Online Monitoring of Seawater Carbon Dioxide Based on an Infrared Rear Beam Splitter
Abstract
:1. Introduction
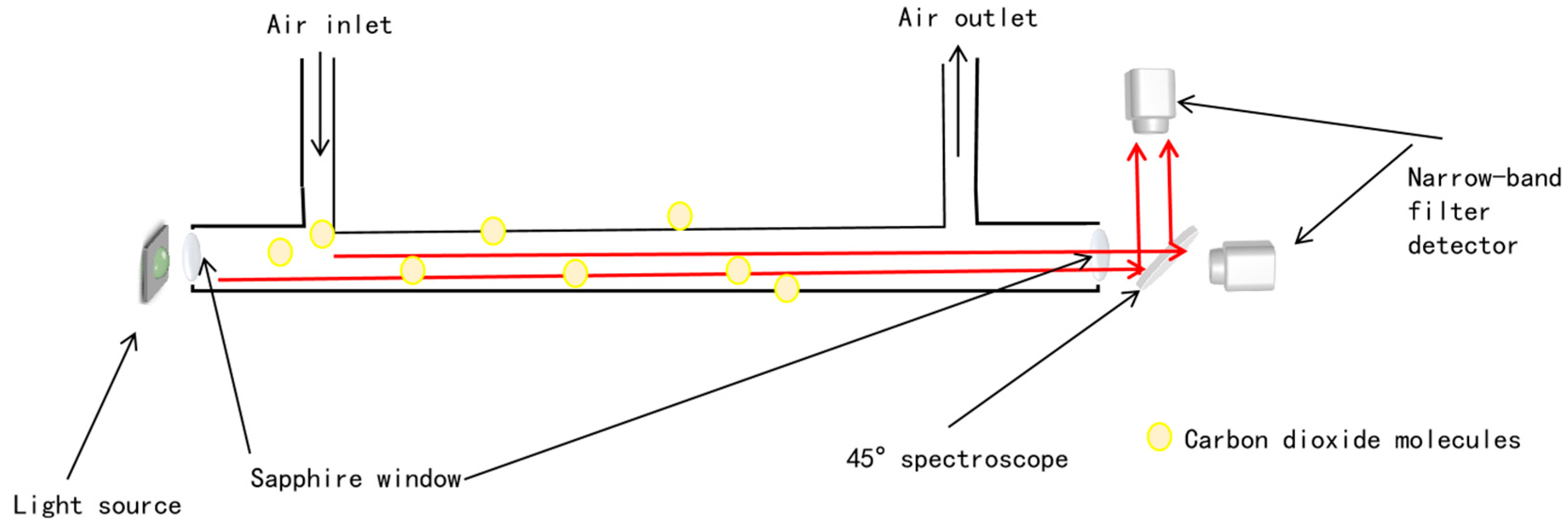
2. Device Principle
2.1. Absorption Spectrum of Carbon Dioxide Molecule
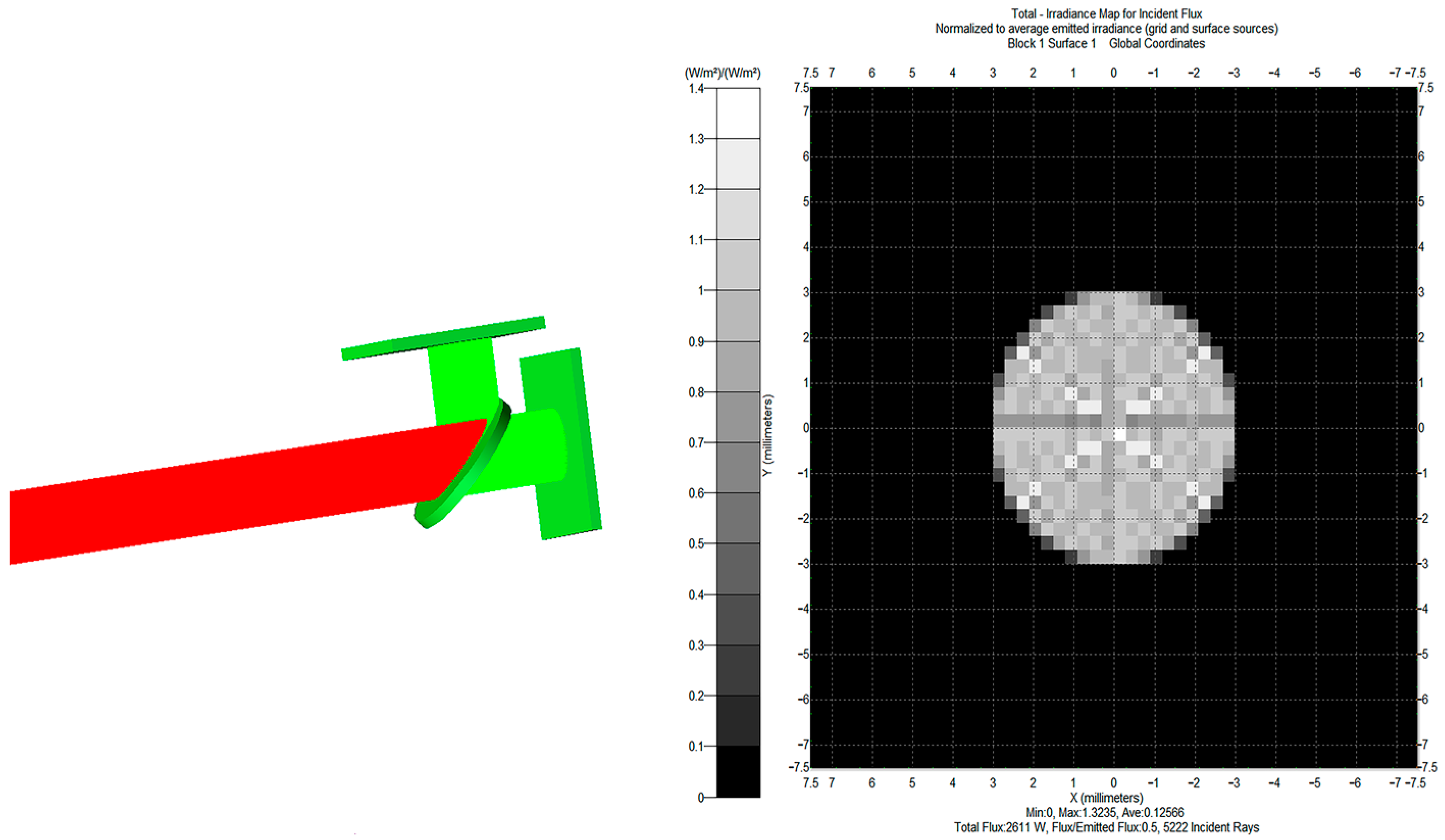
2.2. Optical Path Design and Simulation
3. System Structure and Methods
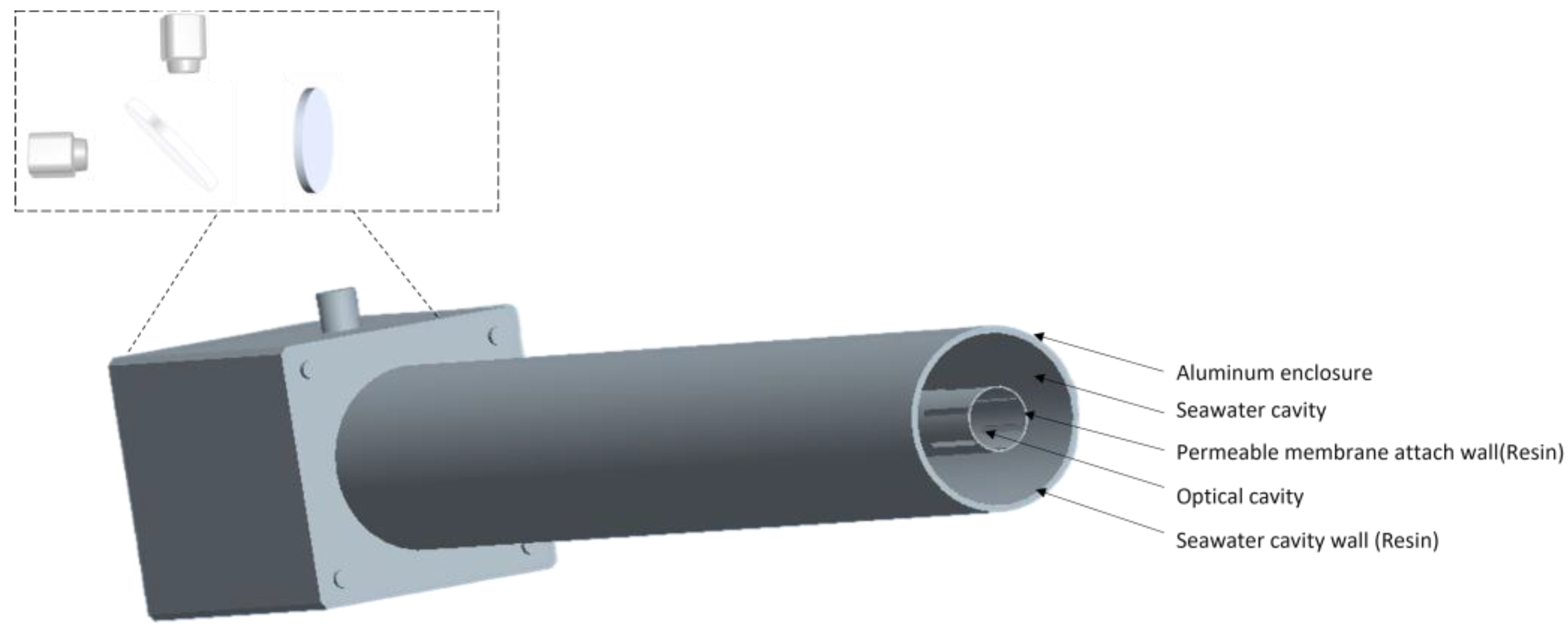
3.1. Device Structure
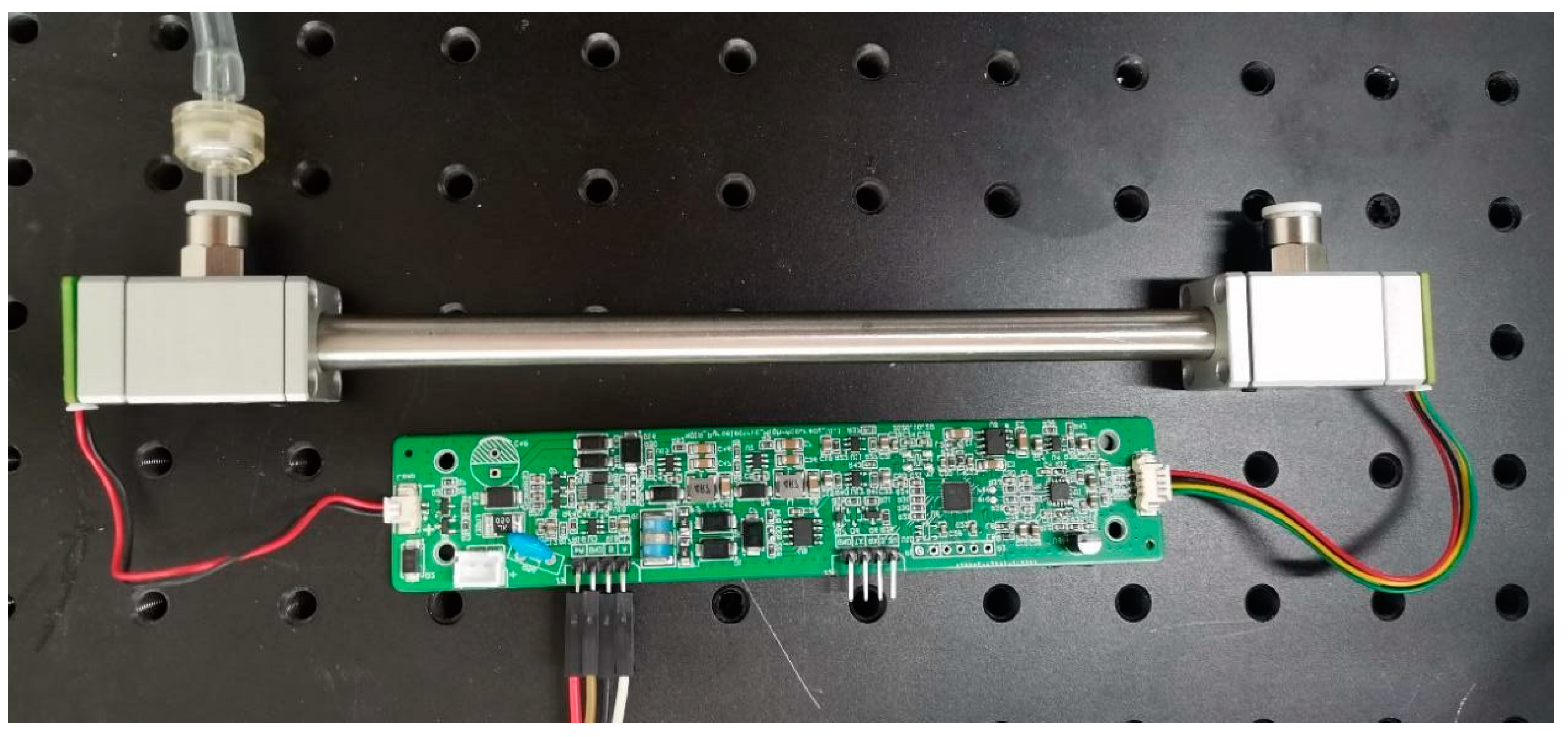
3.2. Experimental System
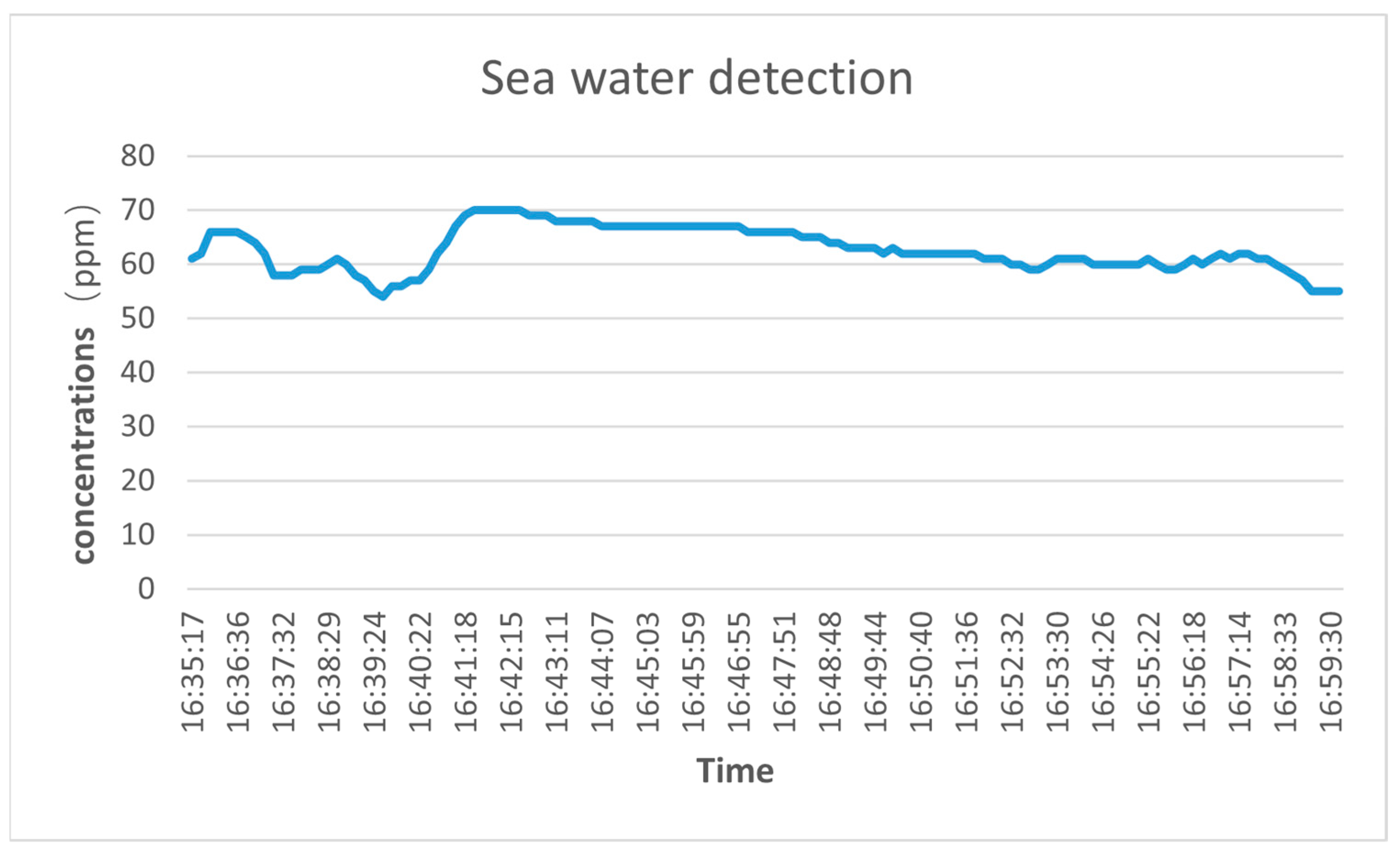
4. Results Analysis and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunk, R.M.; Peltzer, E.T.; Walz, P.M.; Brewer, P.G. Seeing a deep ocean CO2 enrichment experiment in a new light: Laser Raman detection of dissolved CO2 in seawater. Environ. Sci. Technol. 2005, 39, 9630–9636. [Google Scholar] [CrossRef] [PubMed]
- Katkov, E.; Fussmann, G.F. The effect of increasing temperature and pCO(2) on experimental pelagic freshwater communities. Limnol. Oceanogr. 2023. early view. [Google Scholar] [CrossRef]
- Xie, H.X.; Andrews, S.S.; Martin, W.R.; Miller, J.; Ziolkowski, L.; Taylor, C.D.; Zafiriou, O.C. Validated methods for sampling monoxide and headspace analysis of carbon in seawater. Mar. Chem. 2002, 77, 93–108. [Google Scholar] [CrossRef]
