Free-Breathing Phase-Resolved Oxygen-Enhanced Pulmonary MRI Based on 3D Stack-of-Stars UTE Sequence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MR Pulse Sequence and Imaging
2.3. Experiment Protocol and Materials
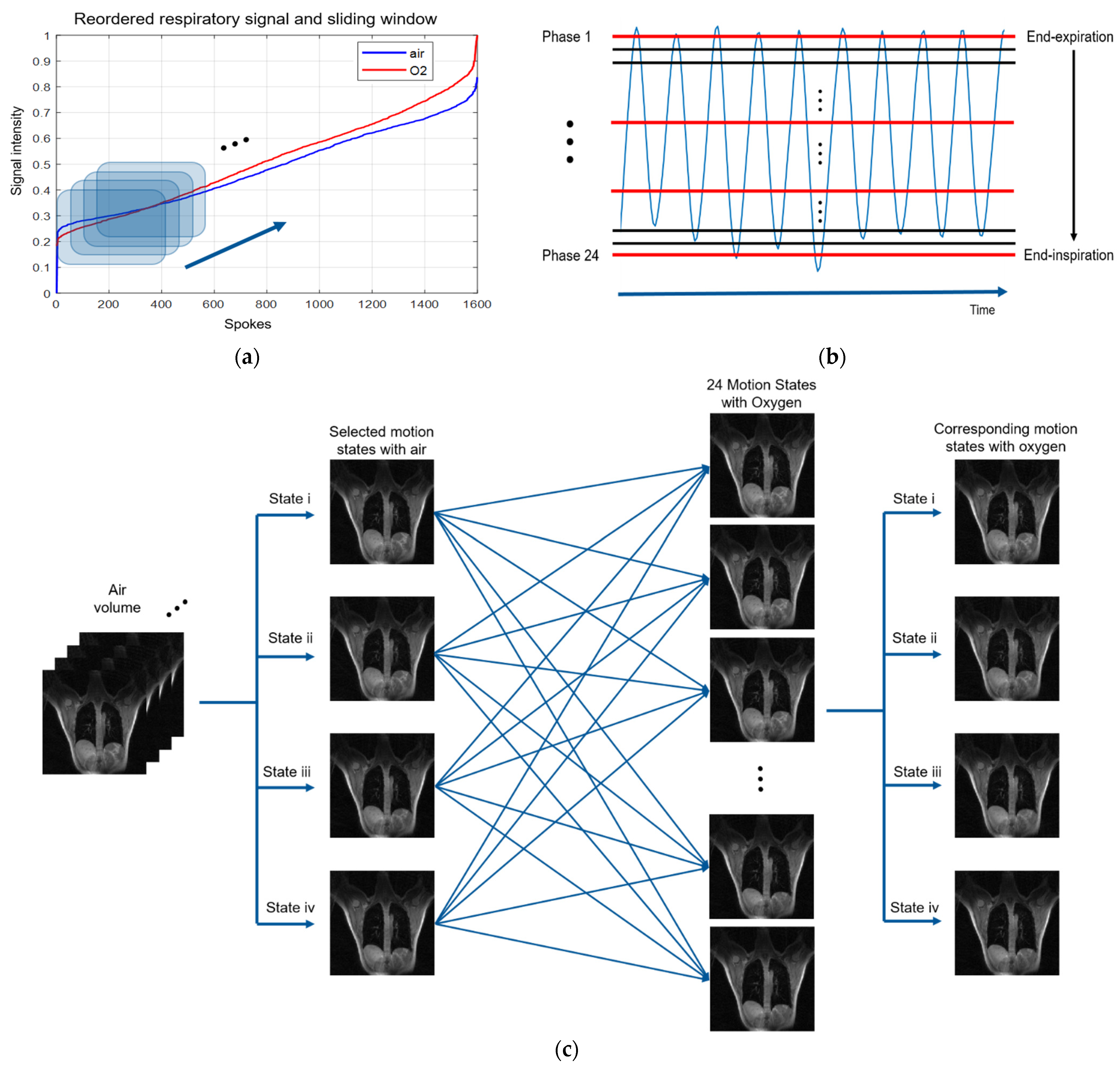
2.4. Respiratory Signal Extraction Method
2.5. Phase-Resolved Pulmonary Imaging
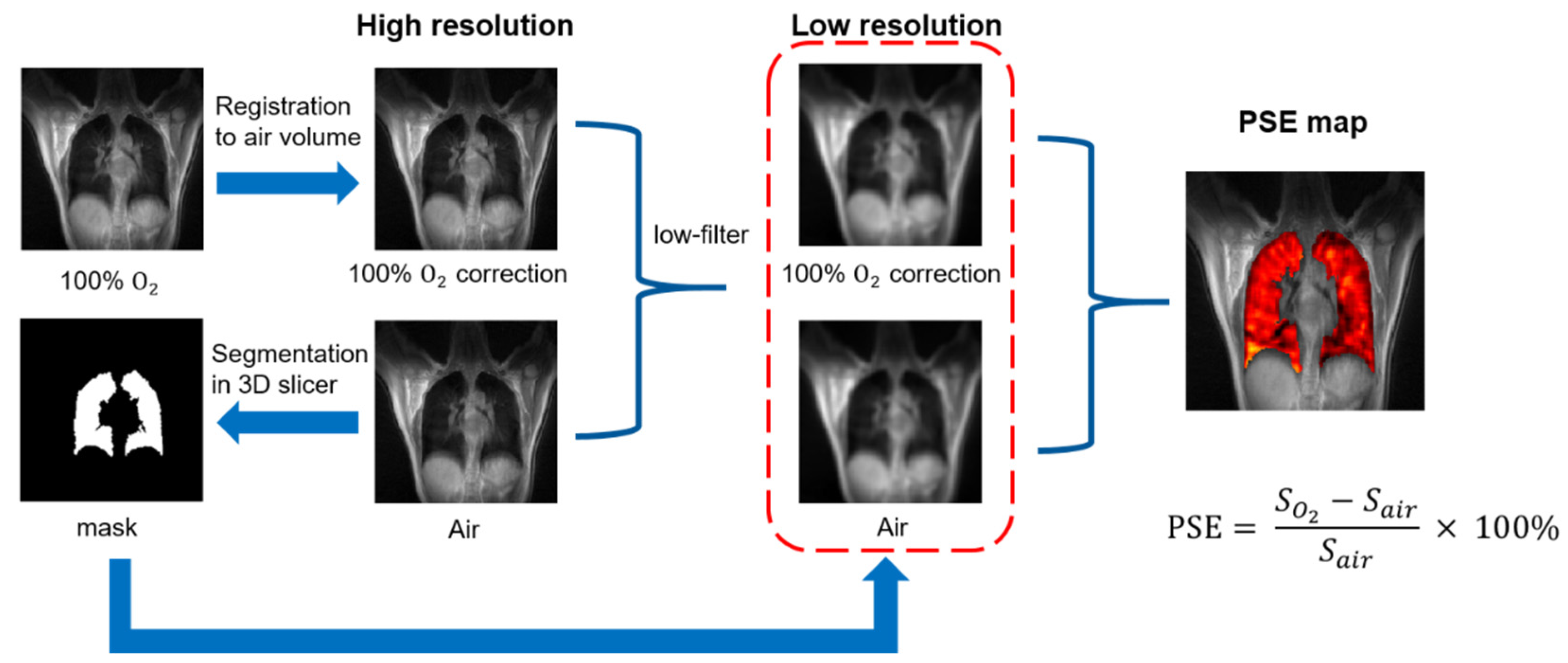
2.6. Image Analysis
3. Results
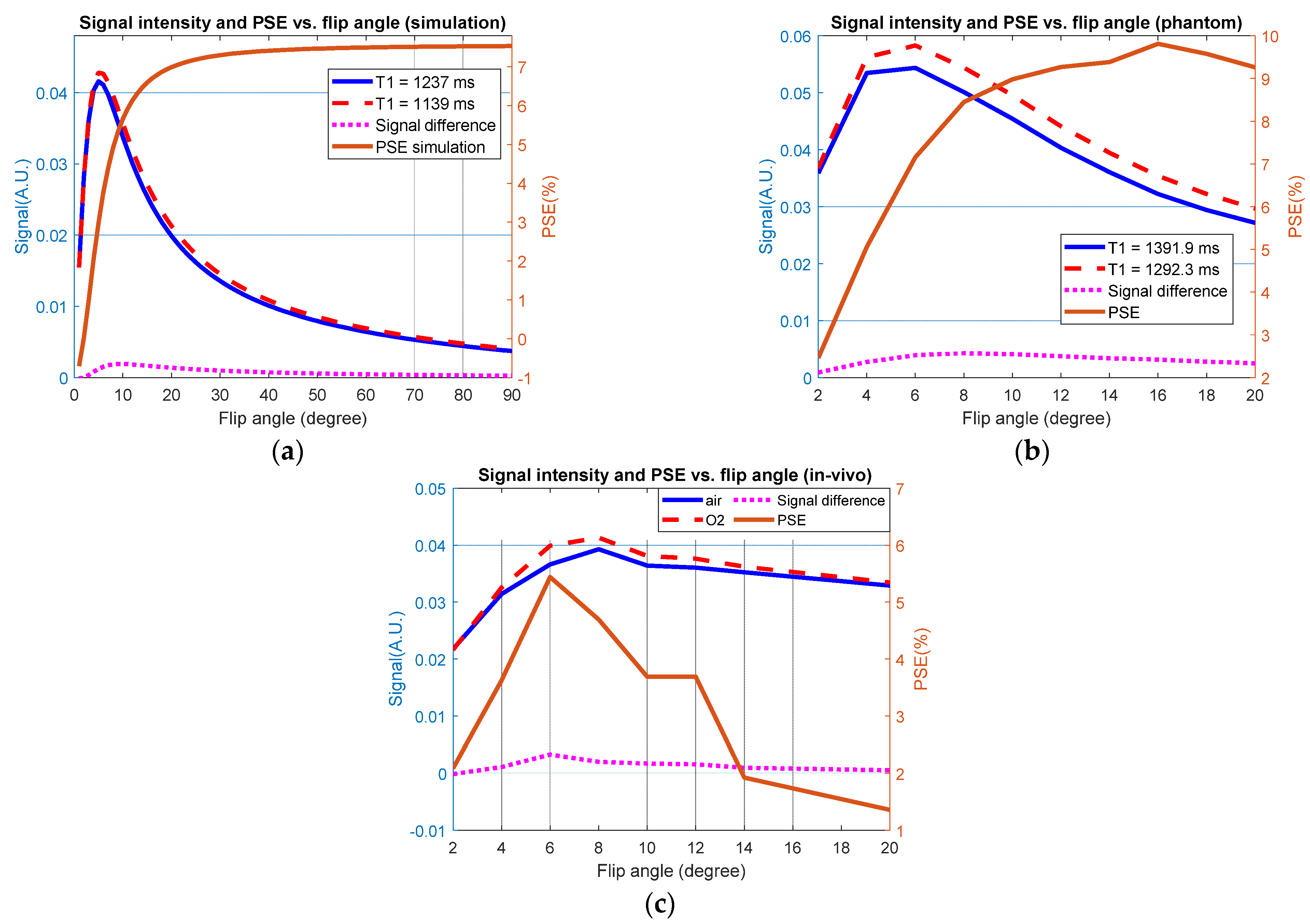
3.1. Simulation Results
3.2. Global Measurements
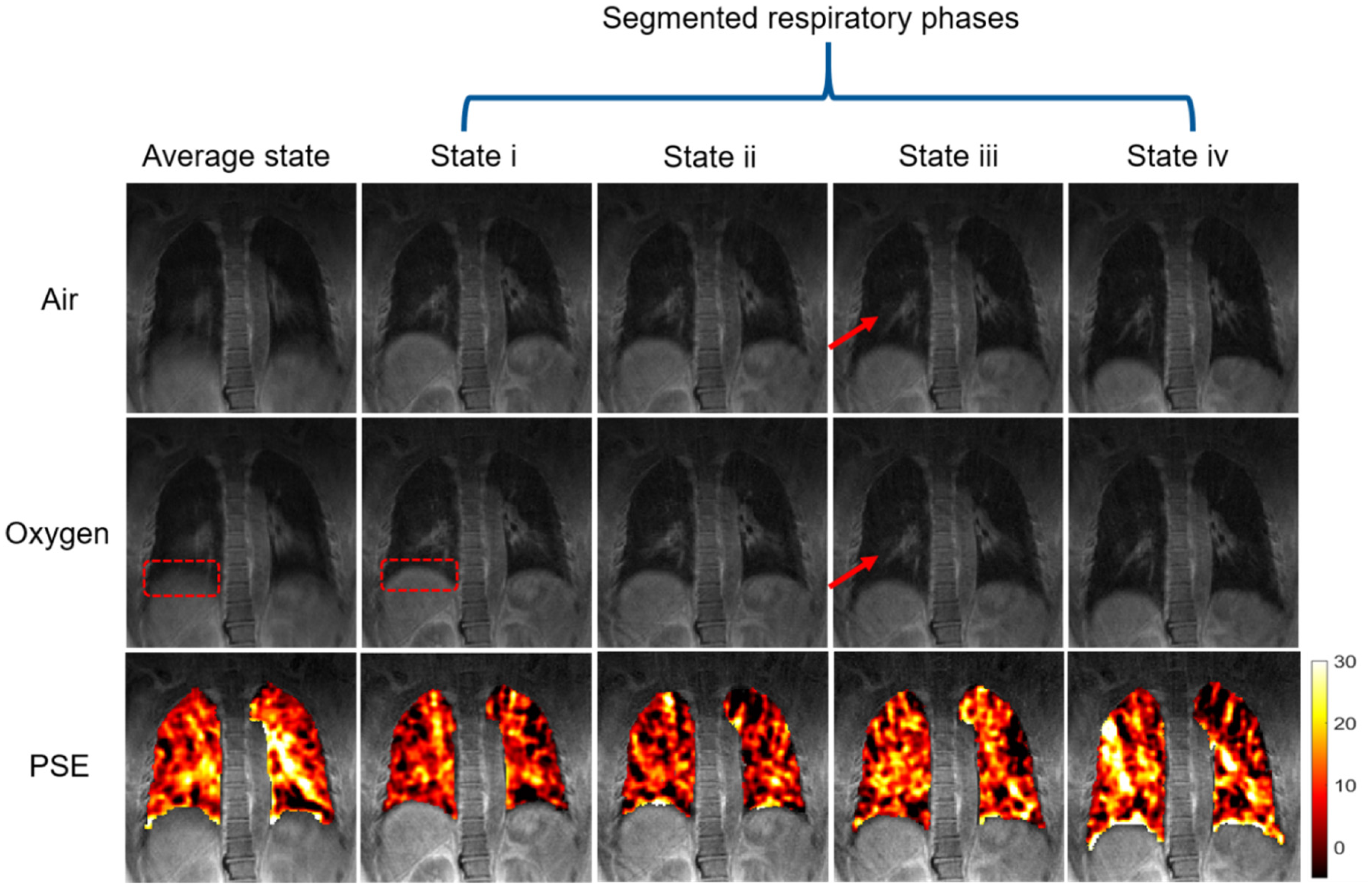
3.3. Phase-Resolved Image and PSE Map
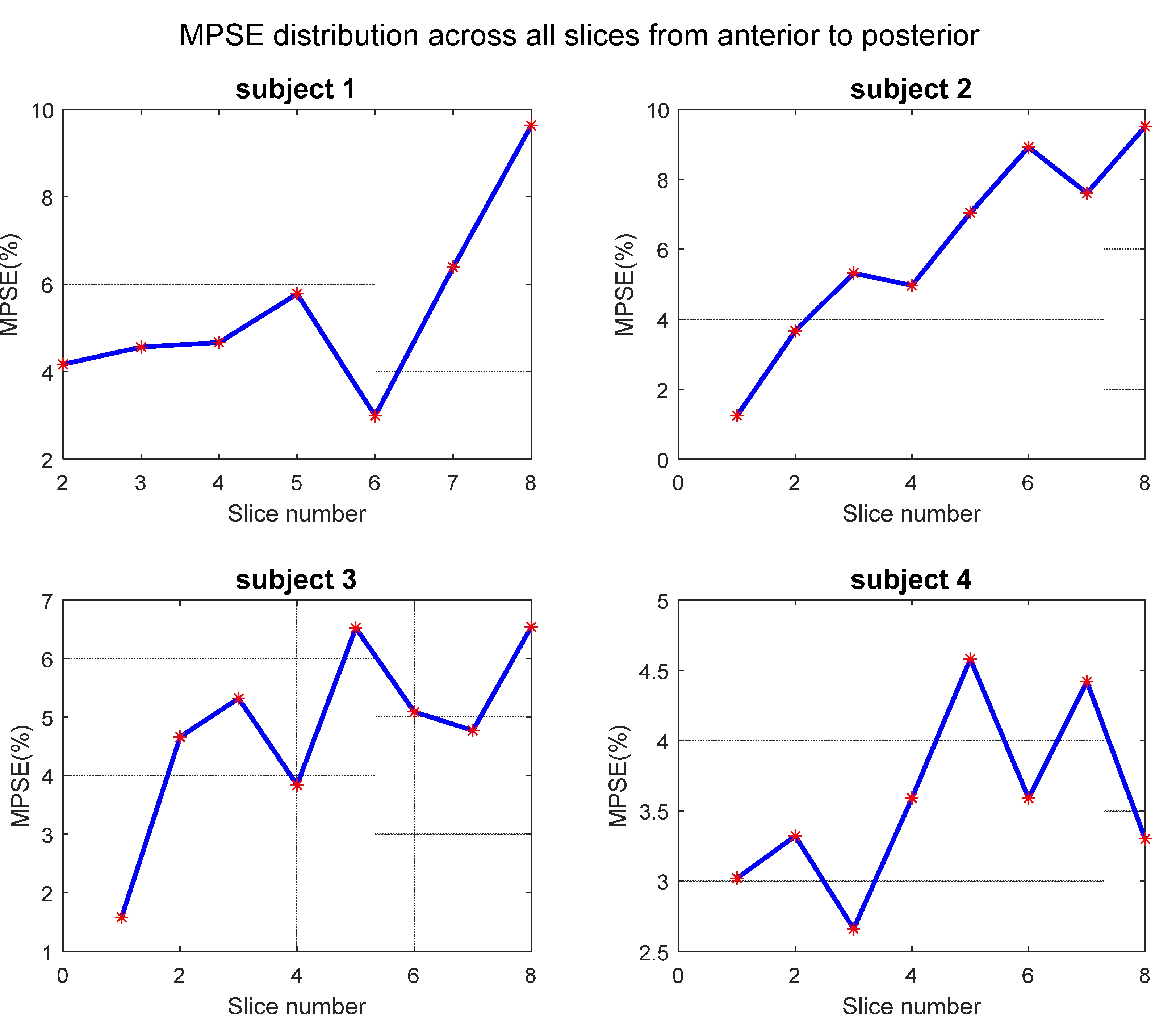
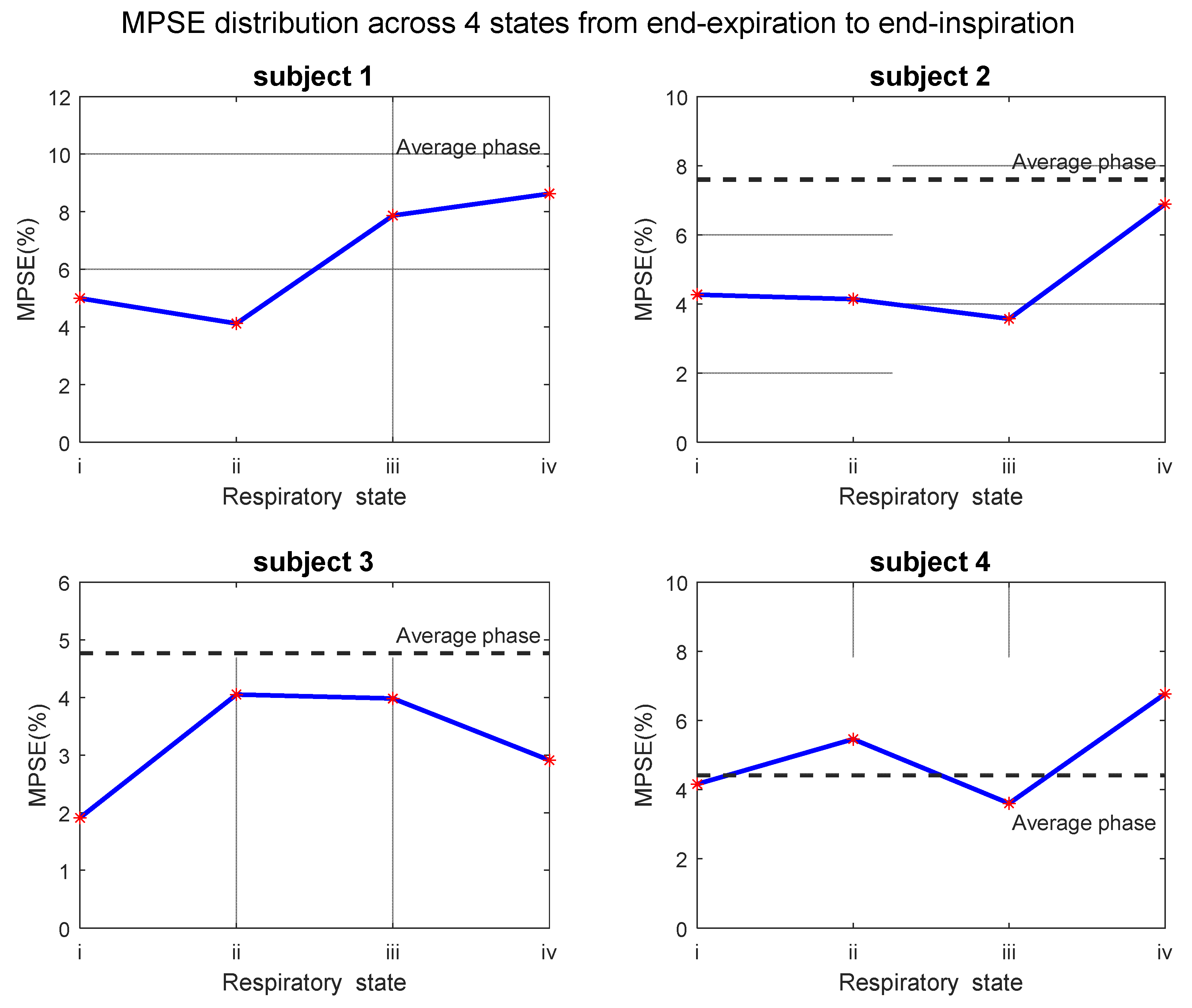
3.4. Test–Retest Repeatability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biederer, J.; Mirsadraee, S.; Beer, M.; Molinari, F.; Hintze, C.; Bauman, G.; Both, M.; Van Beek, E.; Wild, J.; Puderbach, M. MRI of the lung (3/3)—current applications and future perspectives. Insights Imaging 2012, 3, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Woo, M.K.; DelaBarre, L.; Waks, M.; Radder, J.; Choi, U.-S.; Lagore, R.; Ugurbil, K.; Adriany, G. A 16-Channel Dipole Antenna Array for Human Head Magnetic Resonance Imaging at 10.5 Tesla. Sensors 2021, 21, 7250. [Google Scholar] [CrossRef]
- Ohno, Y.; Hanamatsu, S.; Obama, Y.; Ueda, T.; Ikeda, H.; Hattori, H.; Murayama, K.; Toyama, H. Overview of MRI for pulmonary functional imaging. Br. J. Radiol. 2021, 94, 20201053. [Google Scholar] [CrossRef] [PubMed]
- Carminati, M.; Fiorini, C. Challenges for Microelectronics in Non-Invasive Medical Diagnostics. Sensors 2020, 20, 3636. [Google Scholar] [CrossRef]
