Contactless Technologies, Sensors, and Systems for Cardiac and Respiratory Measurement during Sleep: A Systematic Review
Abstract
:1. Introduction
1.1. Review Questions
1.1.1. Main Review Question (MRQ)
1.1.2. Specific Review Questions (SRQs)
- Which technologies can be used for contactless measurement of cardiac activity and respiration during sleep?
- Which sensors are used for those technologies?
- Which physiological parameters can be extracted out of those sensors?
- What are the medical applications of contactless cardiac and respiratory monitoring during sleep?
- What are the differences in the quality of the measurements (contactless vs. contact-based/attached devices)?
2. Materials and Methods
2.1. Eligibility Criteria
- Study type: randomised controlled and clinical trials; results of testing the required systems and technologies at home, in labs, or in nursing houses; research journal articles and conference publications;
- Population: subjects who participated in trials measuring cardiac or respiratory movements during sleep;
- The document must have been published in a peer-reviewed format;
- The approach and system should have been implemented and tested at least at the level of a prototype;
- The system should have been developed for human measures (not for animals);
- The system should have been developed to fulfil at least one of the following aspects:
- ○
- Measuring cardiac parameters, heart rate, etc.;
- ○
- Measuring respiratory rate from the contactless sensor/technology meeting the requirements mentioned above;
- ○
- The system was developed to monitor sleep quality;
- ○
- The system was developed to measure or verify a breathing- or cardiac-related disease;
- ○
- The system was developed to measure or verify one of the diseases related to sleep;
- The method of data transmission or processing does not affect the inclusion/exclusion.
