Spatial Smoothing Effect on Group-Level Functional Connectivity during Resting and Task-Based fMRI
Abstract
:1. Introduction
- We constructed the functional connectivity maps for both rs-fMRI and tb-fMRI in detail for each subject. For the whole brain coverage, all major brain networks (Default Mode Network (DMN), Somatomotor Network (SMN), Visual Network (VN), Salience Network (SN), Dorsal Attention Network (DAN), Frontoparietal Network (FPN), Limbic Network (LN), Cerebellar Network (CN) were included to the analysis. To the best of our knowledge, there is no such study that analyzes the functional interactions of all these networks together.
- Although there are several studies that investigate the smoothing effect on the functional connectivity [30] or tb-fMRI [34] in single-subject [33] or on the healthy and diseased groups [35], however, to the best of our knowledge, there are no studies that have performed rs-fMRI and tb-fMRI together in such detail. Moreover, another important issue that differs from the other studies is that the functional images in the dataset involve sequential resting and task images that belong to the same subjects acquired from a single scanner. Thus, the dataset does not include intra-scanner and subject-related artifacts, which allows for observing the changes clearly.
- The main graph metrics, betweenness centrality, global and local efficiency, clustering coefficient, and average path length, are also analyzed for each smoothing level both for rs-fMRI and tb-fMRI.
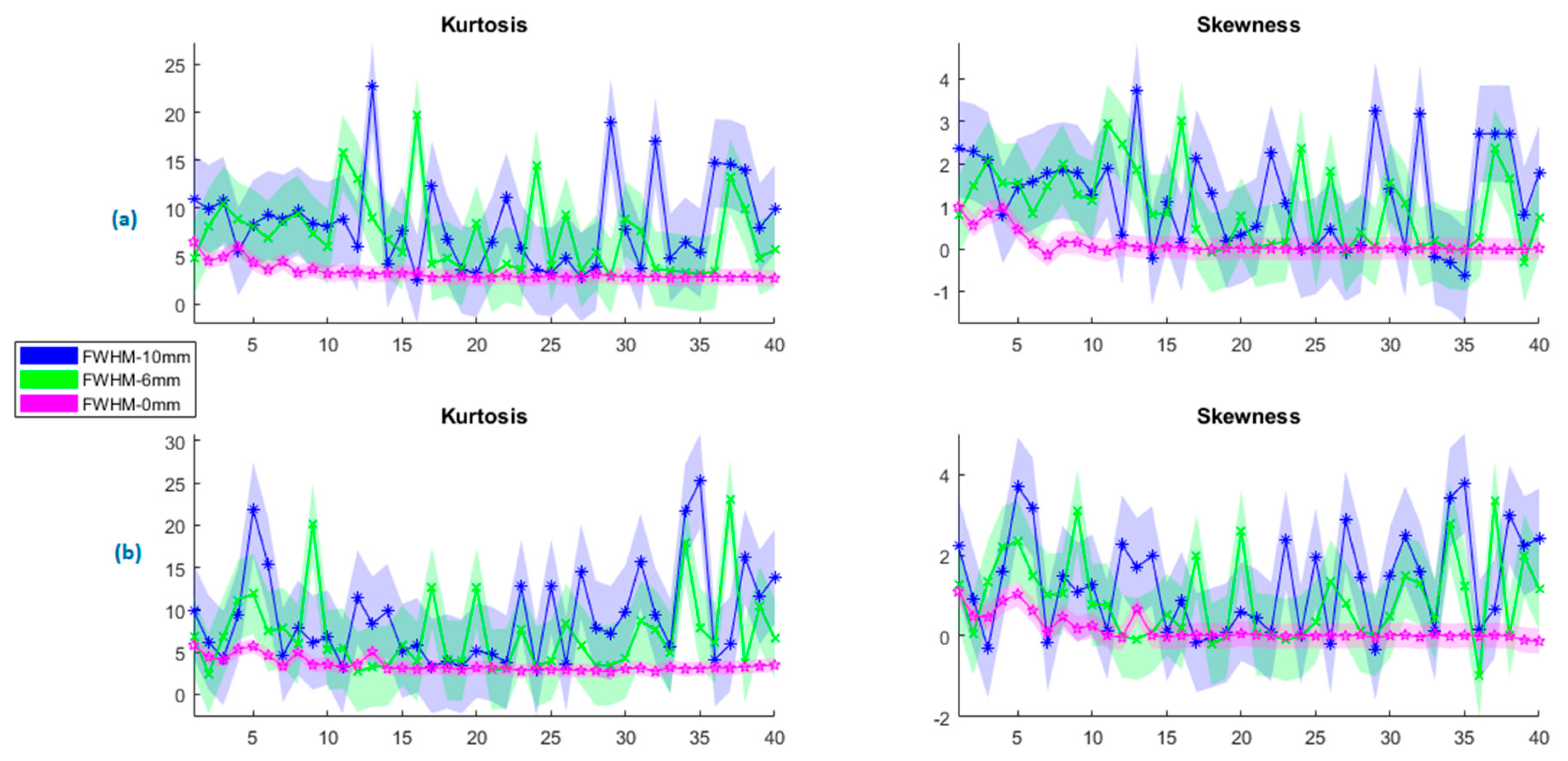
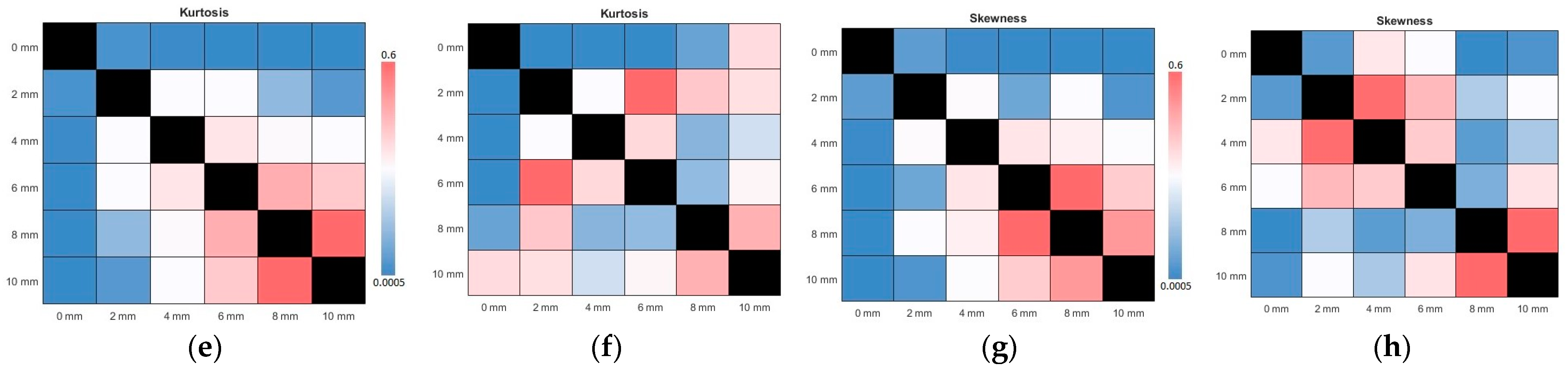
- Besides the functional connectivity analysis, the main component of independent component analysis (ICA) and principal component analysis (PCA), in terms of kurtosis and skewness, are also investigated in detail for both rs-fMRI and tb-fMRI at the group level. We could not find similar studies during the literature search.
