Development of a Smartphone-Integrated Reflective Scatterometer for Bacterial Identification
Abstract
:1. Introduction
2. Materials and Methods
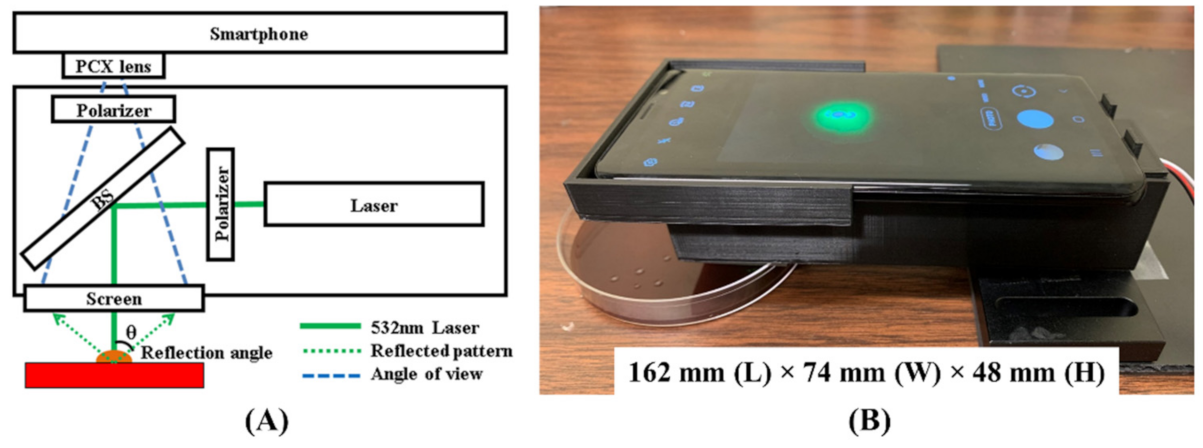
2.1. Instrument Design
2.2. Sample Preparation
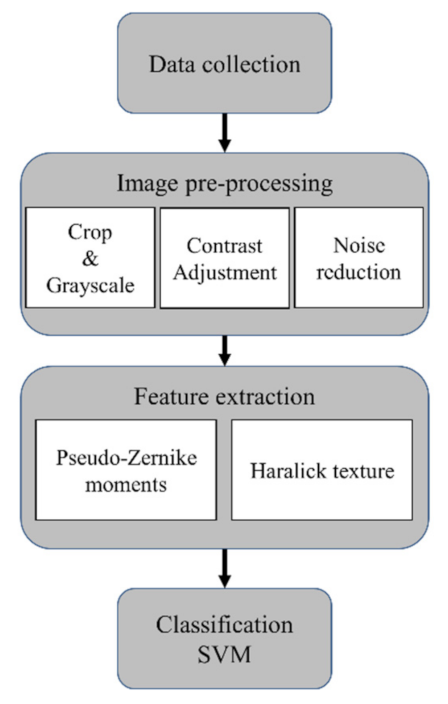
2.3. Measurement and Classification
3. Results
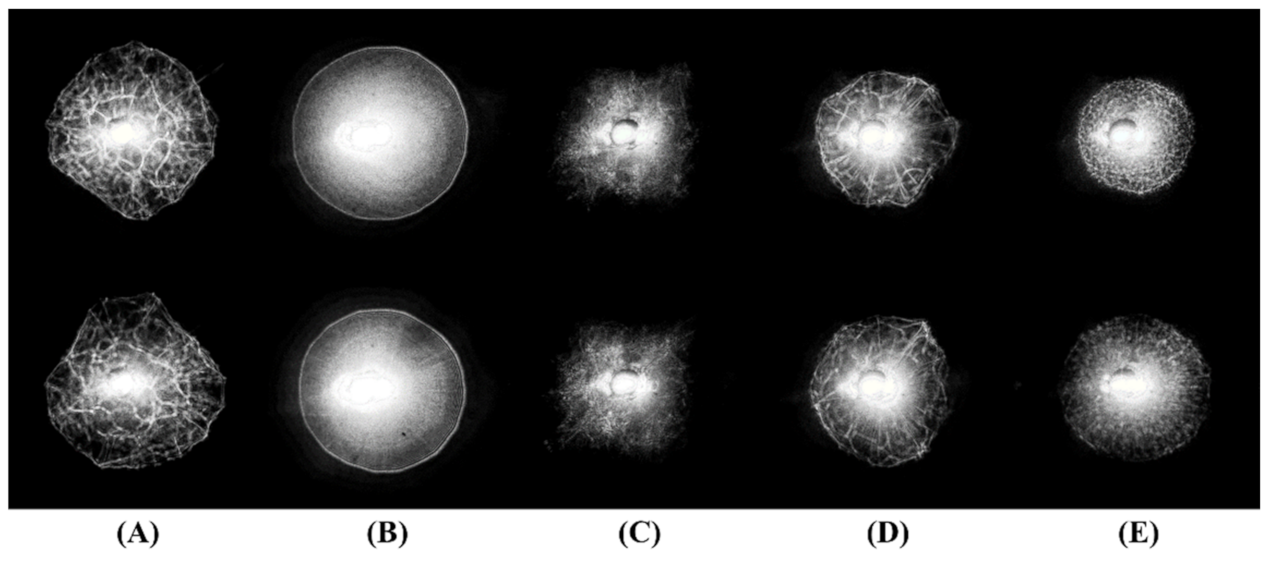
3.1. Reflection Pattern Images
3.2. Genera Level Classification
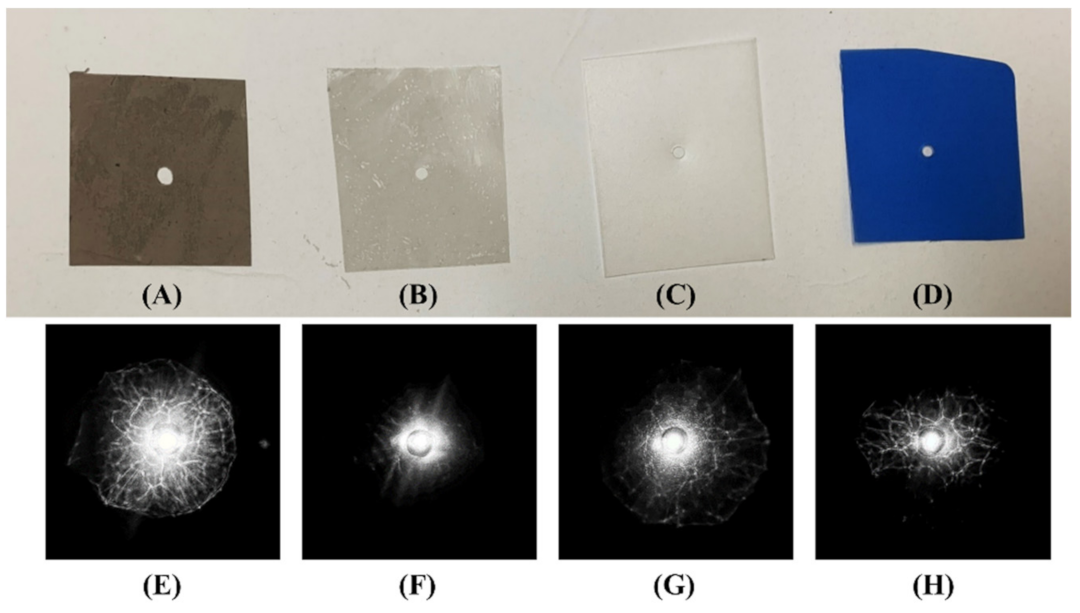
3.3. Colonies on Various Nutrition Media
4. Discussion
4.1. Hardware Design
4.2. Colony Morphology and Reflective Scattering Pattern
4.3. Classification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vidic, J.; Vizzini, P.; Manzano, M.; Kavanaugh, D.; Ramarao, N.; Zivkovic, M.; Radonic, V.; Knezevic, N.; Giouroudi, I.; Gadjanski, I. Point-of-Need DNA Testing for Detection of Foodborne Pathogenic Bacteria. Sensors 2019, 19, 1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajapaksha, P.; Elbourne, A.; Gangadoo, S.; Brown, R.; Cozzolino, D.; Chapman, J. A Review of Methods for the Detection of Pathogenic Microorganisms. Analyst 2019, 144, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Kim, H.; Rajwa, B.; Thomas, J.G.; Robinson, J.P. Current Status and Future Prospects of Using Advanced Computer-Based Methods to Study Bacterial Colonial Morphology. Expert Rev. Anti-Infect. Ther. 2016, 14, 207–218. [Google Scholar] [CrossRef]
- Marcoux, P.R.; Dupoy, M.; Cuer, A.; Kodja, J.-L.; Lefebvre, A.; Licari, F.; Louvet, R.; Narassiguin, A.; Mallard, F. Optical Forward-Scattering for Identification of Bacteria within Microcolonies. Appl. Microbiol. Biotechnol. 2014, 98, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Minoni, U.; Signoroni, A.; Nassini, G. On the Application of Optical Forward-Scattering to Bacterial Identification in an Automated Clinical Analysis Perspective. Biosens. Bioelectron. 