- The exclusion criteria were as follows:
- Book chapters, white papers, editorials, and perspectives;
- Papers not written in English;
- The paper was excluded if only the concept had been presented without any validation;
- The paper was excluded if only using wearable devices;
- Studies not related to measuring vital sign parameters or aforementioned (breathing) movements during sleep;
- Studies with publication dates older than five years when the systematic review was performed (2017–2022);
- Studies that are not focused on the use of contactless systems/methods/technologies for measuring the above parameters;
- Studies that included fewer than five subjects in an experiment;
- Published data not available.
2.2. Search Strategy and Information Sources
2.3. Selection Process and Data Extraction
2.4. Synthesis Methods
3. Results
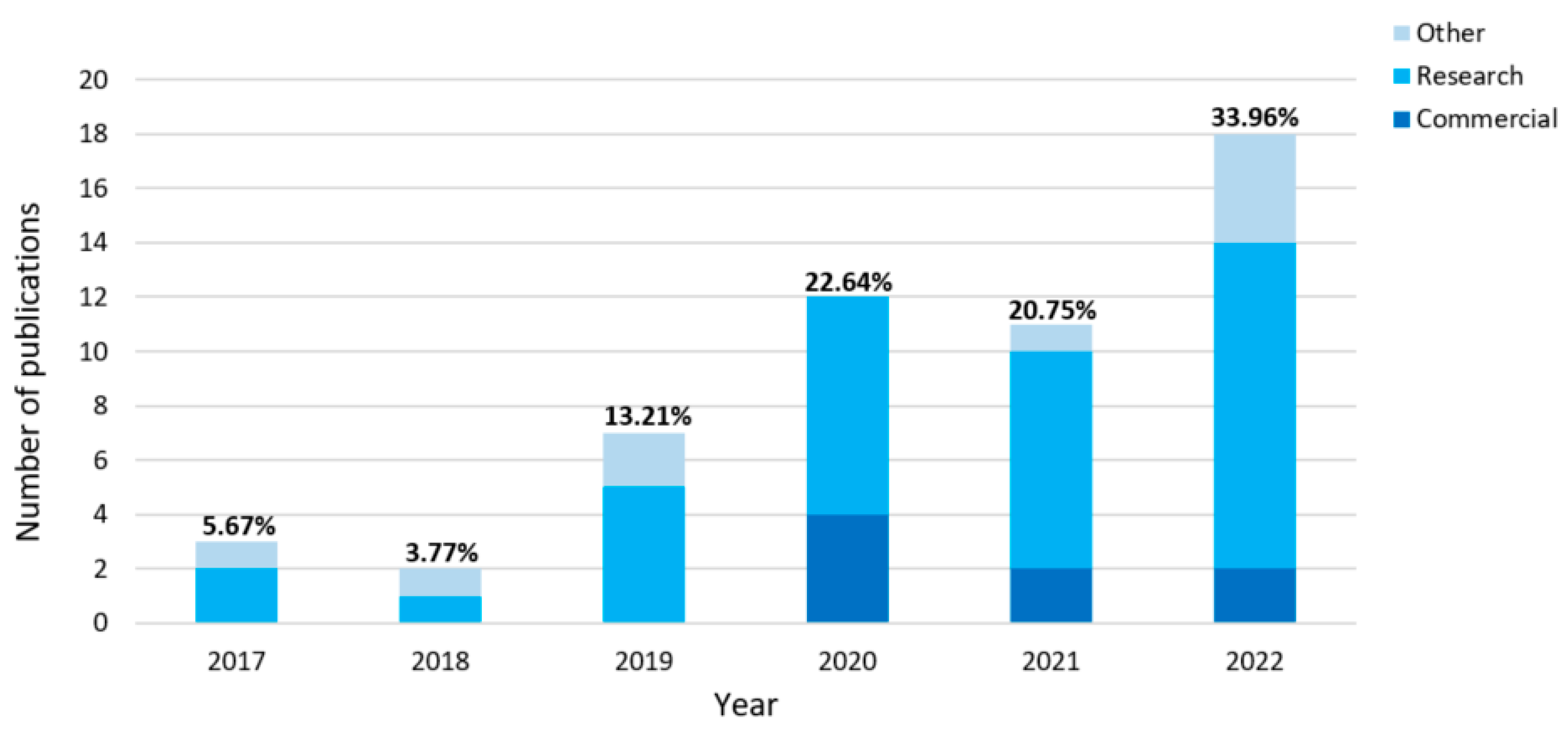
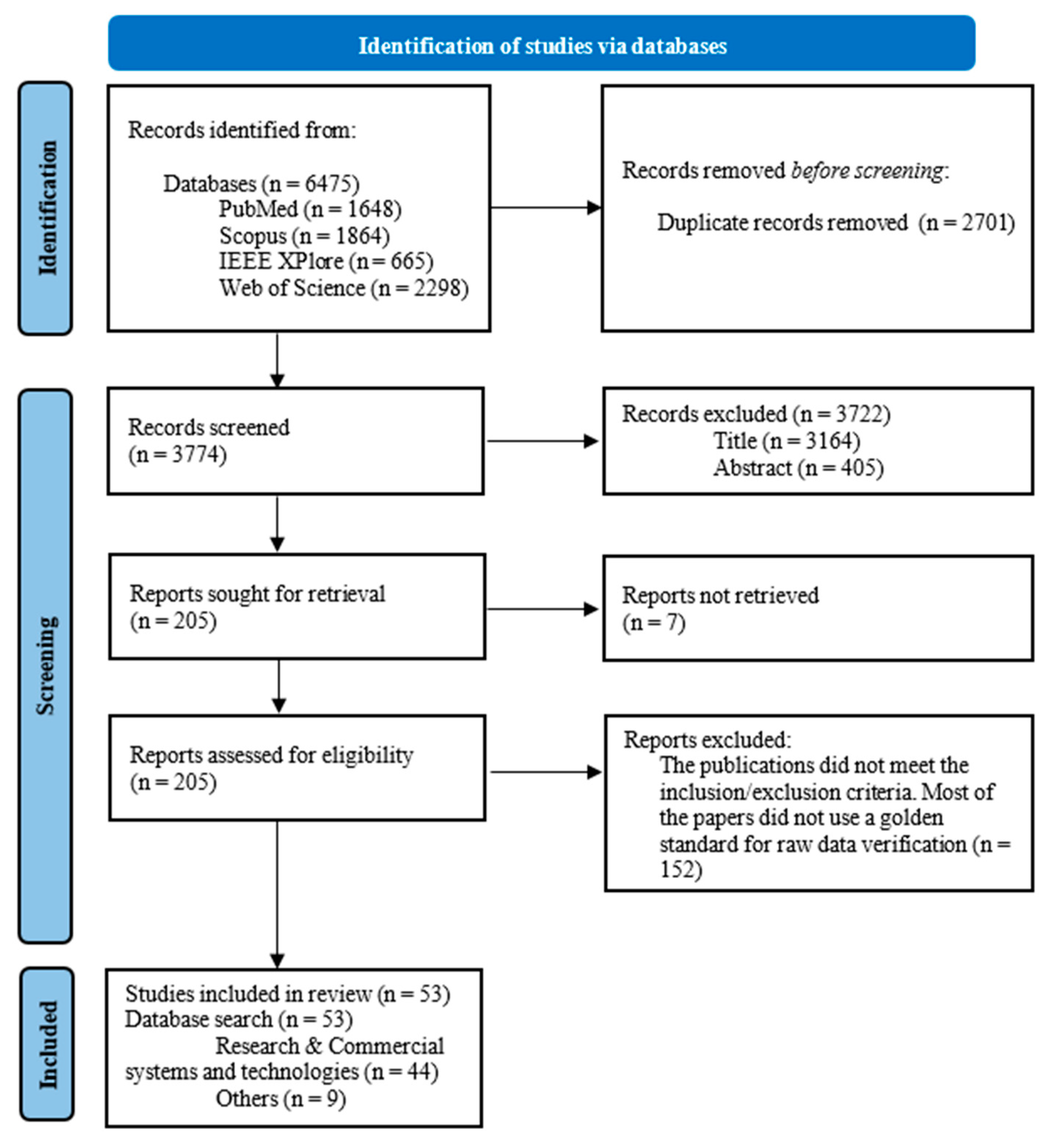
3.1. Study Selection
3.2. Study Characteristics and Individual Publications
3.3. Synthesis Results and Questions of Interest
3.3.1. MRQ: How Can the Cardiac Activity and Respiration Be Contactlessly Monitored during Sleep?
- Video images;
- Movements evoked by cardiac contractions (i.e., heartbeat movements);
- Subject movements (e.g., body, shoulders);
- Temperature maps and thermal images;
- Sounds of cardiac activity (e.g., heart sounds).
3.3.2. SRQ-1: Which Technologies Can Be Used for the Contactless Measurement of Cardiac Activity and Respiration during Sleep?
- Interferometry
- Ballistocardiography (BCG)
- Remote photoplethysmography (rPPG)
- Thermography and Infrared (IR) technologies
3.3.3. SRQ-2: Which Sensors Are Used for Those Technologies?
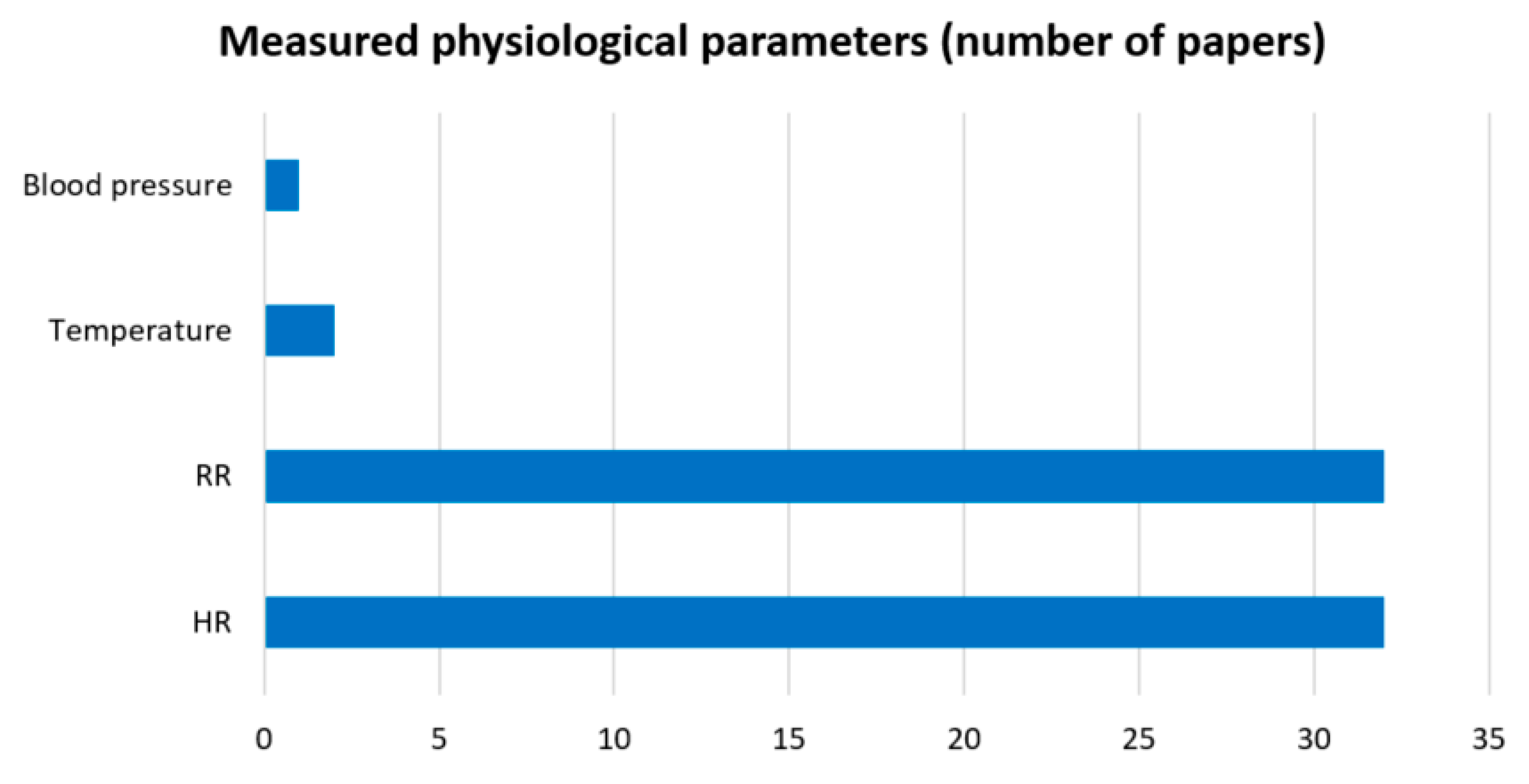
3.3.4. SRQ-3: Which Physiological Parameters Can Be Extracted from Those Sensors?
- Heart rate (HR);
- Respiration rate (RR);
- Temperature;
- Blood pressure.