2. Materials
2.1. Data Preliminaries
2.2. fMRI Task
2.3. Data Preprocessing
3. Methods
3.1. Smoothing
3.2. Functional Connectivity Network Extraction
3.3. Voxel-Based Analysis
3.3.1. Principal Component Analysis (PCA)
3.3.2. Independent Component Analysis (ICA)
3.4. ROI-Based Analysis
4. Results
4.1. Results for Voxel-Based Analysis: Effects on PCA and ICA Components
4.2. Results for ROI-Based Analysis: Effects on Functional Connectivity Networks
5. Conclusions and Future Work
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Default Mode Network (DMN) | |
| Medial Prefrontal Cortex | DMN.MPFC |
| Lateral Parietal left | DMN.LP l |
| Lateral Parietal right | DMN.LP r |
| Posterior Cingulate Cortex | DMN.PCC |
| Visual Network (VN) | |
| Medial Visual area | VN.Medial |
| Occipital Visual area | VN.Occipital |
| Lateral Visual area left | VN.Lateral l |
| Lateral Visual area right | VN.Lateral r |
| Dorsal Attention Network (DAN) | |
| Frontal Eye Fields left | DAN.FEF l |
| Frontal Eye Fields right | DAN.FEF r |
| Intraparietal Sulcus left | DAN.IPS l |
| Intraparietal Sulcus right | DAN.IPS r |
| Sensori Motor Network (SMN) | |
| Lateral Sensorimotor area left | SMN.Lateral l |
| Lateral Sensorimotor area right | SMN.Lateral r |
| Superior Sensorimotor area | SMN.Superior |
| Language Network (LN) | |
| Inferior Frontal Gyrus left | L.IFG l |
| Inferior Frontal Gyrus right | L.IFG r |
| Posterior Superior Temporal Gyrus left | L.pSTG l |
| Posterior Superior Temporal Gyrus right | L.pSTG r |
| Fronto Parietal Network (FPN) | |
| Lateral Prefrontal Cortex left | FPN.LPFC l |
| Posterior Parietal Cortex left | FPN.PPC l |
| Lateral Prefrontal Cortex right | FPN.LPFC r |
| Posterior Parietal Cortex right | FPN.PPC r |
| Salience Network (SN) | |
| Anterior Cingulate Cortex | SN.ACC |
| Anterior Insula left | SN.AInsula l |
| Anterior Insula right | SN.AInsula r |
| Rostral Prefrontal Cortex left | SN.RPFC l |
| Rostral Prefrontal Cortex right | SN.RPFC r |
| SupraMarginal Gyrus left | SN.SMG l |
| SupraMarginal Gyrus right | SN.SMG r |
| Cerebellar Network (CN) | |
| Cerebellum Anterior | C.Anterior |
| Cerebellum Posterior | C.Posterior |
References
- Carter, R. The Human Brain Book: An Illustrated Guide to Its Structure, Function, and Disorders; Illustrated edition; DK: New York, NY, USA, 2019; ISBN 978-1-4654-7954-9. [Google Scholar]
- Bandettini, P.A.; Birn, R.M.; Donahue, K.M. Functional MRI: Background, methodology, limits, and implementation. In Handbook of Psychophysiology, 2nd ed.; Cambridge University Press: New York, NY, USA, 2000; pp. 978–1014. ISBN 978-0-521-62634-7. [Google Scholar]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef]
- Cascino, G. Functional MRI for Language Localization. Epilepsy Curr. 2002, 2, 178–179. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Lv, J.; Jiang, X.; Guo, L.; Liu, T. Characterizing and Differentiating Task-based and Resting State FMRI Signals via Two-stage Sparse Representations. Brain Imaging Behav. 2016, 10, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Goebel, R. Localization of Brain Activity using Functional Magnetic Resonance Imaging. In Clinical Functional MRI: Presurgical Functional Neuroimaging; Stippich, C., Ed.; Medical Radiology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 9–51. ISBN 978-3-540-49976-3. [Google Scholar]
- Bijsterbosch, J.; Smith, S.M.; Beckmann, C.F. An Introduction to Resting State fMRI Functional Connectivity; Illustrated edition; Oxford University Press: Oxford, UK; New York, NY, USA, 2017; ISBN 978-0-19-880822-0. [Google Scholar]
- Sporns, O. Networks of the Brain, 1st ed.; The MIT Press: Cambridge, MA, USA, 2010; ISBN 978-0-262-01469-4. [Google Scholar]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.A.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L.; et al. Functional network organization of the human brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef] [Green Version]
- Farahani, F.V.; Karwowski, W.; Lighthall, N.R. Application of Graph Theory for Identifying Connectivity Patterns in Human Brain Networks: A Systematic Review. Front. Neurosci. 2019, 13, 585. [Google Scholar] [CrossRef]
