Simple and Sensitive Detection of Bacterial Hydrogen Sulfide Production Using a Paper-Based Colorimetric Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of H2S-Sensing Paper
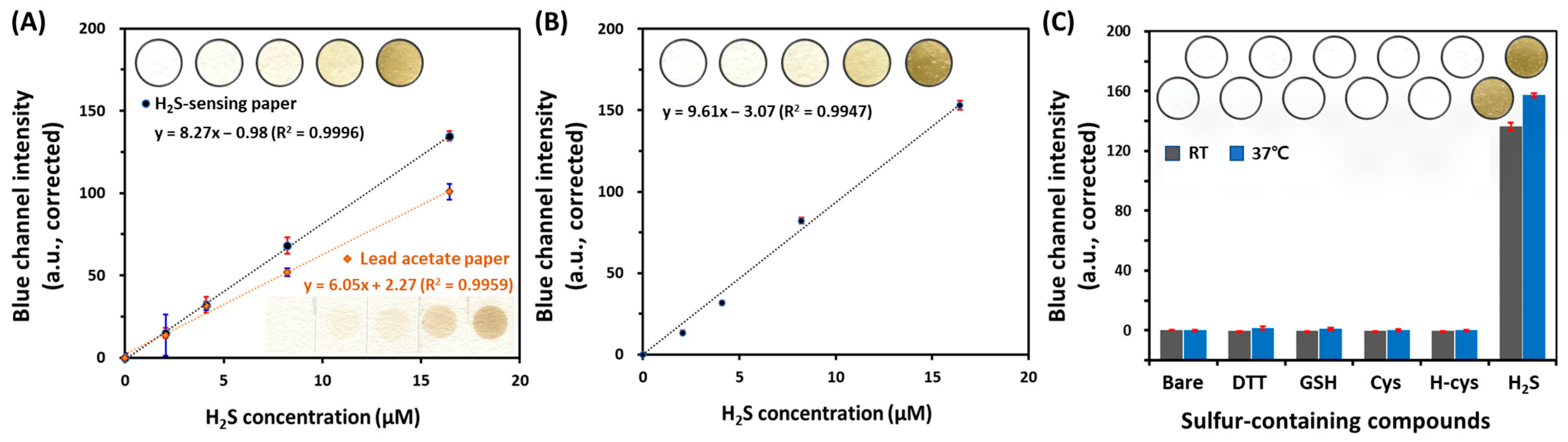
2.3. Evaluation of Analytical Performance of the H2S-Sensing Paper
2.4. Bacterial Strains and Culture Conditions
2.5. Detection of Bacterial H2S Using the H2S-Sensing Paper
3. Results
3.1. Fabrication of H2S-Sensing Paper
3.2. Analytical Performance of H2S-Sensing Paper
3.3. Detection of Bacterial H2S Production Using H2S-Sensing Paper
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giovinazzo, D.; Bursac, B.; Sbodio, J.I.; Nalluru, S.; Vignane, T.; Snowman, A.M.; Albacarys, L.M.; Sedlak, T.W.; Orregrossa, R.; Whiteman, M.; et al. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA 2021, 118, e2017225118. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Cao, L.; Ding, L.; Bian, J.S. A new hope for a devastating disease: Hydrogen sulfide in Parkinson’s disease. Mol. Neurobiol. 2018, 55, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Wang, X.Y.; Bian, J.S. Implications of hydrogen sulfide in liver pathophysiology: Mechanistic insights and therapeutic potential. J. Adv. Res. 2021, 27, 127–135. [Google Scholar] [CrossRef]
- Bhatia, M.; Wong, F.L.; Fu, D.; Lau, H.Y.; Moochhala, S.M.; Moore, P.K. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005, 19, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2012, 17, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Seerangaiyan, K.; van Winkelhoff, A.J.; Harmsen, H.; Rossen, J.; Winkel, E.G. The tongue microbiome in healthy subjects and patients with intra-oral halitosis. J. Breath Res. 2017, 11, 036010. [Google Scholar] [CrossRef]
- Pavolotskaya, A.; McCombs, G.; Darby, M.; Marinak, K.; Dayanand, N.N. Sulcular sulfide monitoring: An indicator of early dental plaque-induced gingival disease. J. Dent. Hyg. 2006, 80, 1–12. [Google Scholar]
- Wu, D.D.; Ngowi, E.E.; Zhai, Y.K.; Wang, Y.Z.; Khan, N.H.; Kombo, A.F.; Khattak, S.; Li, T.; Ji, X.Y. Role of Hydrogen Sulfide in Oral Disease. Oxid. Med. Cell. Longev. 2022, 2022, 1886277. [Google Scholar] [CrossRef]
- Carrero-Ferrer, I.; Molins-Legua, C.; Campíns-Falcó, P. Plasmonic sensor for hydrogen sulphide in saliva: Multisensor platform and bag format. Talanta 2022, 245, 123449. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chung, S.W.; Auh, Q.S.; Hong, S.J.; Lee, Y.A.; Jung, J.; Lee, G.J.; Park, H.J.; Shin, S.I.; Hong, J.Y. Progress in Oral Microbiome Related to Oral and Systemic Diseases: An Update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef]