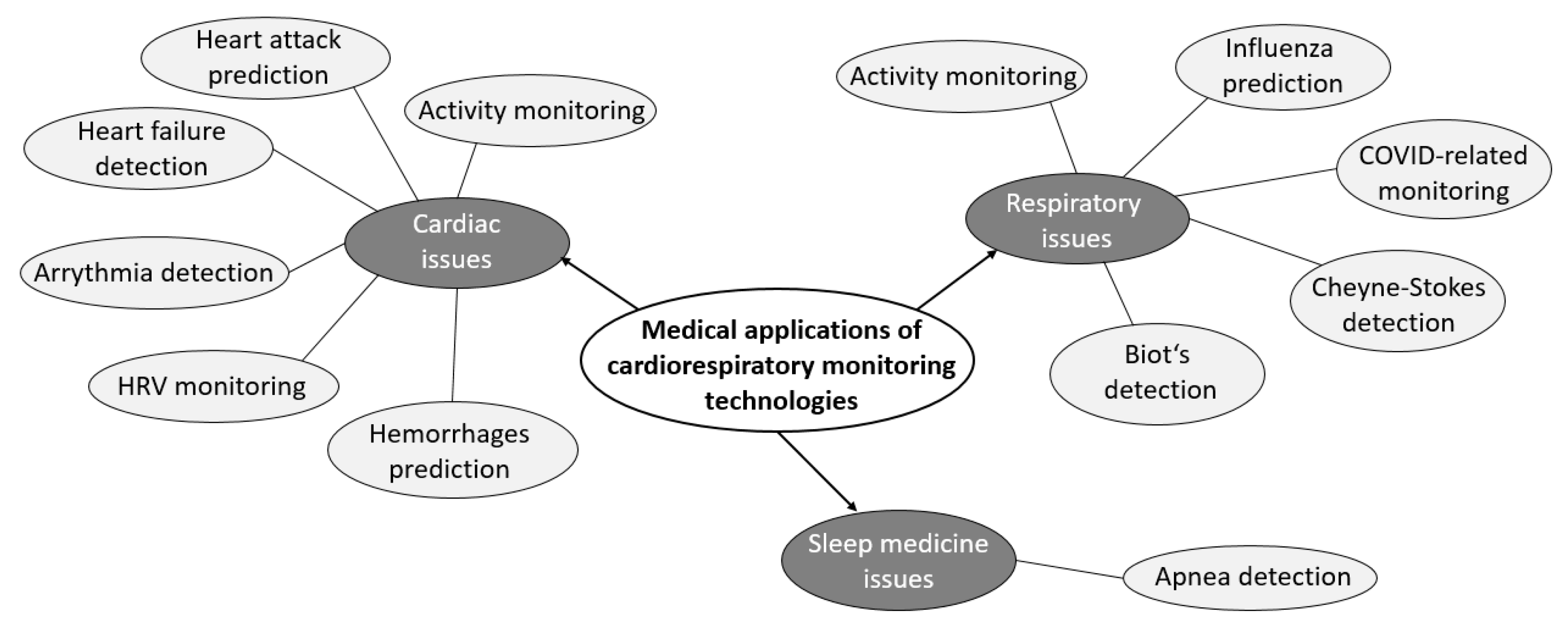
3.3.5. SRQ-4: What Are the Medical Applications of Contactless Cardiac and Respiratory Monitoring during Sleep?
- Cardiac activity;
- Respiratory activity;
- Sleep medicine.
3.3.6. SRQ-5: What Are the Differences in the Quality of the Measurements (Contactless vs. Contact-Based/Attached Devices)?
- Accuracy, sensitivity, specificity (Acc, Sen, Spe);
- Mean absolute error (MAE);
- Mean absolute percentage error (MAPE);
- Pearson’s correlation coefficient (r-Pearson).
3.3.7. Enhancements, Advantages, and Limitations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. IEEE Xplore
Appendix A.2. PubMed
Appendix A.3. Scopus
Appendix A.4. Web of Science
References
- Sadek, I.; Biswas, J.; Abdulrazak, B. Ballistocardiogram signal processing: A review. Health Inf. Sci. Syst. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- De Fazio, R.; Stabile, M.; De Vittorio, M.; Velázquez, R.; Visconti, P. An Overview of Wearable Piezoresistive and Inertial Sensors for Respiration Rate Monitoring. Electronics 2021, 10, 2178. [Google Scholar] [CrossRef]
- Gaiduk, M.; Perea, J.J.; Seepold, R.; Madrid, N.M.; Penzel, T.; Glos, M.; Ortega, J.A. Estimation of Sleep Stages Analyzing Respiratory and Movement Signals. IEEE J. Biomed. Health Inform. 2021, 26, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Gadie, A.; Shafto, M.; Leng, Y.; Kievit, R.A. How are age-related differences in sleep quality associated with health outcomes? An epidemiological investigation in a UK cohort of 2406 adults. BMJ Open 2017, 7, e014920. [Google Scholar] [CrossRef] [PubMed]
- Inan, O.T.; Migeotte, P.-F.; Park, K.-S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and Seismocardiography: A Review of Recent Advances. IEEE J. Biomed. Health Inform. 2014, 19, 1414–1427. [Google Scholar] [CrossRef]
- Stamatakis, E.; de Rezende, L.F.M.; Rey-López, J.P. Sedentary Behaviour and Cardiovascular Disease. In Sedentary Behaviour Epidemiology; Leitzmann, M.F., Jochem, C., Schmid, D., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 215–243. [Google Scholar] [CrossRef]
- Rundo, J.V.; Downey, R. Polysomnography. In Handbook of Clinical Neurology: Clinical Neurophysiology: Basis and Technical Aspects; Levin, K.H., Chauvel, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 381–392. [Google Scholar] [CrossRef]
- Tran, V.P.; Al-Jumaily, A.A.; Islam, S.M.S. Doppler Radar-Based Non-Contact Health Monitoring for Obstructive Sleep Apnea Diagnosis: A Comprehensive Review. Big Data Cogn. Comput. 2019, 3, 3. [Google Scholar] [CrossRef]
- Green, S.F.; Frame, T.; Banerjee, L.V.; Gimson, A.; Blackman, J.; Morrison, H.; Lloyd, K.; Rudd, S.; Fotherby, W.G.F.; Bartsch, U.; et al. A systematic review of the validity of non-invasive sleep-measuring devices in mid-to-late life adults: Future utility for Alzheimer’s disease research. Sleep Med. Rev. 2022, 65, 101665. [Google Scholar] [CrossRef]
- Kagawa, M.; Ueki, K.; Tojima, H.; Matsui, T. Noncontact screening system with two microwave radars for the diagnosis of sleep apnea-hypopnea syndrome. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 2052–2055. [Google Scholar] [CrossRef]
- Ren, L.; Kong, L.; Foroughian, F.; Wang, H.; Theilmann, P.; Fathy, A.E. Comparison Study of Noncontact Vital Signs Detection Using a Doppler Stepped-Frequency Continuous-Wave Radar and Camera-Based Imaging Photoplethysmography. IEEE Trans. Microw. Theory Tech. 2017, 65, 3519–3529. [Google Scholar] [CrossRef]
- Savage, H.O.; Khushaba, R.N.; Zaffaroni, A.; Colefax, M.; Farrugia, S.; Schindhelm, K.; Teschler, H.; Weinreich, G.; Grueger, H.; Neddermann, M.; et al. Development and validation of a novel non-contact monitor of nocturnal respiration for identifying sleep-disordered breathing in patients with heart failure. ESC Heart Fail. 2016, 3, 212–219. [Google Scholar] [CrossRef]
- Hall, T.; Lie, D.Y.C.; Nguyen, T.Q.; Mayeda, J.C.; Lie, P.E.; Lopez, J.; Banister, R.E. Non-Contact Sensor for Long-Term Continuous Vital Signs Monitoring: A Review on Intelligent Phased-Array Doppler Sensor Design. Sensors 2017, 17, 2632. [Google Scholar] [CrossRef]
- Lin, C.-T.; Prasad, M.; Chung, C.-H.; Puthal, D.; El-Sayed, H.; Sankar, S.; Wang, Y.-K.; Singh, J.; Sangaiah, A.K. IoT-Based Wireless Polysomnography Intelligent System for Sleep Monitoring. IEEE Access 2017, 6, 405–414. [Google Scholar] [CrossRef]
- Sadek, I.; Seet, E.; Biswas, J.; Abdulrazak, B.; Mokhtari, M. Nonintrusive Vital Signs Monitoring for Sleep Apnea Patients: A Preliminary Study. IEEE Access 2017, 6, 2506–2514. [Google Scholar] [CrossRef]
- Kim, C.-S.; Ober, S.L.; McMurtry, M.S.; Finegan, B.A.; Inan, O.T.; Mukkamala, R.; Hahn, J.-O. Ballistocardiogram: Mechanism and Potential for Unobtrusive Cardiovascular Health Monitoring. Sci. Rep. 2016, 6, 31297. [Google Scholar] [CrossRef]
- Zhang, X.; Kassem, M.A.M.; Zhou, Y.; Shabsigh, M.; Wang, Q.; Xu, X. A Brief Review of Non-invasive Monitoring of Respiratory Condition for Extubated Patients with or at Risk for Obstructive Sleep Apnea after Surgery. Front. Med. 2017, 4, 26. [Google Scholar] [CrossRef]
- Chung, K.; Song, K.; Shin, K.; Sohn, J.; Cho, S.H.; Chang, J.-H. Noncontact Sleep Study by Multi-Modal Sensor Fusion. Sensors 2017, 17, 1685. [Google Scholar] [CrossRef]
- Tal, A.; Shinar, Z.; Shaki, D.; Codish, S.; Goldbart, A. Validation of Contact-Free Sleep Monitoring Device with Comparison to Polysomnography. J. Clin. Sleep Med. 2017, 13, 517–522. [Google Scholar] [CrossRef]
