A High-Sensitivity Gravimetric Biosensor Based on S1 Mode Lamb Wave Resonator
Abstract
:1. Introduction
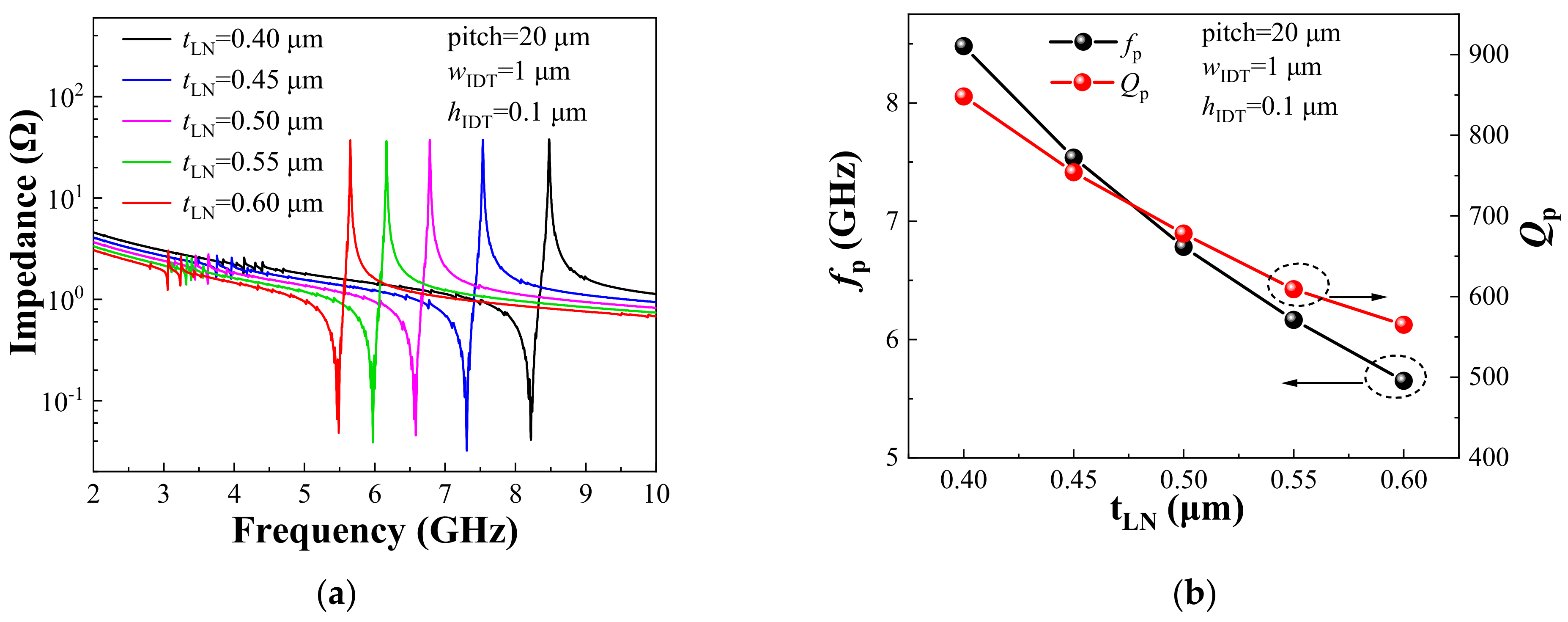
2. Resonators
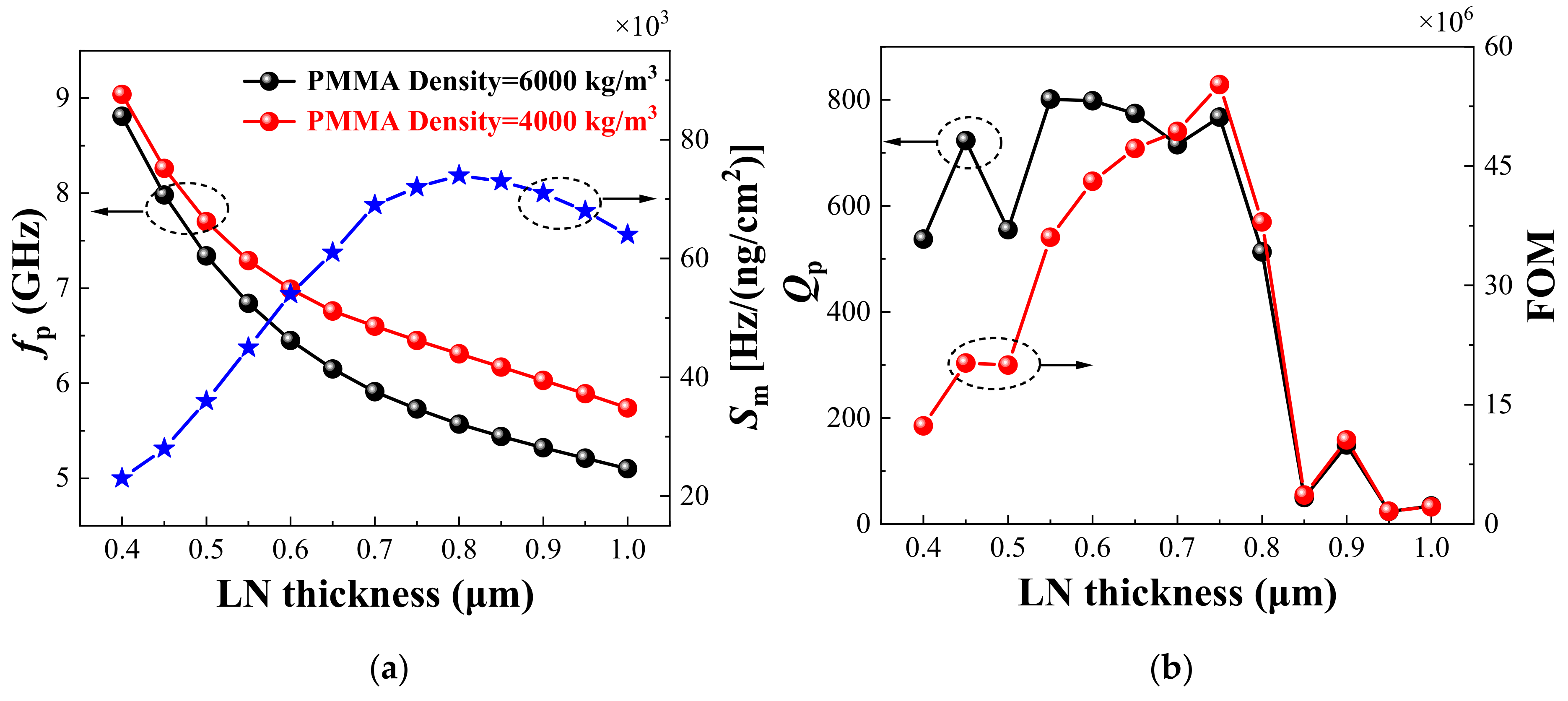
3. Biosensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vigneshvar, S.; Sudhakumari, C.; Senthilkumaran, B.; Prakash, H. Recent advances in biosensor technology for potential applications—An overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Klarák, J.; Andok, R.; Hricko, J.; Klačková, I.; Tsai, H.-Y. Design of the automated calibration process for an experimental laser inspection stand. Sensors 2022, 22, 5306. [Google Scholar] [CrossRef] [PubMed]
- Kuric, I.; Klačková, I.; Domnina, K.; Stenchlák, V.; Sága, M., Jr. Implementation of predictive models in industrial machines with proposed automatic adaptation algorithm. Appl. Sci. 2022, 12, 1853. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastromatteo, U.; Villa, F. High sensitivity acoustic wave AlN/Si mass detectors arrays for artificial olfactory and biosensing applications: A review. Sens. Actuators B Chem. 2013, 179, 319–327. [Google Scholar] [CrossRef]
- Huang, X.-H.; Pan, W.; Hu, J.-G.; Bai, Q.-S. The exploration and confirmation of the maximum mass sensitivity of quartz crystal microbalance. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1888–1892. [Google Scholar] [CrossRef]
- Lim, H.J.; Saha, T.; Tey, B.T.; Tan, W.S.; Ooi, C.W. Quartz crystal microbalance-based biosensors as rapid diagnostic devices for infectious diseases. Biosens. Bioelectron. 2020, 168, 112513. [Google Scholar] [CrossRef]
- Ji, J.; Yang, C.; Zhang, F.; Shang, Z.; Xu, Y.; Chen, Y.; Chen, M.; Mu, X. A high sensitive SH-SAW biosensor based 36 YX black LiTaO3 for label-free detection of Pseudomonas Aeruginosa. Sens. Actuators B Chem. 2019, 281, 757–764. [Google Scholar] [CrossRef]
- Ten, S.; Hashim, U.; Gopinath, S.; Liu, W.; Foo, K.; Sam, S.; Rahman, S.; Voon, C.; Nordin, A. Highly sensitive Escherichia coli shear horizontal surface acoustic wave biosensor with silicon dioxide nanostructures. Biosens. Bioelectron. 2017, 93, 146–154. [Google Scholar] [CrossRef]