2015, 68, 536–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzalewicz, I.; Suchwałko, A.; Korzekwa, K. The Label-Free Optical Biosensor for an Automated, Ultra-Sensitive and Highly Accurate Microorganisms Identification. Measurement 2021, 178, 109408. [Google Scholar] [CrossRef]
- Sasanpour, P.; Dilmaghani-Marand, A.; Montazeri, H.; Ivani, S.; Hajipour, M.J.; Mahmoudi, M. Nanoparticles Affect Bacterial Colonies’ Optical Diffraction Patterns. Nanoscale 2019, 11, 2594–2601. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhao, J.; Chen, Q. Classification of Foodborne Pathogens Using near Infrared (NIR) Laser Scatter Imaging System with Multivariate Calibration. Sci. Rep. 2015, 5, 9524. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Doh, I.-J.; Sturgis, J.; Bhunia, A.K.; Robinson, J.P.; Bae, E. Reflected Scatterometry for Noninvasive Interrogation of Bacterial Colonies. J. Biomed. Opt. 2016, 21, 107004. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shi, Z.; Liu, Q. Smartphone-Based Biosensors for Portable Food Evaluation. Curr. Opin. Food Sci. 2019, 28, 74–81. [Google Scholar] [CrossRef]
- Müller, V.; Sousa, J.M.; Koydemir, H.C.; Veli, M.; Tseng, D.; Cerqueira, L.; Ozcan, A.; Azevedo, N.F.; Westerlund, F. Identification of Pathogenic Bacteria in Complex Samples Using a Smartphone Based Fluorescence Microscope. RSC Adv. 2018, 8, 36493–36502. [Google Scholar] [CrossRef] [Green Version]
- Min, H.J.; Mina, H.A.; Deering, A.J.; Bae, E. Development of a Smartphone-Based Lateral-Flow Imaging System Using Machine-Learning Classifiers for Detection of Salmonella spp. J. Microbiol. Methods 2021, 188, 106288. [Google Scholar] [CrossRef]