- Phan, H.; Mikkelsen, K. Automatic sleep staging of EEG signals: Recent development, challenges, and future directions. Physiol. Meas. 2022, 43, 04TR01. [Google Scholar] [CrossRef]
- Yuda, E.; Yoshida, Y.; Sasanabe, R.; Tanaka, H.; Shiomi, T.; Hayano, J. Sleep Stage Classification by a Combination of Actigraphic and Heart Rate Signals. J. Low Power Electron. Appl. 2017, 7, 28. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Khushaba, R.N.; Armitstead, J.; Schindhelm, K. Monitoring of nocturnal central sleep apnea in Heart failure patients using noncontact respiratory differences. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 1534–1538. [Google Scholar] [CrossRef]
- Kapu, H.; Saraswat, K.; Ozturk, Y.; Cetin, A.E. Resting heart rate estimation using PIR sensors. Infrared Phys. Technol. 2017, 85, 56–61. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.-Y.; Choi, Y.-W.; Park, H.-K.; Cho, S.-H.; Cho, S.H.; Lim, Y.-H. A Novel Non-contact Heart Rate Monitor Using Impulse-Radio Ultra-Wideband (IR-UWB) Radar Technology. Sci. Rep. 2018, 8, 13053. [Google Scholar] [CrossRef] [PubMed]
- Michler, F.; Shi, K.; Schellenberger, S.; Steigleder, T.; Malessa, A.; Hameyer, L.; Neumann, N.; Lurz, F.; Ostgathe, C.; Weigel, R.; et al. A Clinically Evaluated Interferometric Continuous-Wave Radar System for the Contactless Measurement of Human Vital Parameters. Sensors 2019, 19, 2492. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Sun, G.; Sato, S.; Liu, H.; Matsui, T.; Abe, S.; Nishimura, H.; Kirimoto, T. Infection Screening System Using Thermography and CCD Camera with Good Stability and Swiftness for Non-contact Vital-Signs Measurement by Feature Matching and MUSIC Algorithm. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3183–3186. [Google Scholar] [CrossRef]
- Kim, J.D.; Lee, W.H.; Lee, Y.; Lee, H.J.; Cha, T.; Kim, S.H.; Song, K.-M.; Lim, Y.-H.; Cho, S.H.; Cho, S.H.; et al. Non-contact respiration monitoring using impulse radio ultrawideband radar in neonates. R. Soc. Open Sci. 2019, 6, 190149. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, H.-H.; Yang, H.C.; Yoon, J.-S.; Shin, H. Force-Sensing-Based Unobtrusive System for Awakening and Respiration Rate Analysis During Sleep. IEEE Sens. J. 2018, 19, 1917–1924. [Google Scholar] [CrossRef]
- Al-Naji, A.; Al-Askery, A.J.; Gharghan, S.K.; Chahl, J. A System for Monitoring Breathing Activity Using an Ultrasonic Radar Detection with Low Power Consumption. J. Sens. Actuator Netw. 2019, 8, 32. [Google Scholar] [CrossRef]
- Turppa, E.; Kortelainen, J.M.; Antropov, O.; Kiuru, T. Vital Sign Monitoring Using FMCW Radar in Various Sleeping Scenarios. Sensors 2020, 20, 6505. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, X.; Liu, X.; Lu, C.; Wang, L.; Chen, W. Non-Contact Heart Rate Monitoring in Neonatal Intensive Care Unit using RGB Camera. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 5822–5825. [Google Scholar] [CrossRef]
- Villarroel, M.; Jorge, J.; Meredith, D.; Sutherland, S.; Pugh, C.; Tarassenko, L. Non-contact vital-sign monitoring of patients undergoing haemodialysis treatment. Sci. Rep. 2020, 10, 18529. [Google Scholar] [CrossRef]
- Imano, W.; Kameyama, K.; Hollingdal, M.; Refsgaard, J.; Larsen, K.; Topp, C.; Kronborg, S.H.; Gade, J.D.; Dinesen, B. Non-Contact Respiratory Measurement Using a Depth Camera for Elderly People. Sensors 2020, 20, 6901. [Google Scholar] [CrossRef]
- He, S.; Mehta, V.; Bolic, M. A Joint Localization Assisted Respiratory Rate Estimation using IR-UWB Radars. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 489–493. [Google Scholar] [CrossRef]
- Park, J.; Cho, H.; Balan, R.K.; Ko, J. HeartQuake: Accurate Low-Cost Non-Invasive ECG Monitoring Using Bed-Mounted Geophones. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2020, 4, 1–28. [Google Scholar] [CrossRef]
- Shi, K.; Schellenberger, S.; Will, C.; Steigleder, T.; Michler, F.; Fuchs, J.; Weigel, R.; Ostgathe, C.; Koelpin, A. A dataset of radar-recorded heart sounds and vital signs including synchronised reference sensor signals. Sci. Data 2020, 7, 50. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, S.K. Radar-Based Detection of Respiration Rate with Adaptive Harmonic Quefrency Selection. Sensors 2020, 20, 1607. [Google Scholar] [CrossRef]
- Schellenberger, S.; Shi, K.; Steigleder, T.; Malessa, A.; Michler, F.; Hameyer, L.; Neumann, N.; Lurz, F.; Weigel, R.; Ostgathe, C.; et al. A dataset of clinically recorded radar vital signs with synchronised reference sensor signals. Sci. Data 2020, 7, 291. [Google Scholar] [CrossRef]
- Rossol, S.L.; Yang, J.K.; Toney-Noland, C.; Bergin, J.; Basavaraju, C.; Kumar, P.; Lee, H.C. Non-Contact Video-Based Neonatal Respiratory Monitoring. Children 2020, 7, 171. [Google Scholar] [CrossRef]
- Addison, P.S.; Smit, P.; Jacquel, D.; Borg, U.R. Continuous respiratory rate monitoring during an acute hypoxic challenge using a depth sensing camera. J. Clin. Monit. Comput. 2019, 34, 1025–1033. [Google Scholar] [CrossRef]
- Negishi, T.; Abe, S.; Matsui, T.; Liu, H.; Kurosawa, M.; Kirimoto, T.; Sun, G. Contactless Vital Signs Measurement System Using RGB-Thermal Image Sensors and Its Clinical Screening Test on Patients with Seasonal Influenza. Sensors 2020, 20, 2171. [Google Scholar] [CrossRef]
- Xu, C.; Li, H.; Li, Z.; Zhang, H.; Rathore, A.S.; Chen, X.; Wang, K.; Huang, M.-C.; Xu, W. CardiacWave: A mmWave-based Scheme of Non-Contact and High-Definition Heart Activity Computing. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2021, 5, 135. [Google Scholar] [CrossRef]