- Fain, S.; Schiebler, M.L.; McCormack, D.G.; Parraga, G. Imaging of lung function using hyperpolarized helium-3 magnetic resonance imaging: Review of current and emerging translational methods and applications. J. Magn. Reson. Imaging 2010, 32, 1398–1408. [Google Scholar] [CrossRef] [Green Version]
- Adamson, E.B.; Ludwig, K.D.; Mummy, D.G.; Fain, S.B. Magnetic resonance imaging with hyperpolarized agents: Methods and applications. Phys. Med. Biol. 2017, 62, R81. [Google Scholar] [CrossRef] [PubMed]
- Mugler III, J.P.; Altes, T.A. Hyperpolarized 129Xe MRI of the human lung. J. Magn. Reson. Imaging 2013, 37, 313–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couch, M.J.; Ball, I.K.; Li, T.; Fox, M.S.; Biman, B.; Albert, M.S. (19) F MRI of the Lungs Using Inert Fluorinated Gases: Challenges and New Developments. J. Magn. Reson. Imaging 2019, 49, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Middleton, H.; Black, R.D.; Saam, B.; Cates, G.D.; Cofer, G.P.; Guenther, R.; Happer, W.; Hedlund, L.W.; Alan Johnson, G.; Juvan, K. MR imaging with hyperpolarized 3He gas. Magn. Reson. Med. 1995, 33, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Young, I.; Clarke, G.; Bailes, D.; Pennock, J.; Doyle, F.; Bydder, G. Enhancement of relaxation rate with paramagnetic contrast agents in NMR imaging. J. Comput. Tomogr. 1981, 5, 543–547. [Google Scholar] [CrossRef]
- Goodrich, K.; Hackmann, A.; Ganesan, K. Spin-Lattice Relaxation in Excised Rat Lung; Book of Abstracts, 11th Annual Meeting; Society of Magnetic Resonance in Medicine: Berlin, Germany, 1992; p. 1307. [Google Scholar]
