The Principles of Hearable Photoplethysmography Analysis and Applications in Physiological Monitoring–A Review
Abstract
:1. Introduction
- This paper provides an overview of the current technology of the in-ear PPG including its principle, system, processing technology, and its applications in clinical and physiological monitoring.
- This paper compiles published articles related to in-ear PPG technology and applications. It provides a bibliographic analysis of a few selected papers and reviews their principle, processing techniques, and clinical monitoring features applications.
- This paper highlights in-ear PPG processing and applications in physiological monitoring topics. This review will provide a holistic analysis of a few selected papers and can be served as a foundation for further implementation in in-ear PPG research.
2. Materials and Methods
2.1. Search Structure
2.2. Inclusion Criteria
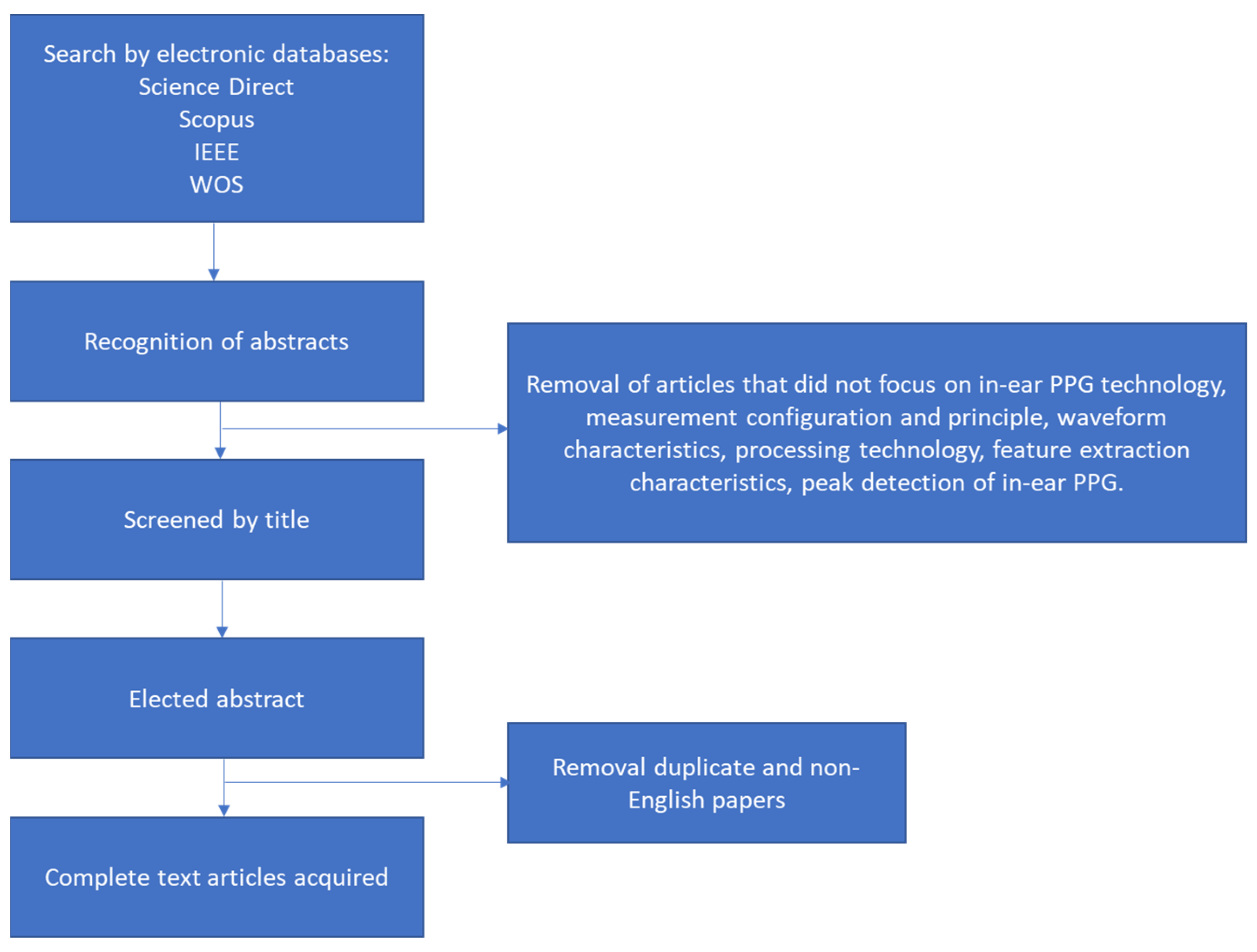
2.3. Review Process
3. Results
3.1. In-Ear PPG Principle
3.2. In-Ear PPG Placement
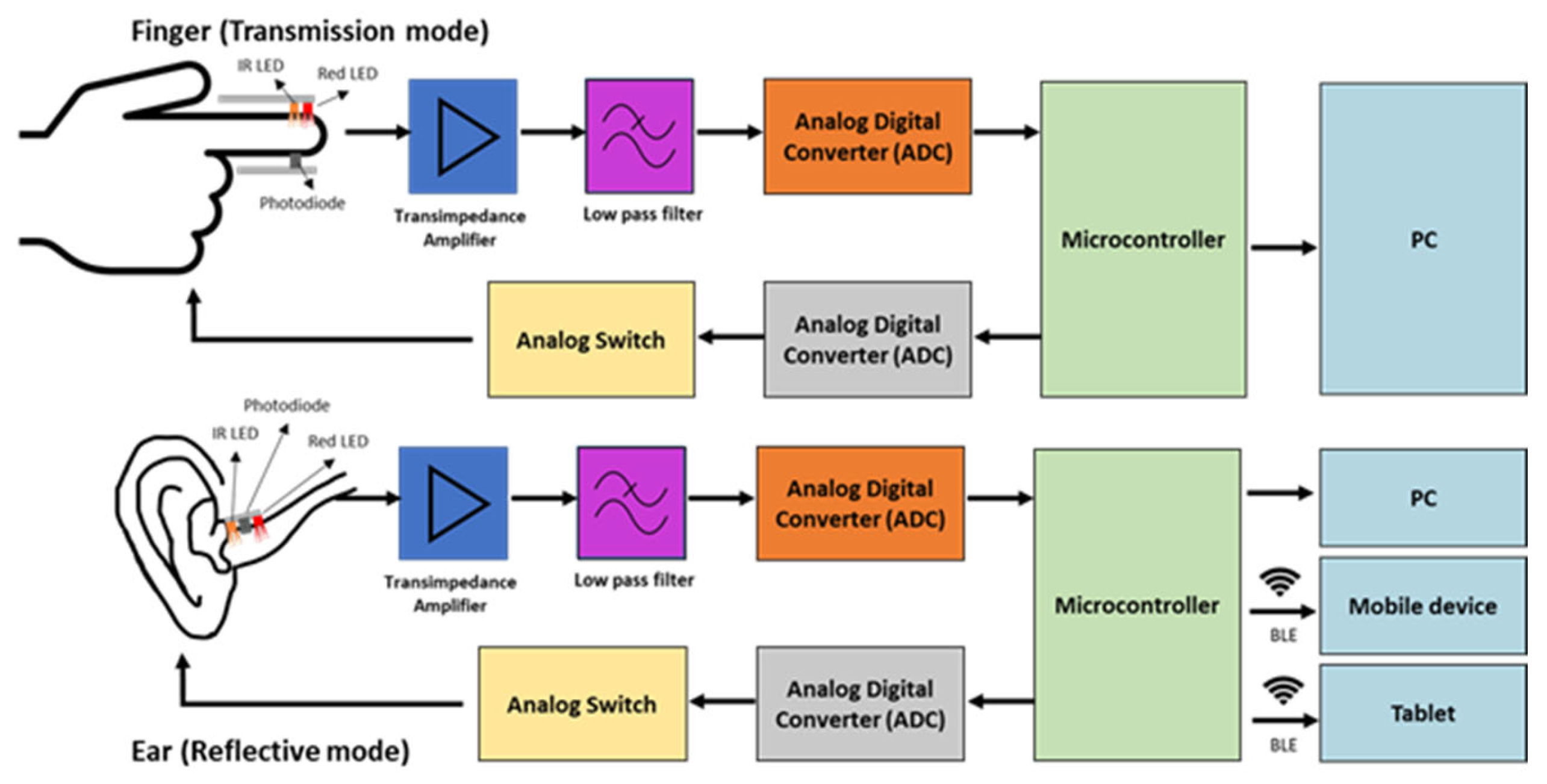
3.3. In-Ear PPG System
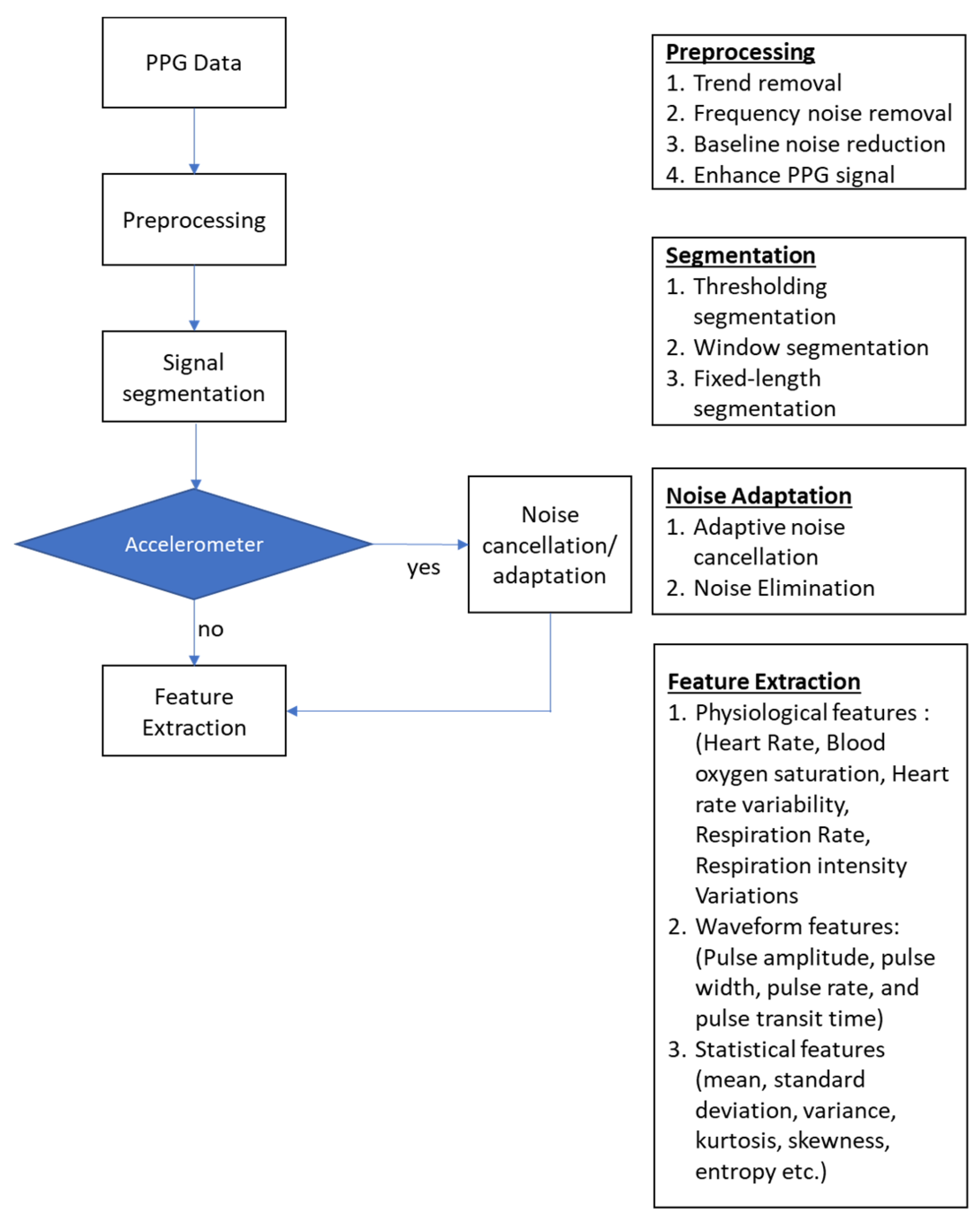
3.4. In-Ear PPG Signal Processing
3.4.1. Waveform Characteristics
3.4.2. Signal Pre-Processing
3.4.3. Feature Extraction
- Waveform features
- Physiological Features
- Motion Artifacts
4. Discussion
4.1. In Ear PPG Principle and Placement
4.2. In-Ear PPG System
4.3. In-Ear PPG Analysis
4.4. In-Ear PPG Applications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lignans Market: Size, Dynamics, Regional Insights and Market Segment. Available online: https://www.maximizemarketresearch.com/market-report/hearables-market/147058/ (accessed on 4 April 2023).