- Malik, A.R.; Boger, J. Zero-Effort Ambient Heart Rate Monitoring Using Ballistocardiography Detected Through a Seat Cushion: Prototype Development and Preliminary Study. JMIR Rehabilitation Assist. Technol. 2021, 8, e25996. [Google Scholar] [CrossRef]
- Mateu-Mateus, M.; Guede-Fernández, F.; Rodriguez-Ibáñez, N.; García-González, M.; Ramos-Castro, J.; Fernández-Chimeno, M. A non-contact camera-based method for respiratory rhythm extraction. Biomed. Signal Process. Control 2021, 66, 102443. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Yang, H.; Qiu, Y.; Li, H.; Chen, Z.; Yu, C. Non-Invasive Measurement of Vital Signs Based on Seven-Core Fiber Interferometer. IEEE Sens. J. 2021, 21, 10703–10710. [Google Scholar] [CrossRef]
- Xia, W.; Li, Y.; Dong, S. Radar-Based High-Accuracy Cardiac Activity Sensing. IEEE Trans. Instrum. Meas. 2021, 70, 1–13. [Google Scholar] [CrossRef]
- Yu, X.; Laurentius, T.; Bollheimer, C.; Leonhardt, S.; Antink, C.H. Noncontact Monitoring of Heart Rate and Heart Rate Variability in Geriatric Patients Using Photoplethysmography Imaging. IEEE J. Biomed. Health Inform. 2020, 25, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Steigleder, T.; Schellenberger, S.; Michler, F.; Malessa, A.; Lurz, F.; Rohleder, N.; Ostgathe, C.; Weigel, R.; Koelpin, A. Contactless analysis of heart rate variability during cold pressor test using radar interferometry and bidirectional LSTM networks. Sci. Rep. 2021, 11, 3025. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Shandhi, M.H.; Li, Y.; Inan, O.T.; Zhang, Y. The Delineation of Fiducial Points for Non-Contact Radar Seismocardiogram Signals Without Concurrent ECG. IEEE J. Biomed. Health Inform. 2020, 25, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Ullal, A.; Su, B.Y.; Enayati, M.; Skubic, M.; Despins, L.; Popescu, M.; Keller, J. Non-invasive monitoring of vital signs for older adults using recliner chairs. Health Technol. 2020, 11, 169–184. [Google Scholar] [CrossRef]
- Wang, W.; Jia, Z.; Xu, C.; Luo, G.; Zhang, D.; An, N.; Zhang, Y. Feasibility study of practical vital sign detection using millimeter-wave radios. CCF Trans. Pervasive Comput. Interact. 2021, 3, 436–452. [Google Scholar] [CrossRef]
- Molinaro, N.; Schena, E.; Silvestri, S.; Massaroni, C. Multi-ROI Spectral Approach for the Continuous Remote Cardio-Respiratory Monitoring from Mobile Device Built-In Cameras. Sensors 2022, 22, 2539. [Google Scholar] [CrossRef]
- Gwak, M.; Vatanparvar, K.; Kuang, J.; Gao, A. Motion-Based Respiratory Rate Estimation with Motion Artifact Removal Using Video of Face and Upper Body. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 1961–1967. [Google Scholar] [CrossRef]
- Do, W.; Russell, R.; Wheeler, C.; Lockwood, M.; De Vos, M.; Pavord, I.; Bafadhel, M. Performance of Contactless Respiratory Rate Monitoring by Albus HomeTM, an Automated System for Nocturnal Monitoring at Home: A Validation Study. Sensors 2022, 22, 7142. [Google Scholar] [CrossRef]
- Svoboda, L.; Sperrhake, J.; Nisser, M.; Zhang, C.; Notni, G.; Proquitté, H. Contactless heart rate measurement in newborn infants using a multimodal 3D camera system. Front. Pediatr. 2022, 10, 897961. [Google Scholar] [CrossRef]
- Ling, Z.; Zhou, W.; Ren, Y.; Wang, J.; Guo, L. Non-Contact Heart Rate Monitoring Based on Millimeter Wave Radar. IEEE Access 2022, 10, 74033–74044. [Google Scholar] [CrossRef]
- Zheng, P.; Zheng, C.; Li, X.; Chen, H.; Wang, A.; Luo, Y. Second Harmonic Weighted Reconstruction for Non-Contact Monitoring Heart Rate. IEEE Sens. J. 2022, 22, 5815–5823. [Google Scholar] [CrossRef]
- Xu, W.; Yu, C.; Dong, B.; Wang, Y.; Zhao, W. Thin Piezoelectric Sheet Assisted PGC Demodulation of Fiber-Optic Integrated MZI and its Application in Under Mattress Vital Signs Monitoring. IEEE Sens. J. 2021, 22, 2151–2159. [Google Scholar] [CrossRef]
- Beltrão, G.; Stutz, R.; Hornberger, F.; Martins, W.A.; Tatarinov, D.; Alaee-Kerahroodi, M.; Lindner, U.; Stock, L.; Kaiser, E.; Goedicke-Fritz, S.; et al. Contactless radar-based breathing monitoring of premature infants in the neonatal intensive care unit. Sci. Rep. 2022, 12, 5150. [Google Scholar] [CrossRef]
- Shokouhmand, A.; Eckstrom, S.; Gholami, B.; Tavassolian, N. Camera-Augmented Non-Contact Vital Sign Monitoring in Real Time. IEEE Sens. J. 2022, 22, 11965–11978. [Google Scholar] [CrossRef]
- He, S.; Han, Z.; Iglesias, C.; Mehta, V.; Bolic, M. A Real-Time Respiration Monitoring and Classification System Using a Depth Camera and Radars. Front. Physiol. 2022, 13, 799621. [Google Scholar] [CrossRef]
- Parchani, G.; Kumar, G.; Rao, R.; Udupa, K.; Saran, V. Efficacy of Non-contact BallistocardiographySystem to Determine Heart Rate Variability. Ann. Neurosci. 2022, 29, 16–20. [Google Scholar] [CrossRef]
- Han, W.; Dai, S.; Yuce, M.R. Real-Time Contactless Respiration Monitoring from a Radar Sensor Using Image Processing Method. IEEE Sens. J. 2022, 22, 19020–19029. [Google Scholar] [CrossRef]
- Kunczik, J.; Hubbermann, K.; Mösch, L.; Follmann, A.; Czaplik, M.; Pereira, C.B. Breathing Pattern Monitoring by Using Remote Sensors. Sensors 2022, 22, 8854. [Google Scholar] [CrossRef]
- Talukdar, D.; De Deus, L.F.; Sehgal, N. Evaluation of a Camera-Based Monitoring Solution Against Regulated Medical Devices to Measure Heart Rate, Respiratory Rate, Oxygen Saturation, and Blood Pressure. Cureus 2022, 14, e31649. [Google Scholar] [CrossRef]
- Cardone, D.; Merla, A. New Frontiers for Applications of Thermal Infrared Imaging Devices: Computational Psychopshysiology in the Neurosciences. Sensors 2017, 17, 1042. [Google Scholar] [CrossRef]