- Wang, J.; Zuo, X.; He, Y. Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci. 2010, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Wang, Z.; Tong, E.; Williams, L.M.; Zaharchuk, G.; Zeineh, M.; Goldstein-Piekarski, A.N.; Ball, T.M.; Liao, C.; Wintermark, M. Resting-State Functional MRI: Everything That Nonexperts Have Always Wanted to Know. Am. J. Neuroradiol. 2018, 39, 1390–1399. [Google Scholar] [CrossRef] [Green Version]
- Friston, K.J. Functional and Effective Connectivity: A Review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef]
- Cribben, I.; Wager, T.D.; Lindquist, M.A. Detecting functional connectivity change points for single-subject fMRI data. Front. Comput. Neurosci. 2013, 7, 143. [Google Scholar] [CrossRef] [Green Version]
- Calhoun, V.D.; Adali, T. Time-Varying Brain Connectivity in fMRI Data: Whole-brain data-driven approaches for capturing and characterizing dynamic states. IEEE Signal Process. Mag. 2016, 33, 52–66. [Google Scholar] [CrossRef]
- Liu, T.T. Noise contributions to the fMRI signal: An overview. NeuroImage 2016, 143, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Candemir, C.; Gonul, A.S.; Selver, A.M. Automatic Detection of Emotional Changes Induced by Social Support Loss using fMRI. IEEE Trans. Affect. Comput. 2021, 14, 706–717. [Google Scholar] [CrossRef]
- Aurich, N.K.; Alves Filho, J.O.; Marques da Silva, A.M.; Franco, A.R. Evaluating the reliability of different preprocessing steps to estimate graph theoretical measures in resting state fMRI data. Front. Neurosci. 2015, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Vergara, V.M.; Mayer, A.R.; Damaraju, E.; Hutchison, K.; Calhoun, V.D. The effect of preprocessing pipelines in subject classification and detection of abnormal resting state functional network connectivity using group ICA. NeuroImage 2017, 145, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Strother, S.C. Evaluating fMRI preprocessing pipelines. IEEE Eng. Med. Biol. Mag. 2006, 25, 27–41. [Google Scholar] [CrossRef]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 2012, 59, 2142–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 2014, 84, 320–341. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.W.; Chen, C.-L.; Liu, P.-Y.; Chao, Y.-P.; Biswal, B.B.; Lin, C.-P. Empirical Evaluations of Slice-Timing, Smoothing, and Normalization Effects in Seed-Based, Resting-State Functional Magnetic Resonance Imaging Analyses. Brain Connect. 2011, 1, 401–410. [Google Scholar] [CrossRef]
- Shirer, W.R.; Jiang, H.; Price, C.M.; Ng, B.; Greicius, M.D. Optimization of rs-fMRI Pre-processing for Enhanced Signal-Noise Separation, Test-Retest Reliability, and Group Discrimination. NeuroImage 2015, 117, 67–79. [Google Scholar] [CrossRef]
- Worsley, K.J.; Friston, K.J. Analysis of fMRI Time-Series Revisited—Again. NeuroImage 1995, 2, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Mikl, M.; Mareček, R.; Hluštík, P.; Pavlicová, M.; Drastich, A.; Chlebus, P.; Brázdil, M.; Krupa, P. Effects of spatial smoothing on fMRI group inferences. Magn. Reson. Imaging 2008, 26, 490–503. [Google Scholar] [CrossRef]
- Sacchet, M.D.; Knutson, B. Spatial smoothing systematically biases the localization of reward-related brain activity. NeuroImage 2013, 66, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.E.; Yanes, J.A.; Kirby, L.A.J.; Reid, M.A.; Robinson, J.L. Left, right, or bilateral amygdala activation? How effects of smoothing and motion correction on ultra-high field, high-resolution functional magnetic resonance imaging (fMRI) data alter inferences. Neurosci. Res. 2020, 150, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Molloy, E.K.; Meyerand, M.E.; Birn, R.M. The influence of spatial resolution and smoothing on the detectability of resting-state and task fMRI. NeuroImage 2014, 86, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Scheinost, D.; Papademetris, X.; Constable, R.T. The impact of image smoothness on intrinsic functional connectivity and head motion confounds. NeuroImage 2014, 95, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Zuo, X.-N.; Xing, X.-X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: A systems neuroscience perspective. Neurosci. Biobehav. Rev. 2014, 45, 100–118. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Aguirre, G.K.; Detre, J.A. To smooth or not to smooth? ROC analysis of perfusion fMRI data. Magn. Reson. Imaging 2005, 23, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Calhoun, V. Effect of Spatial Smoothing on Task fMRI ICA and Functional Connectivity. Front. Neurosci. 2018, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajula, J.; Tohka, J. Effects of spatial smoothing on inter-subject correlation based analysis of FMRI. Magn. Reson. Imaging 2014, 32, 1114–1124. [Google Scholar] [CrossRef]