- Hearables Market Size, Share & Growth | Trends & Analysis-2026. Available online: https://www.alliedmarketresearch.com/hearables-market (accessed on 27 October 2022).
- Best 10 Wireless Earbuds With Heart Rate Monitor in 2023. Available online: https://weartotrack.com/wireless-earbuds-heart-rate-monitor/ (accessed on 4 April 2023).
- Matsumoto, K.; Temiz, Y.; Taghavi, H.; Cornelius, E.L.; Mori, H.; Michel, B. An Earbud-Type Wearable (A Hearable) with Vital Parameter Sensors for Early Detection and Prevention of Heat-Stroke. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 7049–7055. [Google Scholar] [CrossRef]
- Nakamura, T.; Alqurashi, Y.D.; Morrell, M.J.; Mandic, D.P. Hearables: Automatic Overnight Sleep Monitoring with Standardized In-Ear EEG Sensor. IEEE Trans. Biomed. Eng. 2020, 67, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Hwang, T.; Kim, M.; Hong, S.; Park, K.S. Driver Drowsiness Detection Using the In-Ear EEG. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, FL, USA, 16–20 August 2016; pp. 4646–4649. [Google Scholar] [CrossRef]
- Occhipinti, E.; Davies, H.J.; Hammour, G.; Mandic, D.P. Hearables: Artefact Removal in Ear-EEG for Continuous 24/7 Monitoring. In Proceedings of the 2022 International Joint Conference on Neural Networks (IJCNN), Padua, Italy, 18–23 July 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Goverdovsky, V.; Looney, D.; Kidmose, P.; Mandic, D.P. In-Ear EEG From Viscoelastic Generic Earpieces: Robust and Unobtrusive 24/7 Monitoring. IEEE Sens. J. 2016, 16, 271–277. [Google Scholar] [CrossRef]