- Chesnokova, T.Y.; Chentsov, A.V.; Rokotyan, N.V.; Zakharov, V.I. Impact of difference in absorption line parameters in spectroscopic databases on CO2 and CH4 atmospheric content retrievals. J. Mol. Spectrosc. 2016, 327, 171–179. [Google Scholar] [CrossRef]
- Chesnokova, T.Y.; Chentsov, A.V.; Rokotyan, N.V.; Zakharov, V.I.; Firsov, K.M. Retrievals of the atmospheric methane content with use of new spectroscopic absorption line parameters. Proc. Spie 2018, 10833, 108330R. [Google Scholar]
- Chesnokova, T.Y.; Makarova, M.V.; Chentsov, A.V.; Kostsov, V.S.; Poberovskii, A.V.; Zakharov, V.I.; Rokotyan, N.V. Estimation of the impact of differences in the CH4 absorption line parameters on the accuracy of methane atmospheric total column retrievals from ground-based FTIR spectra. J. Quant Spectrosc. Radiat. 2020, 254, 107187. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hargreaves, R.J.; Hashemi, R.; Karlovets, E.V.; Skinner, F.M.; Conway, E.K.; Hill, C.; Kochanov, R.V.; Tan, Y.; et al. The HITRAN2020 molecular spectroscopic database. J. Quant Spectrosc. Radiat. 2022, 277, 107949. [Google Scholar] [CrossRef]
