Assessment of Finger Fat Pad Effect on CSRR-Based Sensor Scattering Parameters for Non-Invasive Blood Glucose Level Detection
Abstract
:1. Introduction
2. Fingertip Tissue Model
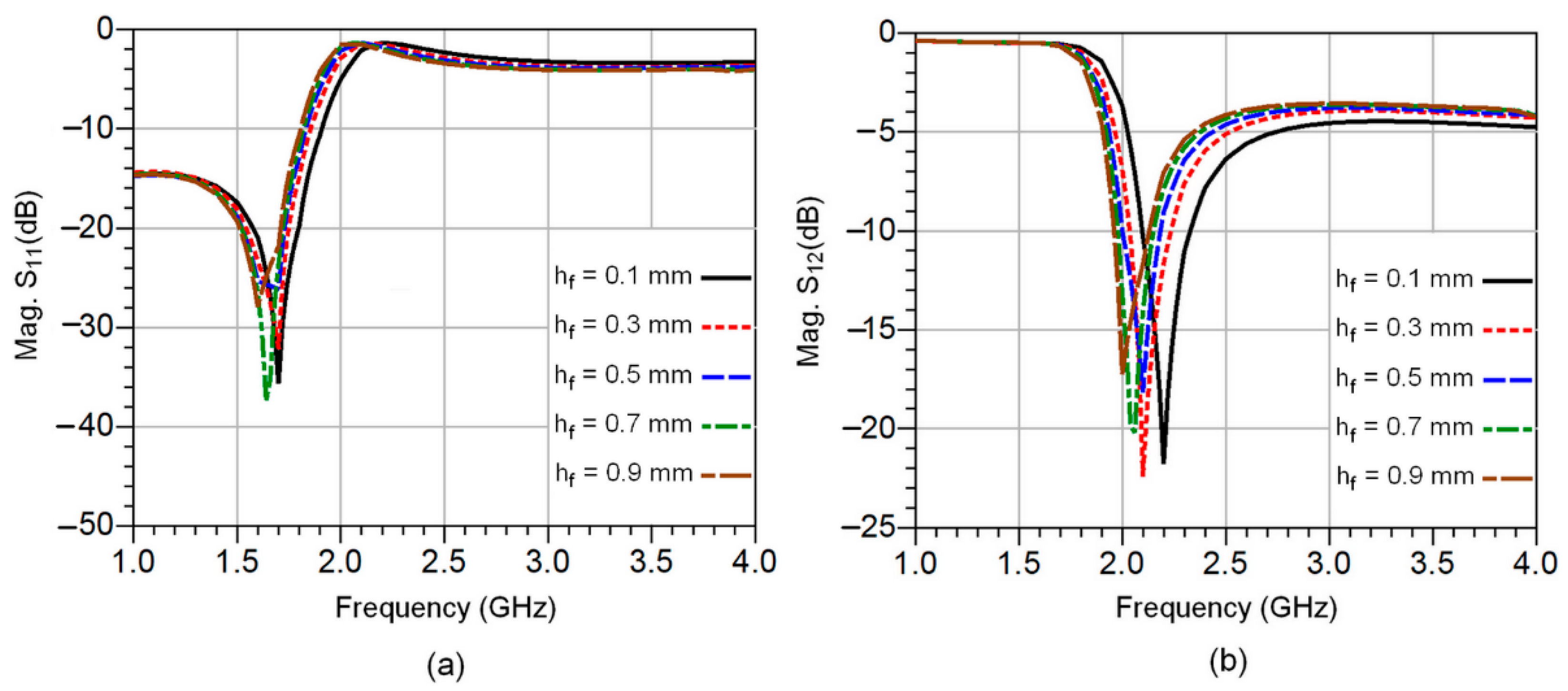
3. CSRR-Based Sensor Design and Analysis
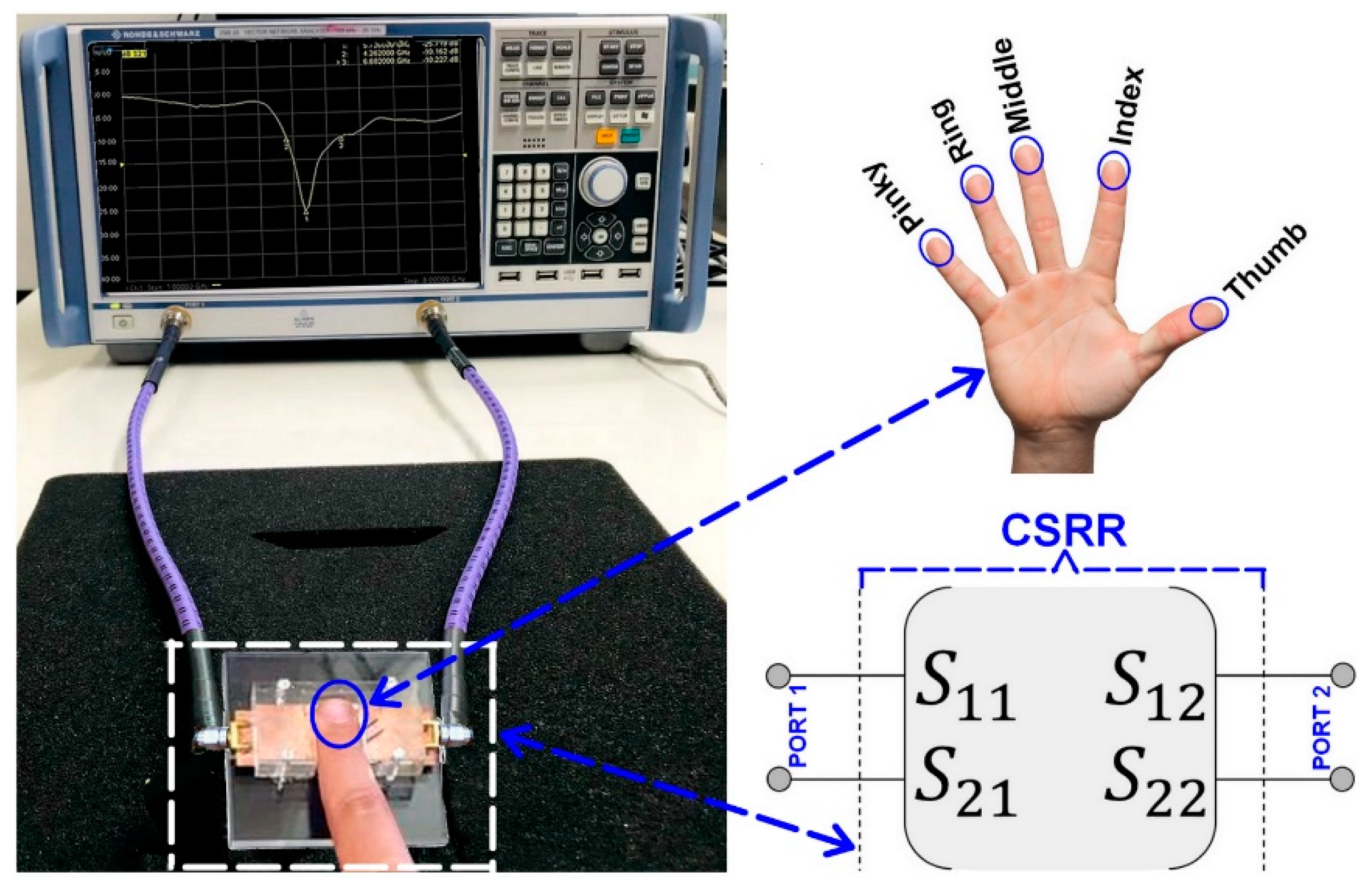
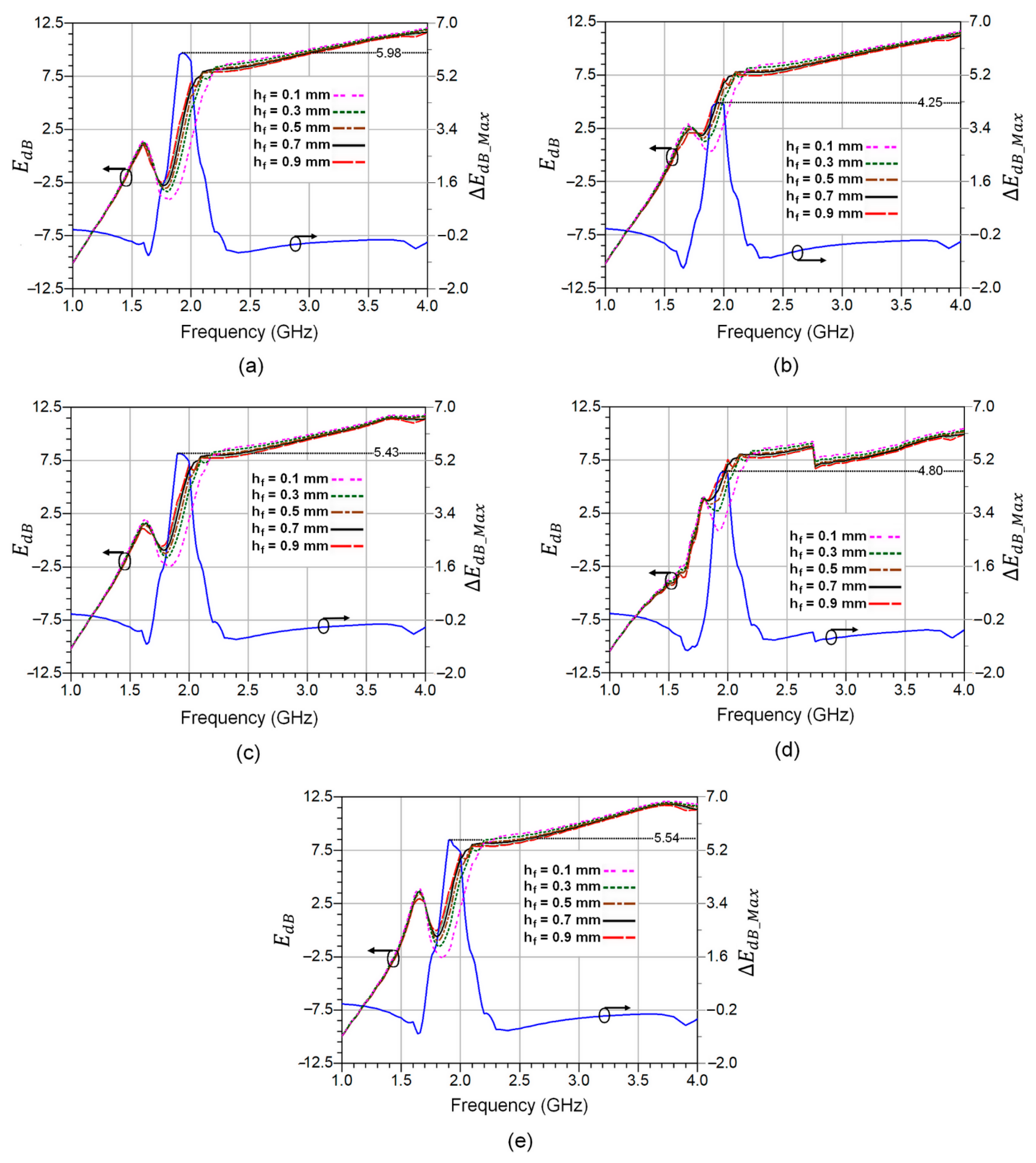
4. Experimental Analysis of Fat Pad Thickness Effect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Koinkar, P.; Fuchiwaki, Y.; Yasuzawa, M. A fine pointed glucose oxidase immobilized electrode for low-invasive amperometric glucose monitoring. Biosens. Bioelectron. 2016, 86, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Gu, S.; Lasri, T. Highly Sensitive Characterization of Glucose Aqueous Solution with Low Concentration: Application to Broadband Dielectric Spectroscopy. Sens. Actuators A Phys. 2017, 267, 318–326. [Google Scholar] [CrossRef]
- Yi, S.W.; Park, S.; Lee, Y.h.; Balkau, B.; Yi, J.J. Fasting Glucose and All-Cause Mortality by Age in Diabetes: A Prospective Cohort Study. Diabetes Care 2018, 41, 623–626. [Google Scholar] [CrossRef] [Green Version]
- Polivka, J. An Overview of Microwave Sensor Technology. High Freq. Electron. 2007, 1, 32–42. [Google Scholar]
- Hensley, S.; Farr, T. Microwave Remote Sensing and Surface Characterization; Elsevier Ltd.: San Diego, CA, USA, 2013; Volume 3, ISBN 9780080885223. [Google Scholar]