- Edelman, R.R.; Hatabu, H.; Tadamura, E.; Li, W.; Prasad, P.V. Noninvasive assessment of regional ventilation in the human lung using oxygen–enhanced magnetic resonance imaging. Nat. Med. 1996, 2, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Oshio, K.; Uematsu, H.; Nakatsu, M.; Gefter, W.B.; Hatabu, H. Single-shot half-Fourier RARE sequence with ultra-short inter-echo spacing for lung imaging. J. Magn. Reson. Imaging 2004, 20, 336–339. [Google Scholar] [CrossRef]
- Chen, Q.; Jakob, P.M.; Griswold, M.A.; Levin, D.L.; Hatabu, H.; Edelman, R.R. Oxygen enhanced MR ventilation imaging of the lung. Magn. Reson. Mater. Phys. Biol. Med. 1998, 7, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Pracht, E.D.; Arnold, J.F.; Wang, T.; Jakob, P.M. Oxygen-enhanced proton imaging of the human lung using T2. Magn. Reson. Med. 2005, 53, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.; Kammerman, J.; Hahn, A.D.; Zha, W.; Nagle, S.K.; Johnson, K.; Sandbo, N.; Meyer, K.; Schiebler, M.; Fain, S.B. Structure-function imaging of lung disease using ultrashort echo time MRI. Acad. Radiol. 2019, 26, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Ernst, R.R.; Anderson, W.A. Application of Fourier transform spectroscopy to magnetic resonance. Rev. Sci. Instrum. 1966, 37, 93–102. [Google Scholar] [CrossRef]
- Stadler, A.; Schima, W.; Ba-Ssalamah, A.; Kettenbach, J.; Eisenhuber, E. Artifacts in body MR imaging: Their appearance and how to eliminate them. Eur. Radiol. 2007, 17, 1242–1255. [Google Scholar] [CrossRef]
- Chandarana, H.; Block, T.K.; Rosenkrantz, A.B.; Lim, R.P.; Kim, D.; Mossa, D.J.; Babb, J.S.; Kiefer, B.; Lee, V.S. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: A viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Investig. Radiol. 2011, 46, 648–653. [Google Scholar] [CrossRef] [Green Version]