- Wong, J.Y.; Schell, M. Zero drift NDIR gas sensors. Sens. Rev. 2011, 31, 70–77. [Google Scholar] [CrossRef]
- Zhao, D.; Miller, D.; Shao, D.; Xian, X.; Tsow, F.; Iglesias, R.A.; Forzani, E.S. A personal device for analyzing carbon dioxide in real time and real breath: Experimental investigation and computational simulation. Sens. Actuators B Chem. 2013, 183, 627–635. [Google Scholar] [CrossRef]
- Besson, J.P.; Schilt, S.; Thevenaz, L. Sub-ppm multi-gas photoacoustic sensor. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 899–904. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Gao, C.; Zhou, Y.; Guo, Y. Temperature-compensated ppb-level sulfur dioxide detection system based on fourier transform ultraviolet differential optical absorption spectrum method. Sens. Actuators B Chem. 2020, 312, 127988. [Google Scholar] [CrossRef]
- Peng, B.; Zhou, Y.; Liu, G.; He, Y.; Gao, C.; Guo, Y. An ultra-sensitive detection system for sulfur dioxide and nitric oxide based on improved differential optical absorption spectroscopy method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 233, 118169. [Google Scholar] [CrossRef]
- Vincent, T.A.; Gardner, J.W. A low cost MEMS based NDIR system for the monitoring of carbon dioxide in breath analysis at ppm levels. Sens. Actuators B Chem. 2016, 236, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liu, Q.; Yang, D.; Guo, J.; Si, G.; Wu, L.; Zheng, R. Underwater In Situ Dissolved Gas Detection Based on Multi-Reflection Raman Spectroscopy. Sensors 2021, 21, 4831. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.W.; Zheng, C.T.; Zhang, T.Y.; Zhang, Y.; Wang, Y.D.; Tittel, F.K. High-precision methane isotopic abundance analysis using near-infrared absorption spectroscopy at 100 Torr. Analyst 2021, 146, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Schroder, S.; Wastine, B.; Bryzgalov, M.; Hummelgard, C.; Rodjegard, H.; Martin, H. Highly Compact multi-Spectral non-Dispersive Infrared Gas Sensor for large-Scale Deployment: K96 Sensor Core Concept. In Proceedings of the 2022 IEEE Sensors, Dallas, TX, USA, 30 October–2 November 2022; pp. 1–4. [Google Scholar]
- Goldschmidt, J.; Nitzsche, L.; Wolf, S.; Lambrecht, A.; Wollenstein, J. Rapid Quantitative Analysis of IR Absorption Spectra for Trace Gas Detection by Artificial Neural Networks Trained with Synthetic Data. Sensors 2022, 22, 857. [Google Scholar] [CrossRef]