- Mendonça, F.; Mostafa, S.S.; Ravelo-García, A.G.; Morgado-Dias, F.; Penzel, T. Devices for home detection of obstructive sleep apnea: A review. Sleep Med. Rev. 2018, 41, 149–160. [Google Scholar] [CrossRef]
- Wang, J.; Spicher, N.; Warnecke, J.M.; Haghi, M.; Schwartze, J.; Deserno, T.M. Unobtrusive Health Monitoring in Private Spaces: The Smart Home. Sensors 2021, 21, 864. [Google Scholar] [CrossRef]
- Heiden, E.; Jones, T.; Maczka, A.B.; Kapoor, M.; Chauhan, M.; Wiffen, L.; Barham, H.; Holland, J.; Saxena, M.; Wegerif, S.; et al. Measurement of Vital Signs Using Lifelight Remote Photoplethysmography: Results of the VISION-D and VISION-V Observational Studies. JMIR Form. Res. 2022, 6, e36340. [Google Scholar] [CrossRef]
- Beltrão, G.; Martins, W.A.A.; Bhavani Shankar, M.R.; Alaee-Kerahroodi, M.; Schroeder, U.; Tatarinov, D. Adaptive Nonlinear Least Squares Framework for Contactless Vital Sign Monitoring. IEEE Trans. Microw. Theory Tech. 2022, 71, 1696–1710. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, B.; Li, S.; Zhang, F.; Zhang, W. ECG-grained Cardiac Monitoring Using UWB Signals. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2022, 6, 186. [Google Scholar] [CrossRef]
- Qiao, J.-H.; Qi, F.-G.; Liang, F.-L.; Ma, J.; Lv, H.; Yu, X.; Xue, H.-J.; An, Q.; Yan, K.-D.; Shi, D.; et al. Contactless multiscale measurement of cardiac motion using biomedical radar sensor. Front. Cardiovasc. Med. 2022, 9, 1057195. [Google Scholar] [CrossRef]
- Bunch, B.H.; Hellemans, A. The History of Science and Technology: A Browser’s Guide to the Great Discoveries, Inventions, and the People who Made Them, from the Dawn of Time to Today; Houghton Mifflin: Boston, MA, USA, 2004; ISBN 9780618221233. [Google Scholar]
- Pinheiro, E.; Postolache, O.; Girão, P. Theory and Developments in an Unobtrusive Cardiovascular System Representation: Ballistocardiography. Open Biomed. Eng. J. 2010, 4, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Vogt, E.; MacQuarrie, D.; Neary, J.P. Using ballistocardiography to measure cardiac performance: A brief review of its history and future significance. Clin. Physiol. Funct. Imaging 2012, 32, 415–420. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Saraste, A.; Tuominen, J.; Koskinen, J.; Teräs, M.; Airaksinen, J.; Pänkäälä, M.; Koivisto, T. Gyrocardiography: A New Non-invasive Monitoring Method for the Assessment of Cardiac Mechanics and the Estimation of Hemodynamic Variables. Sci. Rep. 2017, 7, 6823. [Google Scholar] [CrossRef]
- Botina-Monsalve, D.; Benezeth, Y.; Miteran, J. Performance analysis of remote photoplethysmography deep filtering using long short-term memory neural network. Biomed. Eng. Online 2022, 21, 69. [Google Scholar] [CrossRef]
- Kamshilin, A.A.; Nippolainen, E.; Sidorov, I.S.; Vasilev, P.V.; Erofeev, N.P.; Podolian, N.P.; Romashko, R.V. A new look at the essence of the imaging photoplethysmography. Sci. Rep. 2015, 5, srep10494. [Google Scholar] [CrossRef]
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote plethysmographic imaging using ambient light. Opt. Express 2008, 16, 21434–21445. [Google Scholar] [CrossRef]
- Trumpp, A.; Lohr, J.; Wedekind, D.; Schmidt, M.; Burghardt, M.; Heller, A.R.; Malberg, H.; Zaunseder, S. Camera-based photoplethysmography in an intraoperative setting. Biomed. Eng. Online 2018, 17, 33. [Google Scholar] [CrossRef]
- Wang, W.; den Brinker, A.C.; Stuijk, S.; de Haan, G. Algorithmic Principles of Remote PPG. IEEE Trans. Biomed. Eng. 2017, 64, 1479–1491. [Google Scholar] [CrossRef]
- Hassan, M.; Malik, A.; Fofi, D.; Saad, N.; Karasfi, B.; Ali, Y.; Meriaudeau, F. Heart rate estimation using facial video: A review. Biomed. Signal Process. Control 2017, 38, 346–360. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Wong, K.-L.; Chin, J.-W.; Chan, T.-T.; So, R.H.Y. Deep Learning Methods for Remote Heart Rate Measurement: A Review and Future Research Agenda. Sensors 2021, 21, 6296. [Google Scholar] [CrossRef]
- Pereira, C.B.; Czaplik, M.; Blazek, V.; Leonhardt, S.; Teichmann, D. Monitoring of Cardiorespiratory Signals Using Thermal Imaging: A Pilot Study on Healthy Human Subjects. Sensors 2018, 18, 1541. [Google Scholar] [CrossRef]
- Pereira, C.B.; Czaplik, M.; Blanik, N.; Rossaint, R.; Blazek, V.; Leonhardt, S. Contact-free monitoring of circulation and perfusion dynamics based on the analysis of thermal imagery. Biomed. Opt. Express 2014, 5, 1075–1089. [Google Scholar] [CrossRef]
- Lahiri, B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical applications of infrared thermography: A review. Infrared Phys. Technol. 2012, 55, 221–235. [Google Scholar] [CrossRef]
- Perpetuini, D.; Di Credico, A.; Filippini, C.; Izzicupo, P.; Cardone, D.; Chiacchiaretta, P.; Ghinassi, B.; Di Baldassarre, A.; Merla, A. Is It Possible to Estimate Average Heart Rate from Facial Thermal Imaging? Eng. Proc. 2021, 8, 10. [Google Scholar] [CrossRef]
- Ioannou, S.; Gallese, V.; Merla, A. Thermal infrared imaging in psychophysiology: Potentialities and limits. Psychophysiology 2014, 51, 951–963. [Google Scholar] [CrossRef]
- Magalhaes, C.; Mendes, J.; Vardasca, R. Meta-Analysis and Systematic Review of the Application of Machine Learning Classifiers in Biomedical Applications of Infrared Thermography. Appl. Sci. 2021, 11, 842. [Google Scholar] [CrossRef]
- Di Credico, A.; Perpetuini, D.; Izzicupo, P.; Gaggi, G.; Cardone, D.; Filippini, C.; Merla, A.; Ghinassi, B.; Di Baldassarre, A. Estimation of Heart Rate Variability Parameters by Machine Learning Approaches Applied to Facial Infrared Thermal Imaging. Front. Cardiovasc. Med. 2022, 9, 893374. [Google Scholar] [CrossRef] [PubMed]