- Li, S.; Richardson, M.; Sankaranarayanan, S.K.; Fan, C.; Su, Y.; Bhethanabotla, V.R. Design and fabrication of SiO 2 waveguide-based SAW Sensors with filled Microcavities. In Proceedings of the 2015 IEEE SENSORS, Busan, Korea, 1–4 November 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–4. [Google Scholar]
- Zhang, H.; Kim, E.S. Micromachined acoustic resonant mass sensor. J. Microelectromech. Syst. 2005, 14, 699–706. [Google Scholar] [CrossRef]
- Guo, P.; Xiong, J.; Zheng, D.; Zhang, W.; Liu, L.; Wang, S.; Gu, H. A biosensor based on a film bulk acoustic resonator and biotin–avidin system for the detection of the epithelial tumor marker mucin 1. RSC Adv. 2015, 5, 66355–66359. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, J.; Flewitt, A.J.; Cai, Z.; Zhao, X. Film bulk acoustic resonators (FBARs) as biosensors: A review. Biosens. Bioelectron. 2018, 116, 1–15. [Google Scholar] [CrossRef]
- Xu, W.; Choi, S.; Chae, J. A contour-mode film bulk acoustic resonator of high quality factor in a liquid environment for biosensing applications. Appl. Phys. Lett. 2010, 96, 053703. [Google Scholar] [CrossRef] [Green Version]
- Voiculescu, I.; Nordin, A.N. Acoustic wave based MEMS devices for biosensing applications. Biosens. Bioelectron. 2012, 33, 1–9. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Dickert, F.L. An overview of high frequency acoustic sensors—QCMs, SAWs and FBARs—Chemical and biochemical applications. Sensors 2019, 19, 4395. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Shih, W.-C.; Chang, W.-T.; Yang, C.-H.; Kao, K.-S.; Cheng, C.-C. Biosensor for human IgE detection using shear-mode FBAR devices. Nanoscale Res. Lett. 2015, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Al-Qahtani, A.M.; Khelif, A.; Boussaid, F.; Benchabane, S.; Cheng, Y.; El Agnaf, O.; Bermak, A. Towards acoustic radiation free lamb wave resonators for high-resolution gravimetric biosensing. IEEE Sens. J. 2020, 21, 2725–2733. [Google Scholar] [CrossRef]
- Gao, F.; Bermak, A.; Benchabane, S.; Robert, L.; Khelif, A. Acoustic radiation-free surface phononic crystal resonator for in-liquid low-noise gravimetric detection. Microsyst. Nanoeng. 2021, 7, 8. [Google Scholar] [CrossRef]
