Non-Specific Responsive Nanogels and Plasmonics to Design MathMaterial Sensing Interfaces: The Case of a Solvent Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Nanogels
2.3. Dynamic Light Scattering
2.4. Measurement of the Swelling Ratio
2.5. SPR POF Platform
2.6. Preparation of the Nanogel–POF Platform
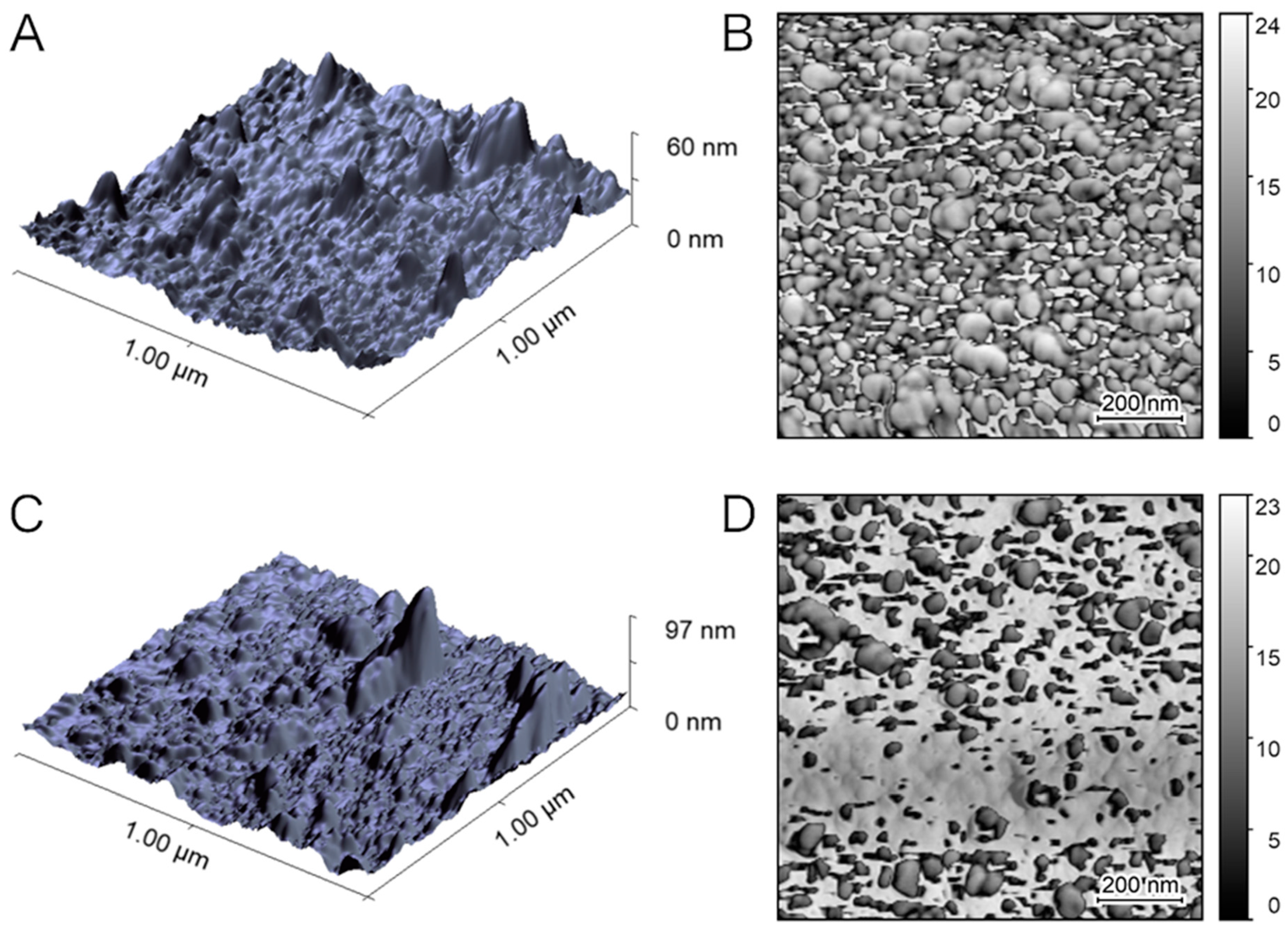
2.7. Analysis of the Nanogel–POF by Atomic Force Microscopy
2.8. Preparation of the Solutions for Test-Measurements
2.9. MathMaterial Sensor Measurements
3. Results and Discussion
3.1. Responsive Behaviour of the Soft Nanogels in Solvent
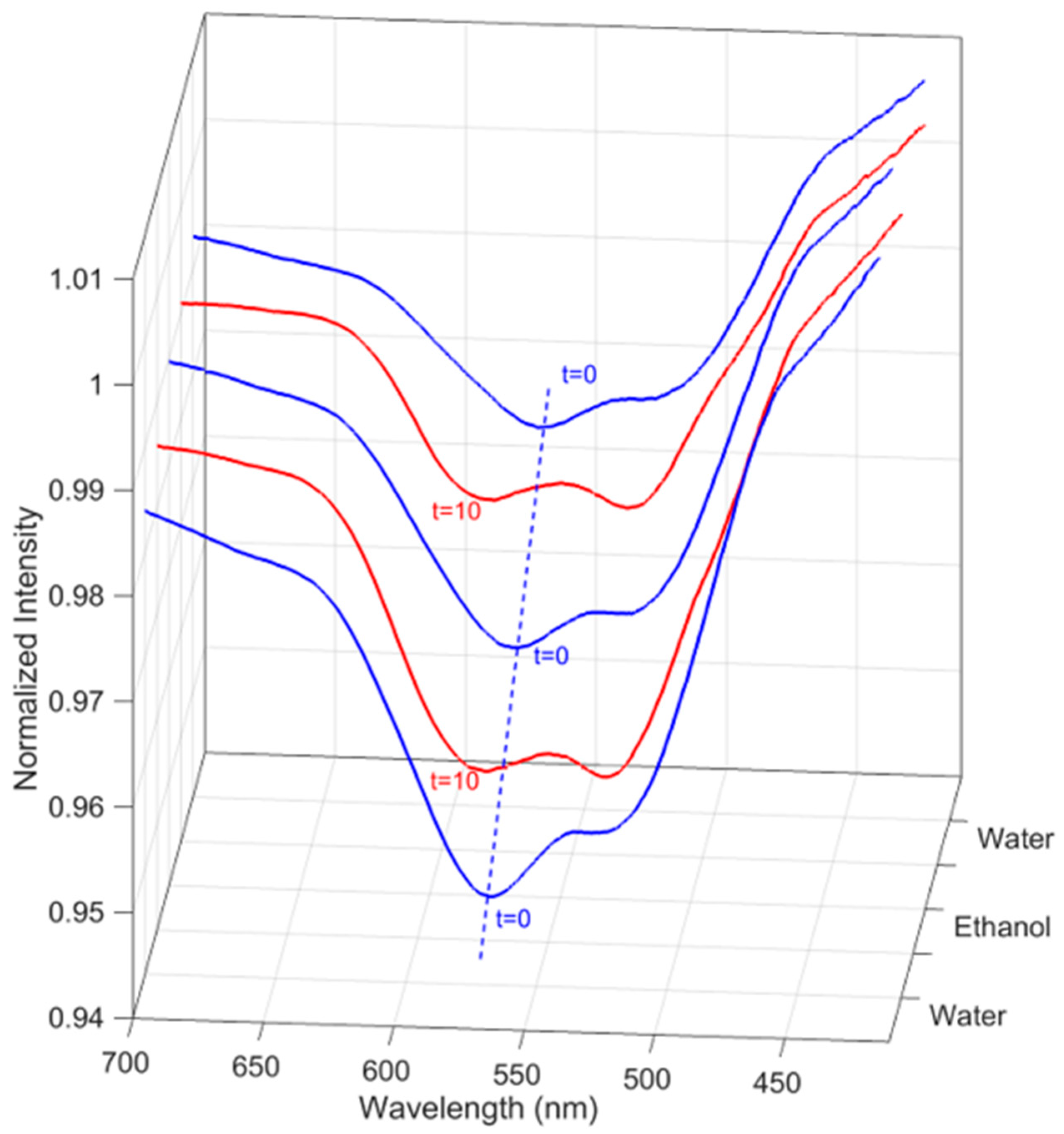
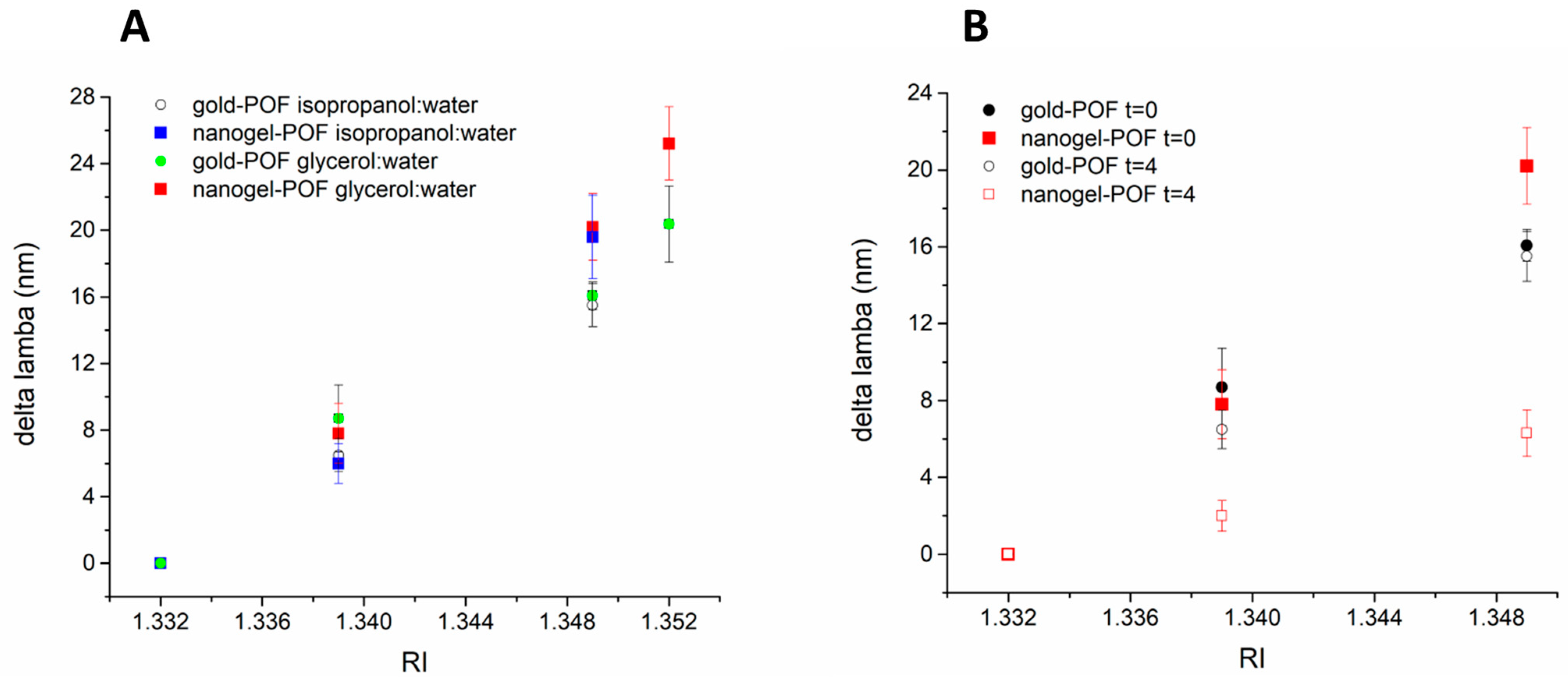
3.2. Responsive Behaviour of the Soft Nanogel–POF Interface
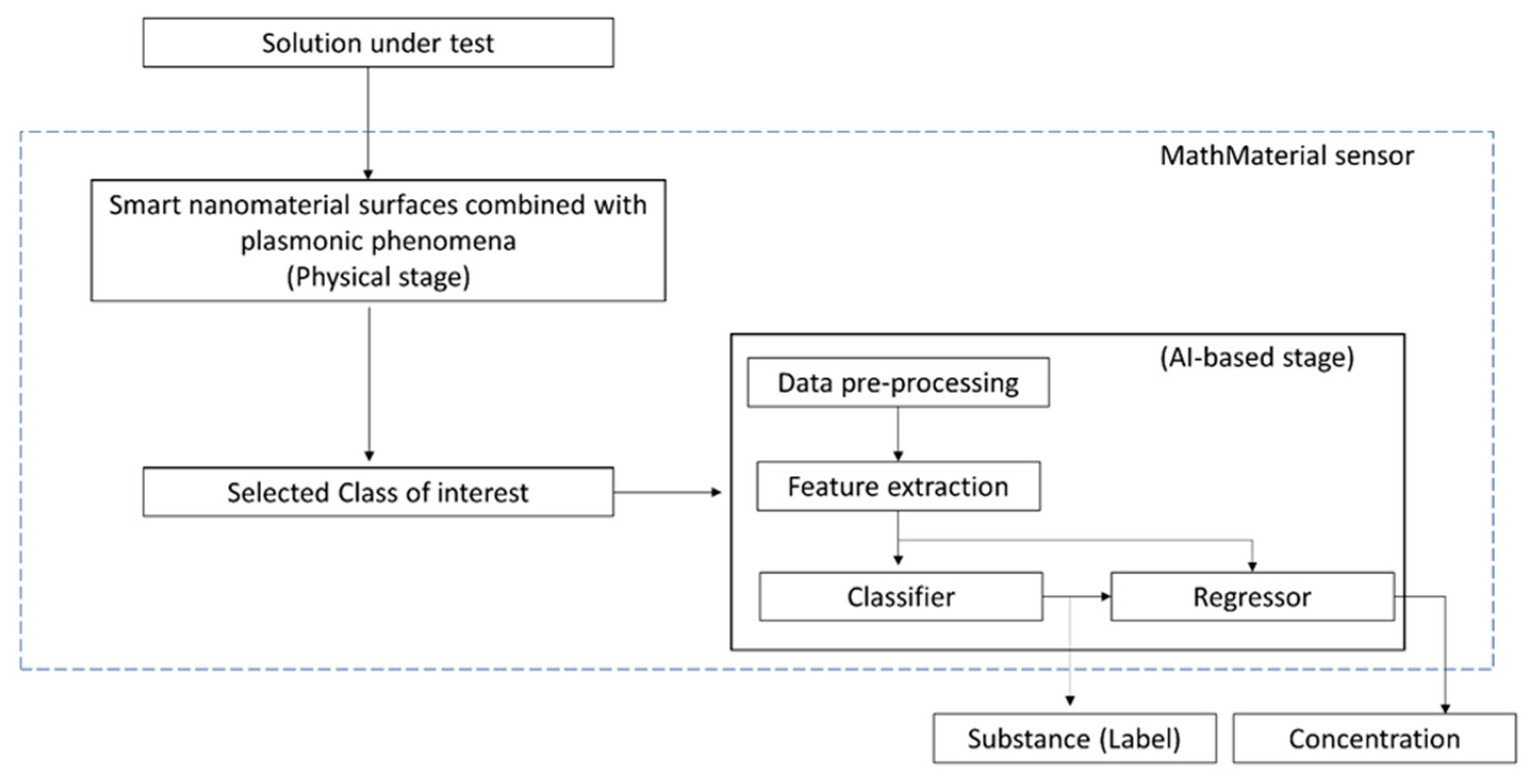
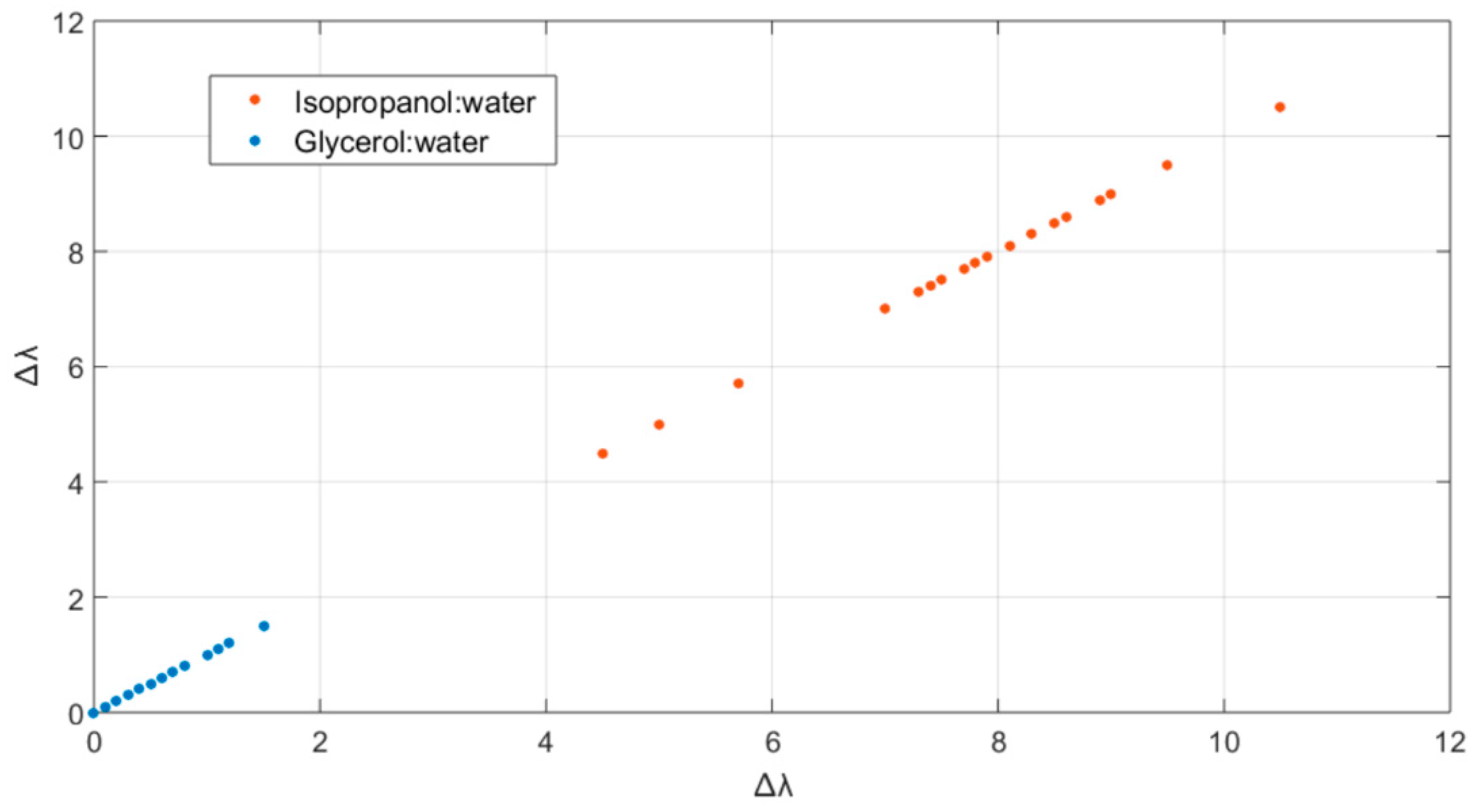
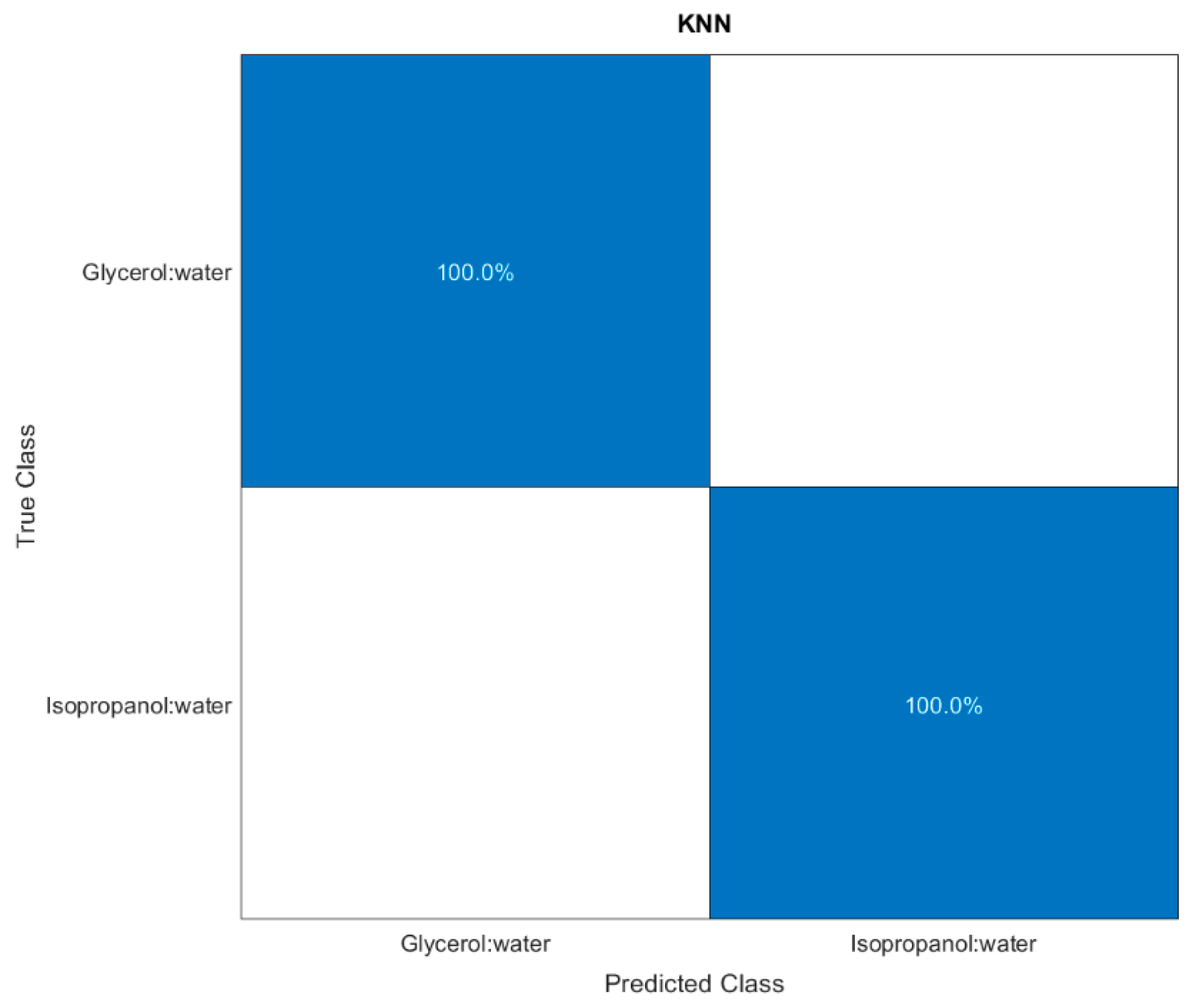