- Boiko, A.; Scherz, W.D.; Gaiduk, M.; Gentili, A.; Conti, M.; Orcioni, S.; Seepold, R.; Madrid, N.M. Sleep Respiration Rate Detection Using an Accelerometer Sensor with Special Holder Setup. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Haghi, M.; Asadov, A.; Boiko, A.; Ortega, J.A.; Madrid, N.M.; Seepold, R. Validating Force Sensitive Resistor Strip Sensors for Cardiorespiratory Measurement during Sleep: A Preliminary Study. Sensors 2023, 23, 3973. [Google Scholar] [CrossRef] [PubMed]





| Publication, Year | Monitoring Type | Measured Physiological Parameters | Number of Subjects | Type of Device |
|---|---|---|---|---|
| [23], 2017 | Body movement analysis | RR | 52 | Research |
| [24], 2017 | Chest movement analysis | HR | 30 | Research |
| [25], 2018 | Chest movement analysis | HR | 22 | Research |
| [26], 2019 | Heart sounds analysis | HR, RR | 30 | Research |
| [27], 2019 | Face image analysis | HR, RR, temperature (facial) | 25 | Research |
| [28], 2019 | Chest movement analysis | RR | 42 | Research |
| [29], 2019 | Body movement analysis | RR | 30 | Research |
| [30], 2019 | Chest movement analysis | RR | 12 | Research |
| [31], 2020 | Heart sounds analysis | HR, RR | 10 | Research |
| [32], 2020 | Face image analysis | HR | 5 | Research |
| [33], 2020 | Face image analysis | HR, RR | 40 | Commercial |
| [34], 2020 | Body image analysis | RR | 39 | Commercial |
| [35], 2020 | Body movement analysis | RR | 5 | Research |
| [36], 2020 | Heartbeat movement analysis | HR | 41 | Commercial |
| [37], 2020 | Heart sounds analysis | HR, RR | 11 | Research |
| [38], 2020 | Chest movement analysis | RR | 16 | Research |
| [39], 2020 | Chest movement analysis | HR, RR | 30 | Research |
| [40], 2020 | Body image analysis | RR | 17 | Research |
| [41], 2020 | Body image analysis | RR | 14 | Commercial |
| [42], 2020 | Body and face images analysis | HR, RR, temperature (body) | 50 | Research |
| [43], 2021 | Chest movement analysis | HR | 40 | Commercial |
| [44], 2021 | Heartbeat movement analysis | HR | 20 | Research |
| [45], 2021 | Body image analysis | RR | 21 | Research |
| [46], 2021 | Heartbeat movement analysis | HR, RR | 11 | Research |
| [47], 2021 | Body movement analysis | HR | 6 | Research |
| [48], 2021 | Face image analysis | HR | 20 | Research |
| [49], 2021 | Heart sounds analysis | HR | 25 | Research |
| [50], 2021 | Body movement analysis | HR | 22 | Research |
| [51], 2021 | Heartbeat movement analysis | HR, RR | 45 | Commercial |
| [52], 2021 | Body movement analysis | HR, RR | 12 | Research |
| [53], 2022 | Body image analysis | HR, RR | 18 | Research |
| [54], 2022 | Body and face images analysis | RR | 30 | Research |
| [55], 2022 | Chest movement analysis | RR | 32 | Commercial |
| [56], 2022 | Skin colour image analysis | HR | 42 | Research |
| [57], 2022 | Body movement analysis | HR | 20 | Research |
| [58], 2022 | Chest movement analysis | HR | 15 | Research |
| [59], 2022 | Heartbeat movement analysis | HR, RR | 6 | Research |
| [60], 2022 | Chest movement analysis | RR | 30 | Research |
| [61], 2022 | Postures and chest movement analysis | HR, RR | 10 | Research |
| [62], 2022 | Body movement and images analysis | RR | 17 | Research |
| [63], 2022 | Heartbeat movement analysis | HR | 24 | Research |
| [64], 2022 | Body movement analysis | RR | 15 | Research |
| [65], 2022 | Face image analysis | RR | 10 | Research |
| [66], 2022 | Face image analysis | HR, RR, blood pressure | 463 | Commercial |
| Publication, Year | Objective | Type of Publication |
|---|---|---|
| [67], 2017 | Review of the principal achievements of thermal infrared imaging in computational psychophysiology, focusing on the capability of the technique for providing ubiquitous and unwired monitoring of psychophysiological activity and affective states | Review article |
| [68], 2018 | Reviewing publications that show the performances of different devices for the ambulatory diagnosis of sleep apnoea | Review article |
| [8], 2019 | A comprehensive review of the current state of non-contact Doppler radar sleep monitoring technology, providing an outline of current challenges and making recommendations on future research directions to practically realise and commercialise the technology for everyday usage | Review article |
| [1], 2019 | Review of the sensors used for obtaining BCG signals. Review of the signal processing methods as applied to the various sensors to analyse the BCG signal and extract physiological parameters such as heart rate and breathing rate, as well as determining sleep stages | Review article |
| [69], 2021 | A retrospective literature review and summarised the state-of-the-art research on leveraging sensor technology for unobtrusive in-home health monitoring | Review article |
| [70], 2022 | Performance of validation results on the use of Lifelight software to accurately cardiorespiratory signal measurement | Commercial article |
| [71], 2022 | A complete framework for vital sign processing using a 77 GHz FMCW radar | Research article |
| [72], 2022 | Development of two complementary heartbeat signal restoration methods to perfectly recover heartbeat signal variation based on the analysis of the properties of UWB signals containing heartbeats and respiration | Research article |
| [73], 2022 | A contactless and multiscale cardiac motion detection method is proposed, with no blind detection of segments during the entire cardiac cycle | Research article |