- Hattori, M.; Shirane, S.; Matsuda, T.; Nagayama, K.; Nagai, T. Smartphone-Based Portable Bioluminescence Imaging System Enabling Observation at Various Scales from Whole Mouse Body to Organelle. Sensors 2020, 20, 7166. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Coronel-Aguilera, C.; Doh, I.-J.; Min, H.J.; Lim, T.; Applegate, B.M.; Bae, E. Design and Application of a Portable Luminometer for Bioluminescence Detection. Appl. Opt. 2020, 59, 801. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, Q.; Li, D.; Xie, J.; Ke, D.; Song, Q.; Tang, Y.; Wang, H. A Smartphone Colorimetric Reader Integrated with an Ambient Light Sensor and a 3D Printed Attachment for On-Site Detection of Zearalenone. Anal. Bioanal. Chem. 2017, 409, 6567–6574. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, J.; Guo, Y.; Xie, L.; Zhang, G. Digital Image Colorimetry on Smartphone for Chemical Analysis: A Review. Measurement 2021, 171, 108829. [Google Scholar] [CrossRef]
- Das, A.J.; Wahi, A.; Kothari, I.; Raskar, R. Ultra-Portable, Wireless Smartphone Spectrometer for Rapid, Non-Destructive Testing of Fruit Ripeness. Sci. Rep. 2016, 6, 32504. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Yao, Y.; Chen, T.; Shen, W.; Tang, S.; Lee, H.K. Application of Smartphone-Based Spectroscopy to Biosample Analysis: A Review. Biosens. Bioelectron. 2021, 172, 112788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Q. Biosensors and Bioelectronics on Smartphone for Portable Biochemical Detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Kanchi, S.; Sabela, M.I.; Mdluli, P.S.; Inamuddin; Bisetty, K. Smartphone Based Bioanalytical and Diagnosis Applications: A Review. Biosens. Bioelectron. 