- De Lucia, R.; Zucchelli, G.; Barletta, V.; Di Cori, A.; Giannotti Santoro, M.; Parollo, M.; Segreti, L.; Viani, S.; Della Tommasina, V.; Paperini, L.; et al. The In-Ear Region as a Novel Anatomical Site for ECG Signal Detection: Validation Study on Healthy Volunteers. J. Interv. Card. Electrophysiol. 2021, 60, 93–100. [Google Scholar] [CrossRef]
- Hammour, G.; Yarici, M.; Von Rosenberg, W.; Mandic, D.P. Hearables Feasibility and Validation of In-Ear Electrocardiogram. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5777–5780. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, X.; Hu, W.; Zhou, D. A Machine Learning-Empowered System for Long-Term Motion-Tolerant Wearable Monitoring of Blood Pressure and Heart Rate with Ear-ECG/PPG. IEEE Access 2017, 5, 10547–10561. [Google Scholar] [CrossRef]
- Poh, M.Z.; Kim, K.; Goessling, A.; Swenson, N.; Picard, R. Cardiovascular Monitoring Using Earphones and a Mobile Device. IEEE Pervasive Comput. 2012, 11, 18–26. [Google Scholar] [CrossRef]
- Venema, B.; Gehring, H.; Michelsen, I.; Blanik, N.; Blazek, V.; Leonhardt, S. Robustness, Specificity, and Reliability of an in-Ear Pulse Oximetric Sensor in Surgical Patients. IEEE J. Biomed. Health Inform. 2014, 18, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Ferlini, A.; Montanari, A.; Min, C.; Li, H.; Sassi, U.; Kawsar, F. In-Ear PPG for Vital Signs. IEEE Pervasive Comput. 2022, 21, 65–74. [Google Scholar] [CrossRef]
- Selvaraj, N.; Shelley, K.H.; Silverman, D.G.; Stachenfeld, N.; Galante, N.; Florian, J.P.; Mendelson, Y.; Chon, K.H. A Novel Approach Using Time-Frequency Analysis of Pulse-Oximeter Data to Detect Progressive Hypovolemia in Spontaneously Breathing Healthy Subjects. IEEE Trans. Biomed. Eng. 2011, 58, 2272–2279. [Google Scholar] [CrossRef]