- Kayal, S.; Shaw, T.; Mitra, D. Design of Metamaterial-Based Compact and Highly Sensitive Microwave Liquid Sensor. Appl. Phys. A Mater. Sci. Process. 2020, 126, 13. [Google Scholar] [CrossRef]
- Liu, W.; Sun, H.; Xu, L. A Microwave Method for Dielectric Characterization Measurement of Small Liquids Using a Metamaterial-Based Sensor. Sensors 2018, 18, 1438. [Google Scholar] [CrossRef] [Green Version]
- Shafi, K.M.; Jha, A.K.; Akhtar, M.J. Improved planar resonant RF sensor for retrieval of permittivity and permeability of materials. IEEE Sens. J. 2017, 17, 5479–5486. [Google Scholar] [CrossRef]
- Haq, T.; Ruan, C.; Zhang, X.; Ullah, S. Complementary metamaterial sensor for non-destructive evaluation of dielectric substrates. Sensors 2019, 19, 2100. [Google Scholar] [CrossRef] [Green Version]
- Qiang, T.; Wang, C.; Kim, N.Y. Quantitative detection of glucose level based on radiofrequency patch biosensor combined with volume-fixed structures. Biosens. Bioelectron. 2017, 98, 357–363. [Google Scholar] [CrossRef]
- Ji, J.h.; Shin, K.s.; Kang, S.; Lee, S.H.; Kang, J.Y.; Kim, S.; Jun, S.C. Fundamental monomeric biomaterial diagnostics by radio frequency signal analysis. Biosens. Bioelectron. 2016, 82, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Lee, J.I. High-sensitivity microwave sensor based on an interdigital-capacitor-shaped defected ground structure for permittivity characterization. Sensors 2019, 19, 498. [Google Scholar] [CrossRef] [Green Version]