- Kruger, S.J.; Fain, S.B.; Johnson, K.M.; Cadman, R.V.; Nagle, S.K. Oxygen-enhanced 3D radial ultrashort echo time magnetic resonance imaging in the healthy human lung. NMR Biomed. 2014, 27, 1535–1541. [Google Scholar] [CrossRef] [Green Version]
- Block, K.T.; Chandarana, H.; Milla, S.; Bruno, M.; Mulholland, T.; Fatterpekar, G.; Hagiwara, M.; Grimm, R.; Geppert, C.; Kiefer, B. Towards routine clinical use of radial stack-of-stars 3D gradient-echo sequences for reducing motion sensitivity. J. Korean Soc. Magn. Reson. Med. 2014, 18, 87–106. [Google Scholar] [CrossRef] [Green Version]
- Winkelmann, S.; Schaeffter, T.; Koehler, T.; Eggers, H.; Doessel, O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans. Med. Imaging 2006, 26, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Grimm, R.; Block, K.T.; Chandarana, H.; Kim, S.; Xu, J.; Axel, L.; Sodickson, D.K.; Otazo, R. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn. Reson. Med. 2014, 72, 707–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, F.; Eichinger, M.; Risse, F.; Plathow, C.; Puderbach, M.; Ley, S.; Herth, F.; Bonomo, L.; Kauczor, H.U.; Fink, C. Navigator-triggered oxygen-enhanced MRI with simultaneous cardiac and respiratory synchronization for the assessment of interstitial lung disease. J. Magn. Reson. Imaging 2007, 26, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Vaninbroukx, J.; Bosmans, H.; Sunaert, S.; Demedts, M.; Delcroix, M.; Marchal, G.; Verschakelen, J. The use of ECG and respiratory triggering to improve the sensitivity of oxygen-enhanced proton MRI of lung ventilation. Eur. Radiol. 2003, 13, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zheng, L.; Shan, F.; Dai, Y.; Shen, J.; Yang, S.; Shi, Y.; Xue, K.; Zhang, Z. Evaluation of pulmonary ventilation in COVID-19 patients using oxygen-enhanced three-dimensional ultrashort echo time MRI: A preliminary study. Clin. Radiol. 2021, 76, 391.e33–391.e41. [Google Scholar] [CrossRef]