3.3. Towards MathMaterial Sensing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical Sensors: Definitions and Classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Morales, M.A.; Halpern, J.M. Guide to Selecting a Biorecognition Element for Biosensors. Bioconjug. Chem. 2018, 29, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, H.; Mitrovics, J.; Weimar, U.; Göpel, W. Sensor Arrays with Only One or Several Transducer Principles? The Advantage of Hybrid Modular Systems. Sens. Actuators B Chem. 2000, 65, 79–81. [Google Scholar] [CrossRef]
- Hu, W.; Wan, L.; Jian, Y.; Ren, C.; Jin, K.; Su, X.; Bai, X.; Haick, H.; Yao, M.; Wu, W. Electronic Noses: From Advanced Materials to Sensors Aided with Data Processing. Adv. Mater. Technol. 2018, 4, 1800488. [Google Scholar] [CrossRef] [Green Version]
- Dannhauser, D.; Rossi, D.; Memmolo, P.; Finizio, A.; Ferraro, P.; Netti, P.A.; Causa, F. Biophysical Investigation of Living Monocytes in Flow by Collaborative Coherent Imaging Techniques. Biomed. Opt. Express 2018, 9, 5194. [Google Scholar] [CrossRef]
- Paiva, J.S.; Jorge, P.A.S.; Ribeiro, R.S.R.; Sampaio, P.; Rosa, C.C.; Cunha, J.P.S. Optical Fiber-Based Sensing Method for Nanoparticle Detection through Supervised Back-Scattering Analysis: A Potential Contributor for Biomedicine. Int. J. Nanomed. 2019, 2019, 2349–2369. [Google Scholar] [CrossRef] [Green Version]
- Leng, J.; Lan, X.; Liu, Y.; Du, S. Shape-Memory Polymers and Their Composites: Stimulus Methods and Applications. Prog. Mater. Sci. 2011, 56, 1077–1135. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, H.; Priimagi, A.; Ikkala, O. Programmable Responsive Hydrogels Inspired by Classical Conditioning Algorithm. Nat. Commun. 2019, 10, 3267. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Philippova, O.E. Responsive Polymer Gels. Polym. Sci. Ser. C 2000, 42, 208–228. [Google Scholar]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on Hydrogel-Based Ph Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Garner, B.W.; Cai, T.; Ghosh, S.; Hu, Z.; Neogi, A. Refractive