- Hao, H.; Wang, D.; Wang, Z. Design of Substrate-Integrated Waveguide Loading Multiple Complementary Open Resonant Rings (CSRRs) for Dielectric Constant Measurement. Sensors 2020, 20, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, F.; Vélez, P.; Gil, M. Microwave Sensors Based on Resonant Elements. Sensors 2020, 20, 3375. [Google Scholar] [CrossRef] [PubMed]
- Albishi, A.M.; Badawe, M.K.E.; Nayyeri, V.; Ramahi, O.M. Enhancing the Sensitivity of Dielectric Sensors with Multiple Coupled Complementary Split-Ring Resonators. IEEE Trans. Microw. Theory Tech. 2020, 68, 4340–4347. [Google Scholar] [CrossRef]
- Jang, C.; Park, J.; Lee, H.; Yun, G.; Yook, J. Non-Invasive Fluidic Glucose Detection Based on Dual Microwave Complementary Split Ring Resonators with a Switching Circuit for Environmental Effect Elimination. IEEE Sens. J. 2020, 20, 8520–8527. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Ultrahigh-sensitivity microwave sensor for microfluidic complex permittivity measurement. IEEE Trans. Microw. Theory Tech. 2019, 67, 4269–4277. [Google Scholar] [CrossRef]
- Guerchouche, K.; Herth, E.; Calvet, L.E.; Roland, N.; Loyez, C. Dielectric Characterization Based on A Printable Resonant Stub in Air and A Liquid Environment. Phys. Status Solidi Appl. Mater. Sci. 2017, 214, 1700138. [Google Scholar] [CrossRef]
- Dinh, T.H.N.; Serfaty, S.; Joubert, P.Y. Non-contact radiofrequency inductive sensor for the dielectric characterization of burn depth in organic tissues. Sensors 2019, 19, 1220. [Google Scholar] [CrossRef] [Green Version]
- Puentes, M.; Weiss, C.; Schüßler, M.; Jakoby, R. Sensor array based on split ring resonators for analysis of organic tissues. In Proceedings of the IEEEMTT-S International Microwave Symposium Digest, Baltimore, MD, USA, 5–10 June 2011. [Google Scholar]
- Abdolrazzaghi, M.; Katchinskiy, N.; Elezzabi, A.Y.; Light, P.E.; Daneshmand, M. Noninvasive Glucose Sensing in Aqueous Solutions Using an Active Split-Ring Resonator. IEEE Sens. J. 2021, 21, 18742–18755. [Google Scholar] [CrossRef]
- Yang, X.; Xiaojun, J.; Yixuan, Z.; Hui, L. Genetic rules for the dermatoglyphics of human fingertips and their role in spouse selection: A preliminary study. Springer Plus 2016, 5, 1396. [Google Scholar] [CrossRef]
- Huber, P. Inaccurate input on touch devices relating to the fingertip (Proseminar). In Media Informatics Proseminar on Interactive Surfaces; 2015; Available online: https://www.medien.ifi.lmu.de/lehre/ss15/ps/papers/Huber-InputOnTouchDevices.pdf (accessed on 20 November 2022).