- Hemberger, K.R.; Jakob, P.M.; Breuer, F.A. Multiparametric oxygen-enhanced functional lung imaging in 3D. Magn. Reson. Mater. Phys. Biol. Med. 2015, 28, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Axel, L.; Chandarana, H.; Block, K.T.; Sodickson, D.K.; Otazo, R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn. Reson. Med. 2016, 75, 775–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, E.; Yun, J.; Wachowicz, K.; Gabos, Z.; Rathee, S.; Fallone, B. Sliding window prior data assisted compressed sensing for MRI tracking of lung tumors. Med. Phys. 2017, 44, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. Soc. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Jakob, P.M.; Hillenbrand, C.M.; Wang, T.; Schultz, G.; Hahn, D.; Haase, A. Rapid quantitative lung 1H T1 mapping. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2001, 14, 795–799. [Google Scholar]
- Yoshimura, K.; Kato, H.; Kuroda, M.; Yoshida, A.; Hanamoto, K.; Tanaka, A.; Tsunoda, M.; Kanazawa, S.; Shibuya, K.; Kawasaki, S. Development of a tissue-equivalent MRI phantom using carrageenan gel. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2003, 50, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Takahashi, K.; Obara, M. Viability of oxygen-enhanced ventilation imaging of the lungs using ultra-short echo time MRI. Magn. Reson. Med. Sci. 2017, 16, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, V.M.; Liu, B.; Li, W.; Polzin, J.; Kurucay, S.; Chen, Q.; Edelman, R.R. Influence