| Technology | Amount of Papers |
|---|---|
| Interferometry | 21 |
| BCG (incl. SCG) | 7 |
| rPPG (incl. iPPG) | 16 |
| Thermography | 4 |
| IR technologies | 1 |
| Radar Type | Frequency Range, GHz | Number of Papers |
|---|---|---|
| Ultrasound | 4∙10–5 | 1 |
| Doppler | 2.4 … 10.525 24 | 3 |
| UWB radar | 2.9 … 10.1 | 6 |
| CW radar | 24 60 77 | 15 |
| Publication, Year | Evaluation Metrics | Additional Points | Sub | Publication Type | |||||
|---|---|---|---|---|---|---|---|---|---|
| Acc | Sen | Spe | MAE, bpm | MAPE, % | r-Pearson | ||||
| [23], 2017 | 86 | 71 | 88 | 0.38 | - | - | 52 | Research | |
| [26], 2019 | 93 | 93 | - | - | - | 0.914 | 30 | Research | |
| [29], 2019 | 76 | - | - | - | - | - | <1 bpm | 30 | Research |
| 97 | <2 bpm | ||||||||
| 99 | <3 bpm | ||||||||
| [28], 2019 | - | - | - | - | - | 0.893 | Depends on movement level (from lack to maximum) | 42 | Research |
| 0.833 | |||||||||
| 0.749 | |||||||||
| [30], 2019 | - | - | - | - | - | 0.977 | 0.5 m distance | 12 | Research |
| 0.956 | 1 m distance | ||||||||
| 0.844 | 2 m distance | ||||||||
| 0.648 | 3 m distance | ||||||||
| [27], 2019 | - | - | - | - | - | 0.920 | 25 | Research | |
| [42], 2020 | - | 85 | 90 | - | - | 0.87 | 50 | Research | |
| [35], 2020 | - | - | - | 1.52 | - | - | For single subject | 5 | Research |
| 1.32 | For multiple subjects | ||||||||
| [38], 2020 | - | - | - | 1.5 | - | 0.870 | 16 | Research | |
| [33], 2020 | - | - | - | 2.8 | - | 0.980 | 40 | Commercial | |
| [34], 2020 | - | - | - | - | 10.7 | - | With T-shirt | 39 | Commercial |
| 14 | Undressed | ||||||||
| [31], 2020 | - | - | - | - | 9.1 | 0.910 | 10 | Research | |
| [40], 2020 | - | - | - | - | - | 0.948 | 17 | Research | |
| [41], 2020 | - | - | - | - | - | 0.910 | 14 | Commercial | |
| [51], 2021 | 94 | - | - | - | 6.25 | - | 45 | Commercial | |
| [52], 2021 | - | - | - | 0.49 | - | - | 12 | Research | |
| [46], 2021 | - | - | - | 2.16 | - | - | 11 | Research | |
| [45], 2021 | - | - | - | - | 6 | 0.850 | 21 | Research | |
| [60], 2022 | 80 | - | - | - | - | - | 6 bpm accuracy | 30 | Research |
| 97 | 10 bpm accuracy | ||||||||
| [61], 2022 | 98 | - | - | - | - | - | 10 | Research | |
| [62], 2022 | 90 | - | - | 0.61 | - | - | For 1 subject | 17 | Research |
| 0.68 | For 2 subjects | ||||||||
| [64], 2022 | 95 | - | - | 0.18 | - | - | Not in real time | 15 | Research |
| 98 | 0.23 | Real time | |||||||
| [55], 2022 | 98 | - | - | - | - | - | 32 | Commercial | |
| [65], 2022 | 95 | - | - | - | - | - | 10 | Research | |
| [54], 2022 | - | - | - | 1.95 | - | 0.886 | For head | 30 | Research |
| 0.934 | For chest | ||||||||
| [53], 2022 | - | - | - | 1 | - | - | 18 | Research | |
| [66], 2022 | - | - | - | 2.9 | - | - | 463 | Commercial | |
| [59], 2022 | - | - | - | - | - | 0.992 | 6 | Research | |
| Publication, Year | Evaluation metrics | Additional Points | Sub | Publication Type | |||||
|---|---|---|---|---|---|---|---|---|---|
| Acc | Sen | Spe | MAE, bpm | MAPE, % | r-Pearson | ||||
| [24], 2017 | 95 | - | - | - | - | - | 30 | Research | |
| [25], 2018 | - | - | - | - | - | 0.856 | 30 | Research | |
| [27], 2019 | - | - | - | - | - | 0.820 | 25 | Research | |
| [36], 2020 | 97 | - | - | - | - | - | 41 | Commercial | |
| [42], 2020 | - | 85 | 90 | - | - | 0.870 | 50 | Research | |
| [33], 2020 | - | - | - | 2.1 | - | 0.920 | 40 | Commercial | |
| [32], 2020 | - | - | - | 7.4 | 12.46 | - | 5 | Research | |
| [31], 2020 | - | - | - | - | 3.6 | 0.860 | 10 | Research | |
| [37], 2020 | - | - | - | - | - | 0.937 | 11 | Research | |
| [39], 2020 | - | - | - | - | - | 0.961 | 30 | Research | |
| [48], 2021 | 95 | - | - | 0.02 | - | - | For IR-Camera algorithm | 20 | Research |
| 99 | For RGB-Camera algorithm | ||||||||
| [49], 2021 | 97 | 98 | - | - | - | - | 25 | Research | |
| 89 | 94 | ||||||||
| [50], 2021 | 75 | - | - | - | - | - | 22 | Research | |
| [51], 2021 | 97 | - | - | - | 3.6 | - | 45 | Commercial | |
| [47], 2021 | 93 | - | - | - | 1.06 | 0.983 | 6 | Research | |
| 1.15 | 0.987 | ||||||||
| [43], 2021 | 95 | - | - | - | - | 0.892 | 40 | Research | |
| [44], 2021 | 91 | - | - | - | - | - | 20 | Research | |
| 94 | |||||||||
| [52], 2021 | - | - | - | 2.39 | - | - | 12 | Research | |
| [46], 2021 | - | - | - | 1.17 | - | - | 11 | Research | |
| [61], 2022 | 86 | - | - | - | - | - | 10 | Research | |
| [63], 2022 | 97 | - | - | - | - | - | 24 | Research | |
| [53], 2022 | - | - | - | 6.7 | - | - | 18 | Research | |
| [66], 2022 | - | - | - | 2.9 | - | - | 463 | Commercial | |
| [57], 2022 | - | - | - | 1.28 | 1.74 | - | 20 | Research | |
| [58], 2022 | - | - | - | 4.28 | 5.56 | - | 15 | Research | |
| [56], 2022 | - | - | - | 3 | - | - | 42 | Research | |
| 8.6 | |||||||||
| [59], 2022 | - | - | - | - | - | 0.998 | 6 | Research | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boiko, A.; Martínez Madrid, N.; Seepold, R. Contactless Technologies, Sensors, and Systems for Cardiac and Respiratory Measurement during Sleep: A Systematic Review. Sensors 2023, 23, 5038. https://doi.org/10.3390/s23115038
Boiko A, Martínez Madrid N, Seepold R. Contactless Technologies, Sensors, and Systems for Cardiac and Respiratory Measurement during Sleep: A Systematic Review. Sensors. 2023; 23(11):5038. https://doi.org/10.3390/s23115038
Chicago/Turabian StyleBoiko, Andrei, Natividad Martínez Madrid, and Ralf Seepold. 2023. "Contactless Technologies, Sensors, and Systems for Cardiac and Respiratory Measurement during Sleep: A Systematic Review" Sensors 23, no. 11: 5038. https://doi.org/10.3390/s23115038