- Jia, X.N.; Roels, J.; Baets, R.; Roelkens, G. A Miniaturised, Fully Integrated NDIR CO2 Sensor On-Chip. Sensors 2021, 21, 5347. [Google Scholar] [CrossRef]
- Gu, M.S.; Chen, J.J.; Zhang, Y.P.; Tan, T.; Wang, G.S.; Liu, K.; Gao, X.M.; Mei, J.X. Portable TDLAS Sensor for Online Monitoring of CO2 and H2O Using a Miniaturized Multi-Pass Cell. Sensors 2023, 23, 2072. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, S.Y.; Bai, H. Thermodynamically-consistent flash calculation in energy industry: From iterative schemes to a unified thermodynamics-informed neural network. Int. J. Energ. Res. 2022, 46, 15332–15346. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, J.; Ye, W.; Cheng, K.; Qi, F.; Zheng, R.; Sun, Z.; Zhang, X. Development of an Easy-to-Operate Underwater Raman System for Deep-Sea Cold Seep and Hydrothermal Vent In Situ Detection. Sensors 2021, 21, 5090. [Google Scholar] [CrossRef]
- Guo, J.; Luo, Z.; Liu, Q.; Yang, D.; Dong, H.; Huang, S.; Kong, A.; Wu, L. High-Sensitivity Raman Gas Probe for In Situ Multi-Component Gas Detection. Sensors 2021, 21, 3539. [Google Scholar] [CrossRef]
