrochim. Acta 2013, 89, 549–554. [Google Scholar] [CrossRef]
- Chi, Y.S.; Lee, B.S.; Kil, M.; Jung, H.-J.; Oh, E.; Choi, I.S. Asymmetrically Functionalized, Four-Armed, Poly(ethylene glycol) Compounds for Construction of Chemically Functionalizable Non-Biofouling Surfaces. Chem. Asian J. 2009, 4, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Waterboer, T.; Sehr, P.; Pawlita, M. Suppression of non-specific binding in serological Luminex assays. J. Immunol. Methods 2006, 309, 200–204. [Google Scholar] [CrossRef]
- Rodda, D.J.; Yamazaki, H. Poly(Vinyl Alcohol) as a Blocking Agent in Enzyme Immunoassays. Immunol. Investig. 1994, 23, 421–428. [Google Scholar] [CrossRef]
- Meyer, G.D.; Moran-Mirabal, J.M.; Branch, D.W.; Craighead, H.G. Nonspecific binding removal from protein microarrays using thickness shear mode resonators. IEEE Sens. J. 2006, 6, 254–261. [Google Scholar] [CrossRef]
- Cular, S.; Branch, D.W.; Bhethanbotla, V.R.; Meyer, G.D.; Craighead, H.G. Removal of nonspecifically bound proteins on microarrays using surface acoustic waves. IEEE Sens. J. 2008, 8, 314–320. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.K.R.S.; Cular, S.; Bhethanabotla, V.R.; Joseph, B. Flow induced by acoustic streaming on surface-acoustic-wave devices and its application in biofouling removal: A computational study and comparisons to experiment. Phys. Rev. E 2008, 77, 6. [Google Scholar] [CrossRef] [PubMed]
- Celik Cogal, G.; Das, P.K.; Yurdabak Karaca, G.; Bhethanabotla, V.R.; Uygun Oksuz, A. Fluorescence Detection of miRNA-21 Using Au/Pt Bimetallic Tubular Micromotors Driven by Chemical and Surface Acoustic Wave Forces. ACS Appl. Bio Mater. 2021, 4, 7932–7941. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.K.R.S.; Singh, R.; Bhethanabotla, V.R. Acoustic streaming induced elimination of nonspecifically bound proteins from a surface acoustic wave biosensor: Mechanism prediction using fluid-structure interaction models. J. Appl. Phys. 2010, 108, 10. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.K.R.S.; Bhethanabotla, V.R. Enhancement of acoustic streaming induced flow on a focused surface acoustic wave device: Implications for biosensing and microfluidics. J. Appl. Phys. 2010, 107, 9. [Google Scholar]
- Singh, R.; Sankaranarayanan, S.K.R.S.; Bhethanabotla, V.R. Orthogonal surface acoustic wave device based on langasite for simultaneous biosensing and biofouling removal. Appl. Phys. Lett. 2009, 94, 3. [Google Scholar] [CrossRef]
- Kondoh, J.; Shimizu, N.; Matsui, Y.; Shiokawa, S. Liquid Heating Effects by SAW Streaming on the Piezoelectric Substrate. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2005, 52, 1881–1883. [Google Scholar] [CrossRef]
- Park, J.; Ha, B.H.; Destgeer, G.; Jung, J.H.; Sung, H.J. Spatiotemporally controllable acoustothermal heating and its application to disposable thermochromic displays. RSC Adv. 2016, 6, 33937–33944. [Google Scholar] [CrossRef]
- Das, P.K.; Snider, A.D.; Bhethanabotla, V.R. Acoustothermal heating in surface acoustic wave driven microchannel flow. Phys. Fluids 2019, 31, 106106. [Google Scholar] [CrossRef]
- Lighthill, S.J. Acoustic streaming. J. Sound Vib. 1978, 61, 391–418. [Google Scholar] [CrossRef]
- Das, P.K.; Snider, A.D.; Bhethanabotla, V.R. Acoustic streaming in second-order fluids. Phys. Fluids 2020, 32, 123103. [Google Scholar] [CrossRef]
- Muller, P.B.; Barnkob, R.; Jensen, M.J.H.; Bruus, H. A numerical study of microparticle acoustophoresis driven by acoustic radiation forces and streaming-induced drag forces. Lab Chip 2012, 12, 4617–4627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowling, R.A. Particles on Surface-Detection, Adhesion, and Removal; Plenum: New York, NY, USA, 1988. [Google Scholar]
- Krupp, H. Particle Adhesion Theory and Experiment; Elsevier: Amsterdam, The Netherlands, 1967. [Google Scholar]
- Qi, Q.; Brereton, G.J. Mechanisms of Removal of Micron-Sized Particles by High-Frequency Ultrasonic-Waves. IEEE T Ultrason. Ferr. 1995, 42, 619–629. [Google Scholar]