- Budidha, K.; Kyriacou, P.A. In Vivo Investigation of Ear Canal Pulse Oximetry during Hypothermia. J. Clin. Monit. Comput. 2018, 32, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, S.; Hülsbusch, M.; Starke, D.; Leonhardt, S. In-Ear Heart Rate Monitoring Using a Micro-Optic Reflective Sensor. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 1375–1378. [Google Scholar] [CrossRef]
- Davies, H.J.; Bachtiger, P.; Williams, I.; Molyneaux, P.L.; Peters, N.S.; Mandic, D.P. Wearable In-Ear PPG: Detailed Respiratory Variations Enable Classification of COPD. IEEE Trans. Biomed. Eng. 2022, 69, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.J.; Williams, I.; Peters, N.S.; Mandic, D.P. In-Ear SpO2: A Tool for Wearable, Unobtrusive Monitoring of Core Blood Oxygen Saturation. Sensors 2020, 20, 4879. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Hülsbusch, M.; Hennig, T.; Blazek, V.; Leonhardt, S. In-Ear Vital Signs Monitoring Using a Novel Microoptic Reflective Sensor. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Venema, B.; Blanik, N.; Blazek, V.; Gehring, H.; Opp, A.; Leonhardt, S. Advances in Reflective Oxygen Saturation Monitoring with a Novel In-Ear Sensor System: Results of a Human Hypoxia Study. IEEE Trans. Biomed. Eng. 2012, 59, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Boloursaz Mashhadi, M.; Asadi, E.; Eskandari, M.; Kiani, S.; Marvasti, F. Heart Rate Tracking Using Wrist-Type Photoplethysmographic (PPG) Signals during Physical Exercise with Simultaneous Accelerometry. IEEE Signal Process. Lett. 2015, 23, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Biswas, D.; Simoes-Capela, N.; Van Hoof, C.; Van Helleputte, N. Heart Rate Estimation from Wrist-Worn Photoplethysmography: A Review. IEEE Sens. J. 2019, 19, 6560–6570. [Google Scholar] [CrossRef]