- Triana, A.M.; Glerean, E.; Saramäki, J.; Korhonen, O. Effects of spatial smoothing on group-level differences in functional brain networks. Netw. Neurosci. 2020, 4, 556–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candemir, C. A Practical Estimation of the Required Sample Size in fMRI Studies. Mugla J. Sci. Technol. 2023, 118, 706–717. [Google Scholar]
- Ebner, N.C.; Riediger, M.; Lindenberger, U. FACES—A database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behav. Res. Methods 2010, 42, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Poldrack, R.A.; Mumford, J.A.; Nichols, T.E. Handbook of Functional MRI Data Analysis; Cambridge University Press: Cambridge, UK, 2011; ISBN 978-0-521-51766-9. [Google Scholar] [CrossRef]
- Silver, N.C.; Dunlap, W.P. Averaging Correlation Coefficients: Should Fisher’s z Transformation Be Used? J. Appl. Psychol. 1987, 72, 146–148. [Google Scholar] [CrossRef]
- Smith, S.M.; Hyvärinen, A.; Varoquaux, G.; Miller, K.L.; Beckmann, C.F. Group-PCA for very large fMRI datasets. NeuroImage 2014, 101, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Huettel, S.A.; Song, A.W.; McCarthy, G. Functional Magnetic Resonance Imaging, 3rd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2014; ISBN 978-0-87893-627-4. [Google Scholar]
- Ma, X.; Jiang, G.; Fu, S.; Fang, J.; Wu, Y.; Liu, M.; Xu, G.; Wang, T. Enhanced Network Efficiency of Functional Brain Networks in Primary Insomnia Patients. Front. Psychiatry 2018, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massullo, C.; Imperatori, C.; De Vico Fallani, F.; Ardito, R.B.; Adenzato, M.; Palmiero, L.; Carbone, G.A.; Farina, B. Decreased brain network global efficiency after attachment memories retrieval in individuals with unresolved/disorganized attachment-related state of mind. Sci. Rep. 2022, 12, 4725. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Szucs, D.; Ioannidis, J.P.A. Sample size evolution in neuroimaging research: An evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. NeuroImage 2020, 221, 117164. [Google Scholar] [CrossRef] [PubMed]
- Zito, G.A.; Tarrano, C.; Jegatheesan, P.; Ekmen, A.; Béranger, B.; Rebsamen, M.; Hubsch, C.; Sangla, S.; Bonnet, C.; Delorme, C.; et al. Somatotopy of cervical dystonia in motor-cerebellar networks: Evidence from resting state fMRI. Parkinsonism Relat. Disord. 2022, 94, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.-J.; Gong, H.-H.; Wang, Y.-X.; Zhou, F.-Q.; Min, Y.-J.; Zhao, F.; Wang, S.-Y.; Liu, B.-X.; Xiao, X.-Z. Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: A resting-state fMRI study. Sleep Med. 2012, 13, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Parsons, N.; Bowden, S.C.; Vogrin, S.; D’Souza, W.J. Single-subject manual independent component analysis and resting state fMRI connectivity outcomes in patients with juvenile absence epilepsy. Magn. Reson. Imaging 2020, 66, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Mejia, A.F.; Nebel, M.B.; Shou, H.; Crainiceanu, C.M.; Pekar, J.J.; Mostofsky, S.; Caffo, B.; Lindquist, M.A. Improving reliability of subject-level resting-state fMRI parcellation with shrinkage estimators. NeuroImage 2015, 112, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Stickland, R.C.; Bright, M.G. Hemodynamic timing in resting-state and breathing-task BOLD fMRI. NeuroImage 2023, 274, 120120. [Google Scholar] [CrossRef] [PubMed]
- Desmond, J.E.; Glover, G.H. Estimating sample size in functional MRI (fMRI) neuroimaging studies: Statistical power analyses. J. Neurosci. Methods 2002, 118, 115–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, J.; Zhang, Z.; Wang, B.; Li, H.; Xu, J.; Wu, S.; Zhu, C.; Yang, X.; Liu, B.; Wu, Y.; et al. Different memory patterns of digits: A functional MRI study. J. Biomed. Sci. 2019, 26, 22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Naya, Y. A dataset of human fMRI/MEG experiments with eye tracking for spatial memory research using virtual reality. Data Brief 2022, 43, 108380. [Google Scholar] [CrossRef]
- Incidental Encoding Task (Posner Cueing Paradigm). Available online: https://openfmri.org/dataset/ds000110/ (accessed on 24 August 2022).