2018, 102, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Patsekin, V.; On, S.; Sturgis, J.; Bae, E.; Rajwa, B.P.; Aleksandr, A.; Robinson, J.P. Classification of Arcobacter Species Using Variational Autoencoders. In Proceedings of the Sensing for Agriculture and Food Quality and Safety XI, Baltimore, MD, USA, 16–17 April 2019; Kim, M.S., Cho, B.-K., Chin, B.A., Eds.; SPIE: Baltimore, MD, USA, 2019; p. 7. [Google Scholar]
- Rajwa, B.; Dundar, M.M.; Akova, F.; Bettasso, A.; Patsekin, V.; Dan Hirleman, E.; Bhunia, A.K.; Robinson, J.P. Discovering the Unknown: Detection of Emerging Pathogens Using a Label-Free Light-Scattering System. Cytometry 2010, 77A, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-Based Food Diagnostic Technologies: A Review. Sensors 2017, 17, 1453. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Emerging Point-of-Care Technologies for Food Safety Analysis. Sensors 2019, 19, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Wang, X.; Chao, R.; Ren, Y.; Hu, C.; Xu, Z.; Liu, G.L. Smartphone Based Portable Bacteria Pre-Concentrating Microfluidic Sensor and Impedance Sensing System. Sens. Actuators B Chem. 2014, 193, 653–659. [Google Scholar] [CrossRef]
- Huang, X.; Xu, D.; Chen, J.; Liu, J.; Li, Y.; Song, J.; Ma, X.; Guo, J. Smartphone-Based Analytical Biosensors. Analyst 2018, 143, 5339–5351. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bai, N.; Bhunia, A.K.; King, G.B.; Hirleman, E.D.; Bae, E. Development of an Integrated Optical Analyzer for Characterization of Growth Dynamics of Bacterial Colonies: Integrated Optical Analyzer for Bacterial Colony. J. Biophoton. 2013, 6, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Aroonnual, A.; Bhunia, A.K.; Hirleman, E.D. On the Sensitivity of Forward Scattering Patterns from Bacterial Colonies to Media Composition. J. Biophoton. 2011, 4, 236–243. [Google Scholar] [CrossRef] [PubMed]