- Liu, S.H.; Liu, H.C.; Chen, W.; Tan, T.H. Evaluating Quality of Photoplethymographic Signal on Wearable Forehead Pulse Oximeter with Supervised Classification Approaches. IEEE Access 2020, 8, 185121–185135. [Google Scholar] [CrossRef]
- Allen, J.; Oates, C.P.; Lees, T.A.; Murray, A. Photoplethysmography Detection of Lower Limb Peripheral Arterial Occlusive Disease: A Comparison of Pulse Timing, Amplitude and Shape Characteristics. Physiol. Meas. 2005, 26, 811–821. [Google Scholar] [CrossRef]
- Zahedi, E.; Beng, G.K. Applicability of Adaptive Noise Cancellation to Fetal Heart Rate Detection Using Photoplethysmography. Comput. Biol. Med. 2008, 38, 31–41. [Google Scholar] [CrossRef]
- Nilsson, L.; Goscinski, T.; Kalman, S.; Lindberg, L.G.; Johansson, A. Combined Photoplethysmographic Monitoring of Respiration Rate and Pulse: A Comparison between Different Measurement Sites in Spontaneously Breathing Subjects. Acta Anaesthesiol. Scand. 2007, 51, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Longmore, S.K.; Lui, G.Y.; Naik, G.; Breen, P.P.; Jalaludin, B.; Gargiulo, G.D. A Comparison of Reflective Photoplethysmography for Detection of Heart Rate, Blood Oxygen Saturation, and Respiration Rate at Various Anatomical Locations. Sensors 2019, 19, 1874. [Google Scholar] [CrossRef] [Green Version]
- Murray, W.; Foster, P.A. The Peripheral Pulse Wave: Information Overlooked. J. Clin. Monit. 1996, 12, 365–377. [Google Scholar] [CrossRef]
- Chua, E.C.P.; Redmond, S.J.; McDarby, G.; Heneghan, C. Towards Using Photo-Plethysmogram Amplitude to Measure Blood Pressure during Sleep. Ann. Biomed. Eng. 2010, 38, 945–954. [Google Scholar] [CrossRef]
- Elgendi, M. On the Analysis of Fingertip Photoplethysmogram Signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef]