| Kernel (FWHM) | Resting | Encoding | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 mm | 0.65288 | 0.78633 | 0.02614 | 0.60200 | 1.78437 | 0.80481 | 0.87310 | 0.01305 | 0.75113 | 1.39245 |
| 2 mm | 0.65357 | 0.78252 | 0.02639 | 0.60905 | 1.79186 | 0.80388 | 0.87275 | 0.01327 | 0.75002 | 1.40063 |
| 4 mm | 0.65361 | 0.78253 | 0.02640 | 0.60915 | 1.79193 | 0.79424 | 0.86558 | 0.01378 | 0.73639 | 1.41412 |
| 6 mm | 0.65361 | 0.78253 | 0.02639 | 0.60916 | 1.79192 | 0.80447 | 0.86554 | 0.01378 | 0.73650 | 1.41443 |
| 8 mm | 0.65393 | 0.77929 | 0.02623 | 0.60959 | 1.78689 | 0.80488 | 0.86625 | 0.01295 | 0.74221 | 1.40784 |
| 10 mm | 0.65589 | 0.78638 | 0.02614 | 0.61200 | 1.78437 | 0.80492 | 0.87316 | 0.01286 | 0.74183 | 1.40638 |
| Average | 0.65392 | 0.78326 | 0.02629 | 0.60884 | 1.78855 | 0.80287 | 0.86939 | 0.01328 | 0.74301 | 1.40598 |
| Publication | #Subjects | Specification | Description |
|---|---|---|---|
| Somatotopy of cervical dystonia in motor-cerebellar networks: Evidence from resting state fMRI [47] | 18 | Resting | 11 min resting state, in which the gaze monitored with eye tracking |
| Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: A resting-state fMRI study [48] | 16 | Resting | Resting state for 30 min |
| Single-subject manual independent component analysis and resting state fMRI connectivity outcomes in patients with juvenile absence epilepsy [49] | 8 | Resting | Resting state about 14 min |
| Improving reliability of subject-level resting-state fMRI parcellation with shrinkage estimators [50] | 20 | Resting state for 7 min | |
| Hemodynamic timing in resting-state and breathing-task BOLD fMRI [51] | 9 | Resting | Resting state for 7 min 48 s |
| 9 | Task-based +Resting | Breath-hold task and resting-state consecutively | |
| Estimating sample size in functional MRI (fMRI) neuroimagingstudies: Statistical power analyses [52] | 6 | Resting | Resting state with open eyes for 4 min. |
| 12 | Task-based | A verbal working memory task | |
| Different memory patterns of digits: a functional MRI study [53] | 22 | Task-based | Short-term, Long Term and Working Memory on numerical figures |
| A dataset of human fMRI/MEG experiments with eye tracking for spatial memory research using virtual reality [54] | 12 | Task-based | Eye tracking task for spatial memory |
| Incidental encoding task [55] | 18 | Task-based | Participants create new memories without purposely by working in their environment and picking up information in the process. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candemir, C. Spatial Smoothing Effect on Group-Level Functional Connectivity during Resting and Task-Based fMRI. Sensors 2023, 23, 5866. https://doi.org/10.3390/s23135866
Candemir C. Spatial Smoothing Effect on Group-Level Functional Connectivity during Resting and Task-Based fMRI. Sensors. 2023; 23(13):5866. https://doi.org/10.3390/s23135866
Chicago/Turabian StyleCandemir, Cemre. 2023. "Spatial Smoothing Effect on Group-Level Functional Connectivity during Resting and Task-Based fMRI" Sensors 23, no. 13: 5866. https://doi.org/10.3390/s23135866