of oxygen flow rate on signal and T(1) changes in oxygen-enhanced ventilation imaging. J. Magn. Reson. Imaging 2002, 16, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Grimm, R.; Fürst, S.; Dregely, I.; Forman, C.; Hutter, J.M.; Ziegler, S.I.; Nekolla, S.; Kiefer, B.; Schwaiger, M.; Hornegger, J. In Self-gated radial MRI for respiratory motion compensation on hybrid PET/MR systems. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Nagoya, Japan, 22–26 September 2013; Springer: Berlin/Heidelberg, Germany, 2013; pp. 17–24. [Google Scholar]
- Feng, L.; Delacoste, J.; Smith, D.; Weissbrot, J.; Flagg, E.; Moore, W.H.; Girvin, F.; Raad, R.; Bhattacharji, P.; Stoffel, D. Simultaneous evaluation of lung anatomy and ventilation using 4D respiratory-motion-resolved ultrashort echo time sparse MRI. J. Magn. Reson. Imaging 2019, 49, 411–422. [Google Scholar] [CrossRef]
- Lin, W.; Guo, J.; Rosen, M.A.; Song, H.K. Respiratory motion-compensated radial dynamic contrast-enhanced (DCE)-MRI of chest and abdominal lesions. Magn. Reson. Med. 2008, 60, 1135–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, R.; Bauer, S.; Kiefer, B.; Hornegger, J.; Block, T. Optimal channel selection for respiratory self-gating signals. In Proceedings of the 21st Annual Meeting ISMRM, Salt Lake City, UT, USA, 20–26 April 2013; p. 3749. [Google Scholar]
- Bernstein, M.A.; King, K.F.; Zhou, X.J. Handbook of MRI Pulse Sequences; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Kroon, D.-J.; Slump, C.H. In MRI modalitiy transformation in demon registration. In Proceedings of the 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Boston, MA, USA, 28 June–1 July 2009; IEEE: Manhattan, NY, USA, 2009; pp. 963–966. [Google Scholar]