- Basic, A.; Blomqvist, S.; Carlén, A.; Dahlén, G. Estimation of bacterial hydrogen sulfide production in vitro. J. Oral Microbiol. 2015, 7, 28166. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Rapid detection of hydrogen sulfide produced by pathogenic bacteria in focused growth media using SHS-MCC-GC-IMS. Microchem. J. 2018, 140, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, A.; Yoshimura, M.; Ohara, N.; Yoshimura, S.; Nagashima, S.; Takehara, T.; Nakayamai, K. Hydrogen Sulfide Production from Cysteine and Homocysteine by Periodontal and Oral Bacteria. J. Periodontol. 2009, 80, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chu, W. A sensitive visual method for the detection of hydrogen sulfide producing bacteria. J. Vis. Exp. 2022, 184, e64201. [Google Scholar] [CrossRef] [PubMed]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A Universal Defense Against Antibiotics in Bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef]
- Xia, Y.; Lü, C.; Hou, N.; Xin, Y.; Liu, J.; Liu, H.; Xun, L. Sulfide production and oxidation by heterotrophic bacteria under aerobic conditions. ISME J. 2017, 11, 2754–2766. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.; Park, J.H.; Oh, J.; Kim, K.; Kim, M.G. Advanced Colorimetric Paper Sensors Using Color Focusing Effect Based on Asymmetric Flow of Fluid. ACS Sens. 2019, 4, 1103–1108. [Google Scholar] [CrossRef]
- Sher, M.; Zhuang, R.; Demirci, U.; Asghara, W. Paper-based analytical devices for clinical diagnosis: Recent advances in the fabrication techniques and sensing mechanisms. Expert Rev. Mol. Diagn. 2017, 17, 351–366. [Google Scholar] [CrossRef]
- Ham, K.N.; Choi, J.S.; Kwon, J. Three-dimensional paper-based slip device for one-step point-of-care testing. Sci. Rep. 2016, 6, 25710. [Google Scholar]
- Nilghaz, A.; Guan, L.; Tan, W.; Shen, W. Advances of Paper-Based Microfluidics for Diagnostics—The Original Motivation and Current Status. ACS Sens. 2016, 1, 1382–1393. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.J.; Ahn, Y.J.; Choi, S.; Lee, G.J. A simple and facile paper-based colorimetric assay for detection of free hydrogen sulfide in prostate cancer cells. Sens. Actuator B Chem. 2018, 256, 828–834. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.J.; Ahn, Y.J.; Kim, M.; Lee, G.J. In Situ Detection of Hydrogen Sulfide in 3D-Cultured, Live Prostate Cancer Cells Using a Paper-Integrated Analytical Device. Chemosensors 2022, 10, 27. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Lee, Y.J.; Lee, J.; Lee, D.; Park, H.K.; Lee, G.J. Colorimetric detection of endogenous hydrogen sulfide production in living cells utilizing silver-embedded polymer membrane. Spectroc. Acta Part A Mol. Biomol. Spectr. 2017, 177, 118–124. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Gil, Y.G.; Lee, Y.J.; Jang, H.; Lee, G.J. A dual-mode colorimetric and SERS detection of hydrogen sulfide in live prostate cancer cells using a silver nanoplate-coated paper assay. Microchem. J. 2020, 155, 104724. [Google Scholar] [CrossRef]