- Mirea, T.; Yantchev, V.; Olivares, J.; Iborra, E. Influence of liquid properties on the performance of S0-mode Lamb wave sensors II: Experimental validation. Sens. Actuators B Chem. 2016, 229, 331–337. [Google Scholar] [CrossRef]
- Yantchev, V.; Katardjiev, I. Thin film Lamb wave resonators in frequency control and sensing applications: A review. J. Micromech. Microeng. 2013, 23, 043001. [Google Scholar] [CrossRef]
- Bjurström, J.; Katardjiev, I.; Yantchev, V. Lateral-field-excited thin-film Lamb wave resonator. Appl. Phys. Lett. 2005, 86, 925. [Google Scholar] [CrossRef]
- Lissenden, C.J.; Hakoda, C.N.; Shokouhi, P. Control of low-frequency Lamb wave propagation in plates by boundary condition manipulation. J. Appl. Phys. 2021, 129, 094903. [Google Scholar] [CrossRef]
- Bassignot, F.; Courjon, E.; Ulliac, G.; Ballandras, S.; Lesage, J.M.; Petit, R. Acoustic resonator based on periodically poled transducers: Concept and analysis. J. Appl. Phys. 2012, 111, 114107. [Google Scholar] [CrossRef]
- Bassignot, F.; Courjon, E.; Ulliac, G.; Ballandras, S.; Lesage, J.M.; Petit, R. Acoustic resonator based on periodically poled transducers: Fabrication and characterization. J. Appl. Phys. 2012, 112, 074108. [Google Scholar] [CrossRef]
- Zou, J. High-Performance Aluminum Nitride Lamb Wave Resonators for RF Front-End Technology. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2015. [Google Scholar]
- Liu, W.; Zhan, D.; Ma, X.; Song, Z.; Feng, S. Fabrication of single-crystalline LiTaO3 film on silicon substrate using thin film transfer technology. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Processing Meas. Phenom. 2008, 26, 206–208. [Google Scholar] [CrossRef]
- Takigawa, R.; Higurashi, E.; Suga, T.; Kawanishi, T. Room-temperature transfer bonding of lithium niobate thin film on micromachined silicon substrate with Au microbumps. Sens. Actuators A Phys. 2017, 264, 274–281. [Google Scholar] [CrossRef]
- Shimazoe, K.; Nishinaka, H.; Arata, Y.; Tahara, D.; Yoshimoto, M. Phase control of α-and κ-Ga2O3 epitaxial growth on LiNbO3 and LiTaO3 substrates using α-Fe2O3 buffer layers. AIP Adv. 2020, 10, 055310. [Google Scholar] [CrossRef]