- Cherednik, V.I.; Dvoesherstov, M.Y. Propagation of surface acoustic waves in a piezoelectric medium with a periodic groove structure. Acoust. Phys. 2010, 56, 37–46. [Google Scholar] [CrossRef]
- Furukawa, S.; Nomura, T.; Yasuda, T. Characteristic Features of Leaky Surface Acoustic-Waves Propagating on Liquid Piezoelectric Film Glass Structures. J. Phys. D Appl. Phys. 1991, 24, 706–713. [Google Scholar] [CrossRef]
- Moroney, R.M. Ultrasonic Microtransport; University of California: Berkeley, CA, USA, 1995. [Google Scholar]
- Guttenberg, Z.; Rathgeber, A.; Keller, S.; Radler, J.O.; Wixforth, A.; Kostur, M.; Schindler, M.; Talkner, P. Flow profiling of a surface-acoustic-wave nanopump. Phys. Rev. E 2004, 70, 5. [Google Scholar] [CrossRef] [Green Version]
- Sano, A.; Rlatsui, Y.; Shiokawa, S. A New Manipulator Based on Surface Acoustic Wave Streaming. In Proceedings of the 1997 IEEE Ultrasonics Symposium Proceedings An International Symposium, Toronto, ON, Canada, 5–8 October 1997; Volume 1, pp. 467–470. [Google Scholar]
- Shiokawa, S.; Matsui, Y.; Moriizumi, T. Experimental-Study on Liquid Streaming by Saw. Jpn. J. Appl. Phys. 1989, 28, 126–128. [Google Scholar] [CrossRef] [Green Version]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Reverberi, R.; Reverberi, L. Factors affecting the antigen-antibody reaction. Blood Transfus. 2007, 5, 227–240. [Google Scholar]
- Franke, T.; Abate, A.R.; Weitz, D.A.; Wixforth, A. Surface acoustic wave (SAW) directed droplet flow in microfluidics for PDMS devices. Lab Chip 2009, 9, 2625–2627. [Google Scholar] [CrossRef]
- Campbell, C.K. Surface Acoustic Wave Devices for Mobile and Wireless Communications; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Renaudin, A.; Tabourier, P.; Zhang, V.; Camart, J.C.; Druon, C. SAW nanopump for handling droplets in view of biological applications. Sens. Actuators B Chem. 2006, 113, 389–397. [Google Scholar] [CrossRef]
- Raghavan, R.; Friend, J.; Yeo, L. Particle concentration via acoustically driven microcentrifugation: MicroPIV flow visualization and numerical modelling studies. Microfluid. Nanofluid 2010, 8, 73–84. [Google Scholar] [CrossRef]
- Mitsakakis, K.; Gizeli, E. Detection of multiple cardiac markers with an integrated acoustic platform for cardiovascular risk assessment. Anal. Chim. Acta 2011, 699, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, K.K.; Trinchi, A.; Wlodarski, W.; Holland, A. A novel Love-mode device based on a ZnO/ST-cut quartz crystal structure for sensing applications. Sens. Actuators A Phys. 2002, 100, 135–143. [Google Scholar] [CrossRef]
- Deobagkar, D. Acoustic wave immunosensing of Escherichia coli in water. Sens. Actuators B Chem. 2005, 104, 85–89. [Google Scholar] [CrossRef]



| Force | 10 MHz | 50 MHz | 100 MHz |
|---|---|---|---|
| FvdW | 2 × 10−8 | 2 × 10−8 | 2 × 10−8 |
| FSAW | 2 × 10−4 | 8 × 10−5 | 1 × 10−5 |
| FL | 4 × 10−10 | 2 × 10−9 | 4 × 10−8 |
| FST | 4 × 10−10 | 8 × 10−10 | 2 × 10−9 |
| Force | 128° YX LiNbO3 | Langasite (0,22,0) | ST-Quartz |
|---|---|---|---|
| FvdW | 2 × 10−8 | 2 × 10−8 | 2 × 10−8 |
| FSAW | 4 × 10−4 | 3.3 × 10−5 | 5 × 10−6 |
| FL | 2 × 10−14 | 1 × 10−14 | 2 × 10−10 |
| FST | 1 × 10−11 | 4 × 10−11 | 1 × 10−10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, M.; Das, P.K.; Morrill, S.; Suthar, K.J.; Sankaranarayanan, S.K.R.S.; Bhethanabotla, V.R. Removal of Non-Specifically Bound Proteins Using Rayleigh Waves Generated on ST-Quartz Substrates. Sensors 2022, 22, 4096. https://doi.org/10.3390/s22114096
Richardson M, Das PK, Morrill S, Suthar KJ, Sankaranarayanan SKRS, Bhethanabotla VR. Removal of Non-Specifically Bound Proteins Using Rayleigh Waves Generated on ST-Quartz Substrates. Sensors. 2022; 22(11):4096. https://doi.org/10.3390/s22114096
Chicago/Turabian StyleRichardson, Mandek, Pradipta K. Das, Samuel Morrill, Kamlesh J. Suthar, Subramanian K. R. S. Sankaranarayanan, and Venkat R. Bhethanabotla. 2022. "Removal of Non-Specifically Bound Proteins Using Rayleigh Waves Generated on ST-Quartz Substrates" Sensors 22, no. 11: 4096. https://doi.org/10.3390/s22114096