- Nagy, L.; Filotas, D.; Boros, M.; Pozsgai, G.; Pintér, E.; Nagy, G. Amperometric cell for subcutaneous detection of hydrogen sulfide in anesthetized experimental animals. Physiol. Meas. 2014, 35, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Han, S.H.; Lee, G.J. Rapid and simple colorimetric detection of hydrogen sulfide using an etching-resistant effect on silver nanoprisms. Microchim. Acta 2021, 188, 129. [Google Scholar] [CrossRef] [PubMed]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in quality in the analytical laboratory. II. analytical method validation and quality assurance. Trends Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- Mironov, A.; Seregina, T.; Nagornykh, M.; Luhachack, L.G.; Korolkova, N.; Lopes, L.E.; Kotova, V.; Zavilgelsky, G.; Shakulov, R.; Shatalin, K.; et al. Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli. Proc. Natl. Acad. Sci. USA 2017, 114, 6022–6027. [Google Scholar] [CrossRef] [Green Version]
- Hampelska, K.; Jaworska, M.M.; Babalska, Z.Ł.; Karpiński, T.M. The Role of Oral Microbiota in Intra-Oral Halitosis. J. Clin. Med. 2020, 9, 2484. [Google Scholar] [CrossRef]
- Aydin, M.; Gunay, I. Cysteine challenge test as a novel diagnostic tool to distinguish oral halitosis. Aust. Dent. J. 2022, 67, 69–75. [Google Scholar] [CrossRef]
- Persson, S.; Edlund, M.B.; Claesson, R.; Carlsson, J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol. Immunol. 1990, 5, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kleinberg, I.; Codipilly, D.M. Cystein challenge testing: A powerful tool for examining oral malodour processes and treatments in vivo. Int. Dent. J. 2002, 52, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Codipilly, D.; Kleinberg, I. Generation of indole/skatole during malodor formation in the salivary sediment model system and initial examination of the oral bacteria involved. J. Breath Res. 2008, 2, 17017. [Google Scholar] [CrossRef] [PubMed]
- Puig-Silla, M.; Montiel-Company, J.M.; Dasi-Fernandez, F.; Almerich-Silla, J.M. Prevalence of periodontal pathogens as predictor of the evolution of periodontal status. Odontology 2017, 105, 467–476. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, B.-K.; Ahn, Y.-J.; Lee, Y.-J.; Lee, Y.-H.; Lee, G.-J. Simple and Sensitive Detection of Bacterial Hydrogen Sulfide Production Using a Paper-Based Colorimetric Assay. Sensors 2022, 22, 5928. https://doi.org/10.3390/s22155928
Ahn B-K, Ahn Y-J, Lee Y-J, Lee Y-H, Lee G-J. Simple and Sensitive Detection of Bacterial Hydrogen Sulfide Production Using a Paper-Based Colorimetric Assay. Sensors. 2022; 22(15):5928. https://doi.org/10.3390/s22155928
Chicago/Turabian StyleAhn, Byung-Ki, Yong-Jin Ahn, Young-Ju Lee, Yeon-Hee Lee, and Gi-Ja Lee. 2022. "Simple and Sensitive Detection of Bacterial Hydrogen Sulfide Production Using a Paper-Based Colorimetric Assay" Sensors 22, no. 15: 5928. https://doi.org/10.3390/s22155928