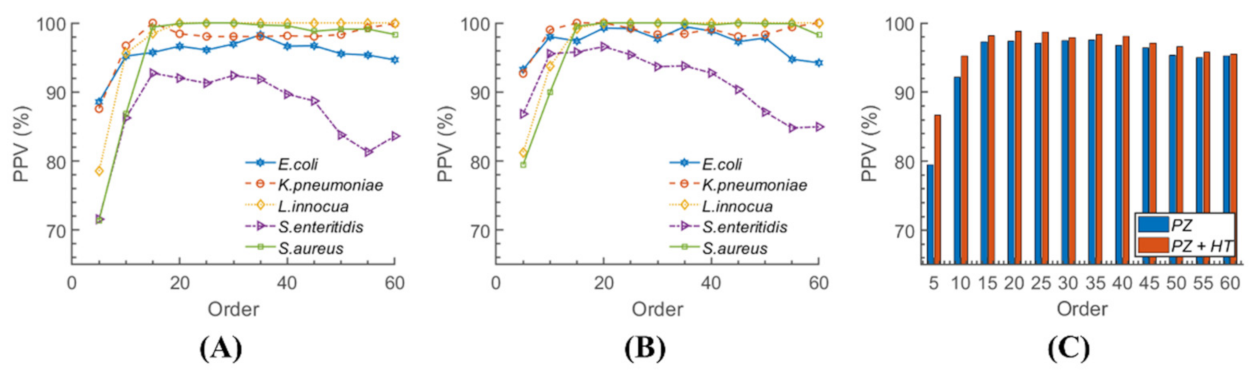
| E. coli | K. pneumoniae | L. innocua | S. enteritidis | S. aureus | |
|---|---|---|---|---|---|
| Accuracy | 98.8 | 100 | 100 | 98.8 | 100 |
| Sensitivity | 97.1 | 100 | 100 | 96.7 | 100 |
| Specificity | 99.2 | 100 | 100 | 99.3 | 100 |
| PPV | 96.7 | 100 | 100 | 97.1 | 100 |
| NPV | 99.3 | 100 | 100 | 99.2 | 100 |
| E. coli | K. pneumoniae | L. innocua | S. enteritidis | S. aureus | |
|---|---|---|---|---|---|
| BCYE Agar | |||||
| Accuracy | 94.8 | 98.7 | 95.4 | 99.6 | 97.7 |
| Sensitivity | 83.9 | 93.7 | 90.0 | 97.9 | 100 |
| Specificity | 97.6 | 100 | 96.7 | 100 | 97.1 |
| PPV | 89.5 | 100 | 87.3 | 100 | 89.6 |
| NPV | 96.0 | 98.5 | 97.5 | 99.5 | 100 |
| Chocolate Agar | |||||
| Accuracy | 94.6 | 96.8 | 99.8 | 96.0 | 97.8 |
| Sensitivity | 90.5 | 90.4 | 98.9 | 85.5 | 97.2 |
| Specificity | 95.6 | 98.5 | 100 | 98.6 | 97.6 |
| PPV | 83.8 | 93.6 | 100 | 94.0 | 92.1 |
| NPV | 97.6 | 97.6 | 99.7 | 96.5 | 99.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doh, I.-J.; Dowden, B.; Patsekin, V.; Rajwa, B.; Robinson, J.P.; Bae, E. Development of a Smartphone-Integrated Reflective Scatterometer for Bacterial Identification. Sensors 2022, 22, 2646. https://doi.org/10.3390/s22072646
Doh I-J, Dowden B, Patsekin V, Rajwa B, Robinson JP, Bae E. Development of a Smartphone-Integrated Reflective Scatterometer for Bacterial Identification. Sensors. 2022; 22(7):2646. https://doi.org/10.3390/s22072646
Chicago/Turabian StyleDoh, Iyll-Joon, Brianna Dowden, Valery Patsekin, Bartek Rajwa, J. Paul Robinson, and Euiwon Bae. 2022. "Development of a Smartphone-Integrated Reflective Scatterometer for Bacterial Identification" Sensors 22, no. 7: 2646. https://doi.org/10.3390/s22072646