- Calloway, D. Beer-Lambert law. J. Chem. Educ. 1997, 74, 744. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.W.; Zeng, Y.; Liu, W.Q.; Xie, P.H.; Chan, K.L.; Li, X.X.; Wang, S.M.; Huang, S.H. Cross-interference correction and simultaneous multi-gas analysis based on infrared absorption. Chin. Phys. B 2012, 21, 090701. [Google Scholar] [CrossRef]
- Seo, J.B.; Jeon, S.B.; Choi, W.J.; Kim, J.W.; Lee, G.H.; Oh, K.J. The absorption rate of CO2/SO2/NO2 into a blended aqueous AMP/ammonia solution. Korean J. Chem. Eng. 2011, 28, 170–177. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Liu, R.; Ma, G.; Feng, S.; Mu, Y.; Meng, D.; Wang, S.; Cai, E. Online Monitoring of Seawater Carbon Dioxide Based on an Infrared Rear Beam Splitter. Sensors 2023, 23, 6273. https://doi.org/10.3390/s23146273
Liu L, Liu R, Ma G, Feng S, Mu Y, Meng D, Wang S, Cai E. Online Monitoring of Seawater Carbon Dioxide Based on an Infrared Rear Beam Splitter. Sensors. 2023; 23(14):6273. https://doi.org/10.3390/s23146273
Chicago/Turabian StyleLiu, Luyin, Ruzhang Liu, Guochao Ma, Shanshan Feng, Yuanhui Mu, Dexi Meng, Shuying Wang, and Enlin Cai. 2023. "Online Monitoring of Seawater Carbon Dioxide Based on an Infrared Rear Beam Splitter" Sensors 23, no. 14: 6273. https://doi.org/10.3390/s23146273