Index Change Due to Volume-Phase Transition in Polyacrylamide Gel Nanospheres for Optoelectronics and Bio-Photonics. Appl. Phys. Express 2009, 2, 057001. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Jiang, N.; Yetisen, A.K.; Yuk, H.; Yang, C.; Khademhosseini, A.; Zhao, X.; Yun, S.-H. Highly Stretchable, Strain Sensing Hydrogel Optical Fibers. Adv. Mater. 2016, 28, 10244–10249. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; MacConaghy, K.I.; Kaar, J.L.; Stoykovich, M.P. Enhanced Optical Sensitivity in Thermoresponsive Photonic Crystal Hydrogels by Operating near the Phase Transition. ACS Appl. Mater. Interfaces 2017, 9, 27927–27935. [Google Scholar] [CrossRef]
- Cennamo, N.; Maniglio, D.; Tatti, R.; Zeni, L.; Bossi, A.M. Deformable Molecularly Imprinted Nanogels Permit Sensitivity-Gain in Plasmonic Sensing. Biosens. Bioelectron. 2020, 156, 112126. [Google Scholar] [CrossRef]
- Cennamo, N.; Bossi, A.M.; Arcadio, F.; Maniglio, D.; Zeni, L. On the Effect of Soft Molecularly Imprinted Nanoparticles Receptors Combined to Nanoplasmonic Probes for Biomedical Applications. Front. Bioeng. Biotechnol. 2021, 9, 801489. [Google Scholar] [CrossRef]
- Matsui, J.; Akamatsu, K.; Hara, N.; Miyoshi, D.; Nawafune, H.; Tamaki, K.; Sugimoto, N. SPR Sensor Chip for Detection of Small Molecules Using Molecularly Imprinted Polymer with Embedded Gold Nanoparticles. Anal. Chem. 2005, 77, 4282–4285. [Google Scholar] [CrossRef]
- Cennamo, N.; Massarotti, D.; Conte, L.; Zeni, L. Low Cost Sensors Based on SPR in a Plastic Optical Fiber for Biosensor Implementation. Sensors 2011, 11, 11752–11760. [Google Scholar] [CrossRef]
- Cennamo, N.; Pesavento, M.; Lunelli, L.; Vanzetti, L.; Pederzolli, C.; Zeni, L.; Pasquardini, L. An Easy Way to Realize SPR Aptasensor: A Multimode Plastic Optical Fiber Platform for Cancer Biomarkers Detection. Talanta 2015, 140, 88–95. [Google Scholar] [CrossRef]