- Zha, W.; Nagle, S.K.; Cadman, R.V.; Schiebler, M.L.; Fain, S.B. Three-dimensional Isotropic Functional Imaging of Cystic Fibrosis Using Oxygen-enhanced MRI: Comparison with Hyperpolarized (3)He MRI. Radiology 2019, 290, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sa, R.C.; Cronin, M.V.; Henderson, A.C.; Holverda, S.; Theilmann, R.J.; Arai, T.J.; Dubowitz, D.J.; Hopkins, S.R.; Buxton, R.B.; Prisk, G.K. Vertical distribution of specific ventilation in normal supine humans measured by oxygen-enhanced proton MRI. J. Appl. Physiol. 2010, 109, 1950–1959. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.J.; Horn, F.C.; Sa, R.C.; Rao, M.R.; Collier, G.J.; Theilmann, R.J.; Prisk, G.K.; Wild, J.M. Comparison of quantitative multiple-breath specific ventilation imaging using colocalized 2D oxygen-enhanced MRI and hyperpolarized (3)He MRI. J. Appl. Physiol. 2018, 125, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Kruger, S.J.; Johnson, K.M.; Cadman, R.V.; Bell, L.C.; Liu, F.; Hahn, A.D.; Evans, M.D.; Nagle, S.K.; Fain, S.B. Pulmonary ventilation imaging in asthma and cystic fibrosis using oxygen-enhanced 3D radial ultrashort echo time MRI. J. Magn. Reson. Imaging 2018, 47, 1287–1297. [Google Scholar] [CrossRef]
- Holmes, J.E.; Bydder, G.M. MR imaging with ultrashort TE (UTE) pulse sequences: Basic principles. Radiography 2005, 11, 163–174. [Google Scholar] [CrossRef]