- Nicholls, M.E.; Orr, C.A.; Yates, M.J.; Loftus, A.M. A new means of measuring index/ring finger (2D:4D) ratio and its association with gender and hand preference. Laterality 2008, 13, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Alahnomi, R.A.; Zakaria, Z.; Yussof, Z.M.; Althuwayb, A.A.; Alhegazi, A.; Alsariera, H.; Rahman, N.A. Review of Recent Microwave Planar Resonator-Based Sensors: Techniques of Complex Permittivity Extraction, Applications, Open Challenges and Future Research Directions. Sensors 2021, 21, 2267. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.M. Anatomy of the joints of the fingers. Hand Clin. 1992, 8, 693–700. [Google Scholar] [CrossRef]
- Shores, J.T. Anatomy and physiology of the fingertip. In Fingertip Injuries: Diagnosis, Management and Reconstruction; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–9. [Google Scholar]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, K.; Cole, R. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 4, 341. [Google Scholar] [CrossRef] [Green Version]
- Jensen, P.D.; Meaney, P.M.; Epstien, N.R.; Paulsen, K.D. Cole–Cole Parameter Characterization of Urea and Potassium for Improving Dialysis Treatment Assessment. IEEE Antennas Wirel. Propag. Lett. 2012, 11, 1598–1601. [Google Scholar] [CrossRef]
- Dima, R.; Buonanno, G.; Costanzo, S.; Solimene, R. Robustness for the Starting Point of Two Iterative Methods for Fitting Debye or Cole–Cole Models to a Dielectric Permittivity Spectrum. Appl. Sci. 2022, 12, 5698. [Google Scholar] [CrossRef]
- Karacolak, T.; Moreland, E.C.; Topsakal, E. Cole–Cole model for glucose-dependent dielectric properties of blood plasma for continuous glucose monitoring. Microw. Opt. Technol. Lett. 2013, 55, 1160–1164. [Google Scholar] [CrossRef]