- Çaykara, T.; Turan, E. Effect of the Amount and Type of the Crosslinker on the Swelling Behavior of Temperature-Sensitive Poly(n-Tert-Butylacrylamide-Co-Acrylamide) Hydrogels. Colloid Polym. Sci. 2006, 284, 1038–1048. [Google Scholar] [CrossRef]
- Miner, C.S.; Dalton, N.N. Physical Properties of Glycerine and Its Solutions; Glycerine Producers’ Association: New York, NY, USA, 1963; p. 27. [Google Scholar]
- Washburn, E.W. International Critical Tables of Numerical Data, Physics, Chemistry and Technology; McGraw-Hill: New York, NY, USA, 1933; Volume III. [Google Scholar]
- Cenci, L.; Tatti, R.; Tognato, R.; Ambrosi, E.; Piotto, C.; Bossi, A.M. Synthesis and Characterization of Peptide-Imprinted Nanogels of Controllable Size and Affinity. Eur. Polym. J. 2018, 109, 453–459. [Google Scholar] [CrossRef]
- De Oliveira, R.R.L.; Albuquerque, D.A.C.; Cruz, T.G.S.; Yamaji, F.M.; Leite, F.L. Measurements at the Nanoscale Roughness by Atomic Force Microscopy: Basic Principles and Applications; Bellitto, V., Ed.; Intech Open: Rijeka, Croatia, 2012; ISBN 978-953-51-0414-8. [Google Scholar]