- Tadamura, E.; Hatabu, H.; Li, W.; Prasad, P.V.; Edelman, R.R. Effect of oxygen inhalation on relaxation times in various tissues. J. Magn. Reson. Imaging 1997, 7, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Bauman, G.; Puderbach, M.; Deimling, M.; Jellus, V.; Chefd’hotel, C.; Dinkel, J.; Hintze, C.; Kauczor, H.U.; Schad, L.R. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of Fourier decomposition in proton MRI. Magn. Reson. Med. 2009, 62, 656–664. [Google Scholar] [CrossRef] [PubMed]




| Information | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Mean ± Std |
|---|---|---|---|---|---|
| Age | 46 | 28 | 29 | 28 | 32.8 ± 8.8 |
| Height (cm) | 170 | 178 | 160 | 172 | 170 ± 7.5 |
| Ethnicity | North East Asian | North East Asian | North East Asian | North East Asian | - |
| FEV1 (L/%) 1 | 4.68/126.8 | 4.54/103.2 | 3.6/117.1 | 5.25/128.8 | - |
| FVC (L/%) 1 | 4.83/106.7 | 4.54/85.9 | 3.81/106.6 | 5.25/107.9 | - |
| FEF2575 (L/s/%) 1 | 7.56/205.1 | 7.85/173.1 | 4.05/123.9 | 9.25/216.1 | - |
| FEF75 (L/s/%) 1 | 3.39/212.7 | 4.86/215.2 | 2.09/128.3 | 6.29/299.4 | - |
| FEV1/FVC | 0.97/118.5 | 1.00/119.4 | 0.95/109.6 | 1.00/118.8 | - |
| MPSE (%) | 5.46 | 6.03 | 4.79 | 3.56 | 4.96 ± 1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Zhang, J.; Nan, Z.; Meersmann, T.; Wang, C. Free-Breathing Phase-Resolved Oxygen-Enhanced Pulmonary MRI Based on 3D Stack-of-Stars UTE Sequence. Sensors 2022, 22, 3270. https://doi.org/10.3390/s22093270
Xu P, Zhang J, Nan Z, Meersmann T, Wang C. Free-Breathing Phase-Resolved Oxygen-Enhanced Pulmonary MRI Based on 3D Stack-of-Stars UTE Sequence. Sensors. 2022; 22(9):3270. https://doi.org/10.3390/s22093270
Chicago/Turabian StyleXu, Pengfei, Jichang Zhang, Zhen Nan, Thomas Meersmann, and Chengbo Wang. 2022. "Free-Breathing Phase-Resolved Oxygen-Enhanced Pulmonary MRI Based on 3D Stack-of-Stars UTE Sequence" Sensors 22, no. 9: 3270. https://doi.org/10.3390/s22093270