- Turgul, V.; Kale, I. Influence of fingerprints and finger positioning on accuracy of RF blood glucose measurement from fingertips. IET Electron. Lett. 2016, 53, 218–220. [Google Scholar] [CrossRef]
- Moko Technology. Available online: https://www.mokotechnology.com/fr4-pcb/ (accessed on 15 November 2022).
- Masini, J.; Shahbaz, R.; Deshours, F.; Alquié, G.; El bastami, C.; Kokabi, H. Penetration Depth in Multilayered Biological Tissues using a Compact Microwave Biosensor. In Proceedings of the 52nd European Microwave Conference (EuMC), Milan, Italy, 25–30 September 2022. [Google Scholar]


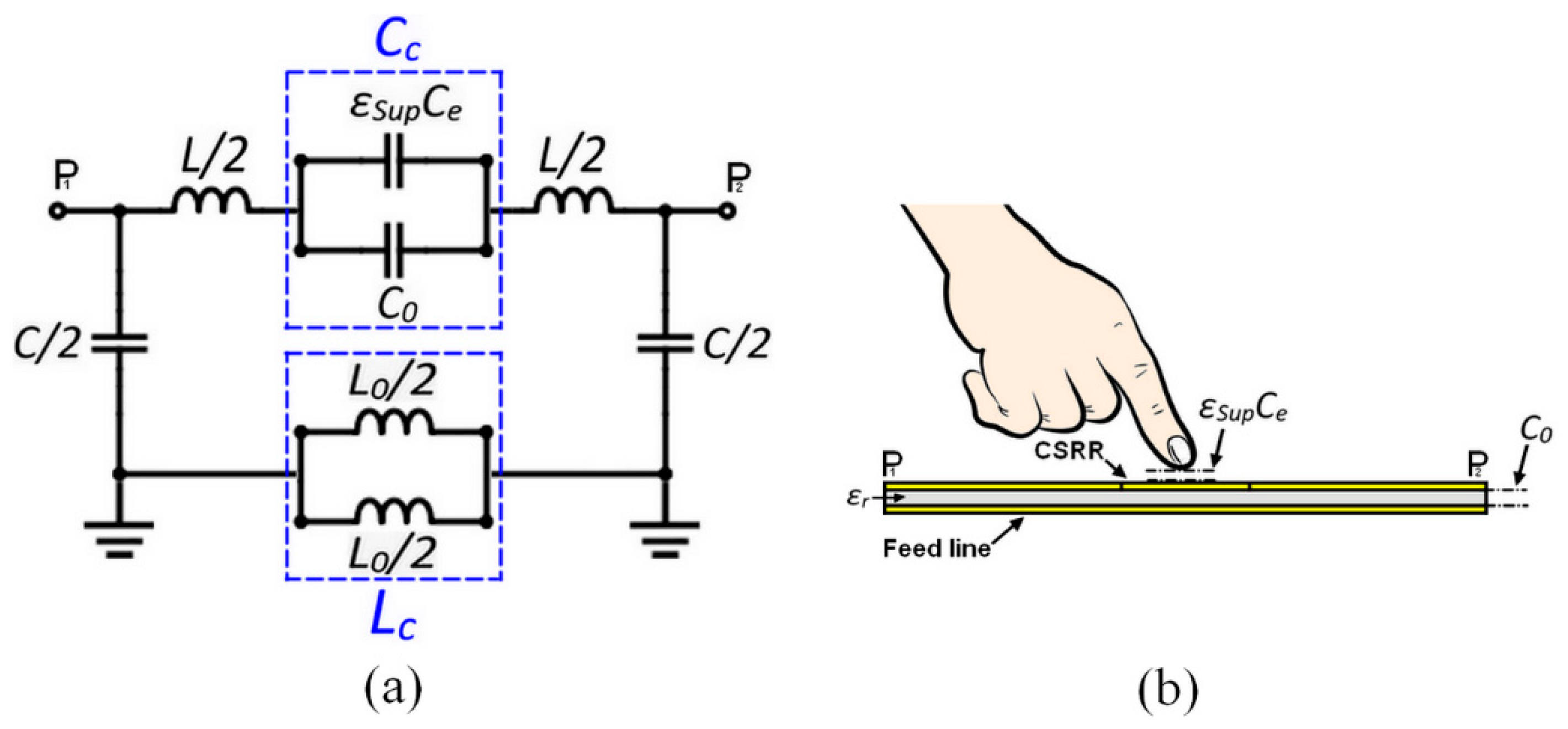


| Parameter | Skin | Fat | Blood | Bone |
|---|---|---|---|---|
| Thickness | hs = 1 mm | hf = 0.5 mm | hb = 5 mm | hB = 4 mm |
| Permittivity | 35 | 5.5 | 59 | 20 |
| Fat Thick. hf (mm) | 0.1 | 0.3 | 0.5 | 0.7 | 0.9 |
| Res. Freq. fr (GHz) | 2.2 | 2.1 | 2.08 | 2.05 | 2 |
| Δfr (GHz) | 0.1 | 0.2 | 0.22 | 0.25 | 0.3 |
| S12 (dB) | −21.78 | −22.34 | −18.16 | −22.76 | −17.27 |
| Permittivity | 4.97 | 6.47 | 6.98 | 7.33 | 8.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannachi, C.; Deshours, F.; Alquie, G.; Kokabi, H. Assessment of Finger Fat Pad Effect on CSRR-Based Sensor Scattering Parameters for Non-Invasive Blood Glucose Level Detection. Sensors 2023, 23, 473. https://doi.org/10.3390/s23010473
Hannachi C, Deshours F, Alquie G, Kokabi H. Assessment of Finger Fat Pad Effect on CSRR-Based Sensor Scattering Parameters for Non-Invasive Blood Glucose Level Detection. Sensors. 2023; 23(1):473. https://doi.org/10.3390/s23010473
Chicago/Turabian StyleHannachi, Chaouki, Frédérique Deshours, George Alquie, and Hamid Kokabi. 2023. "Assessment of Finger Fat Pad Effect on CSRR-Based Sensor Scattering Parameters for Non-Invasive Blood Glucose Level Detection" Sensors 23, no. 1: 473. https://doi.org/10.3390/s23010473