- Ling, Q.-D.; Liaw, D.-J.; Zhu, C.; Chan, D.S.-H.; Kang, E.-T.; Neoh, K.-G. Polymer Electronic Memories: Materials, Devices and Mechanisms. Prog. Polym. Sci. 2008, 33, 917–978. [Google Scholar] [CrossRef]
- Caucheteur, C.; Guo, T.; Albert, J. Review of Plasmonic Fiber Optic Biochemical Sensors: Improving the Limit of Detection. Anal. Bioanal. Chem. 2015, 407, 3883–3897. [Google Scholar] [CrossRef]
- Çaykara, T.; Doğmuş, M. The Effect of Solvent Composition on Swelling and Shrinking Properties of Poly(Acrylamide-Co-Itaconic Acid) Hydrogels. Eur. Polym. J. 2004, 40, 2605–2609. [Google Scholar] [CrossRef]
- Janiesch, C.; Zschech, P.; Heinrich, K. Machine Learning and Deep Learning. Electron. Mark. 2021, 31, 685–695. [Google Scholar] [CrossRef]
- Tkatchenko, A. Machine Learning for Chemical Discovery. Nat. Commun. 2020, 11, 4125. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’Amico, A. Nonspecific Sensor Arrays (“Electronic Tongue”) for Chemical Analysis of Liquids (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cennamo, N.; Arcadio, F.; Capasso, F.; Maniglio, D.; Zeni, L.; Bossi, A.M. Non-Specific Responsive Nanogels and Plasmonics to Design MathMaterial Sensing Interfaces: The Case of a Solvent Sensor. Sensors 2022, 22, 10006. https://doi.org/10.3390/s222410006
Cennamo N, Arcadio F, Capasso F, Maniglio D, Zeni L, Bossi AM. Non-Specific Responsive Nanogels and Plasmonics to Design MathMaterial Sensing Interfaces: The Case of a Solvent Sensor. Sensors. 2022; 22(24):10006. https://doi.org/10.3390/s222410006
Chicago/Turabian StyleCennamo, Nunzio, Francesco Arcadio, Fiore Capasso, Devid Maniglio, Luigi Zeni, and Alessandra Maria Bossi. 2022. "Non-Specific Responsive Nanogels and Plasmonics to Design MathMaterial Sensing Interfaces: The Case of a Solvent Sensor" Sensors 22, no. 24: 10006. https://doi.org/10.3390/s222410006