- Fujimaki, T.; Suzuki, M.; Kakio, S. Analysis of leaky surface acoustic waves on LiNbO3 or LiTaO3 thin plate bonded to similar-material substrates. Jpn. J. Appl. Phys. 2020, 59, SKKC01. [Google Scholar] [CrossRef]
- Zou, J.; Lin, C.-M.; Gao, A.; Pisano, A.P. The multi-mode resonance in AlN Lamb wave resonators. J. Microelectromech. Syst. 2018, 27, 973–984. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, X.; Cheng, H.; Xiao, S.; Sun, H.; Zuo, C. Ultra high Q lithium niobate resonator at 15-degree three-dimensional Euler angle. IEEE Electron Device Lett. 2022, 43, 1105–1108. [Google Scholar] [CrossRef]
- Cherng, J.; Chen, T.; Lin, C. Pulsed-DC sputtering of molybdenum bottom electrode and piezoelectric aluminum nitride films for bulk acoustic resonator applications. Thin Solid Film. 2011, 519, 6797–6800. [Google Scholar] [CrossRef]
- Mansfeld, G.; Alekseev, S.; Kotelyansky, I. Acoustic HBAR spectroscopy of metal (W, Ti, Mo, Al) thin films. In Proceedings of the 2001 IEEE Ultrasonics Symposium. Proceedings. An International Symposium (Cat. No. 01CH37263), Atlanta, GA, USA, 7–10 October 2001; IEEE: Piscataway, NJ, USA, 2001; pp. 415–418. [Google Scholar]
- Temel, F.; Ozaytekin, I. The enhanced humidity sensing performance of calixarene/PMMA hybrid layers: QCM sensing mechanism. J. Mater. Sci. Mater. Electron. 2022, 33, 2801–2815. [Google Scholar] [CrossRef]
- Pandey, P.S.; Singh, Y.; Raghuwanshi, S.K. Theoretical analysis of the LRSPR sensor with enhance FOM for low refractive index detection using mxene and fluorinated graphene. IEEE Sens. J. 2021, 21, 23979–23986. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Liu, W.; Wen, Z.; Xie, Y.; Tong, X.; Cai, Y.; Liu, Y.; Sun, C. A High-Sensitivity Gravimetric Biosensor Based on S1 Mode Lamb Wave Resonator. Sensors 2022, 22, 5912. https://doi.org/10.3390/s22155912
Luo T, Liu W, Wen Z, Xie Y, Tong X, Cai Y, Liu Y, Sun C. A High-Sensitivity Gravimetric Biosensor Based on S1 Mode Lamb Wave Resonator. Sensors. 2022; 22(15):5912. https://doi.org/10.3390/s22155912
Chicago/Turabian StyleLuo, Tiancheng, Wenjuan Liu, Zhiwei Wen, Ying Xie, Xin Tong, Yao Cai, Yan Liu, and Chengliang Sun. 2022. "A High-Sensitivity Gravimetric Biosensor Based on S1 Mode Lamb Wave Resonator" Sensors 22, no. 15: 5912. https://doi.org/10.3390/s22155912