- Awad, A.A.; Haddadin, A.S.; Tantawy, H.; Badr, T.M.; Stout, R.G.; Silverman, D.G.; Shelley, K.H. The Relationship between the Photoplethysmographic Waveform and Systemic Vascular Resistance. J. Clin. Monit. Comput. 2007, 21, 365–372. [Google Scholar] [CrossRef]
- Gil, E.; Orini, M.; Bailón, R.; Vergara, J.M.; Mainardi, L.; Laguna, P. Photoplethysmography Pulse Rate Variability as a Surrogate Measurement of Heart Rate Variability during Non-Stationary Conditions. Physiol. Meas. 2010, 31, 1271. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, H.; Ju, K.; Shin, K.; Lee, M.; Shelley, K.; Chon, K.H. Can Photoplethysmography Variability Serve as an Alternative Approach to Obtain Heart Rate Variability Information? J. Clin. Monit. Comput. 2007, 22, 23–29. [Google Scholar] [CrossRef]
- Dorlas, J.C.; Nijboer, J.A. Photo-electric plethysmography as a monitoring device in anaesthesia Application and Interpretation. Br. J. Anaesth. 1985, 57, 524–530. [Google Scholar] [CrossRef]
- Shobitha, S.; Amita, P.M.; Krupa, B.N.; Beng, G.K. Cuffless Blood Pressure Prediction from PPG Using Relevance Vector Machine. In Proceedings of the 2017 International Conference on Electrical Electronics, Communication, Computer, and Optimization Techniques (ICEECCOT), Mysuru, India, 15–16 December 2017; pp. 75–78. [Google Scholar] [CrossRef]
- Budidha, K.; Kyriacou, P.A. Investigation of Pulse Transit Times Utilizing Multisite Reflectance Photoplethysmography under Conditions of Artificially Induced Peripheral Vasoconstriction. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1965–1968. [Google Scholar] [CrossRef]
- Venema, B.; Blazek, V.; Leonhardt, S. In-Ear Photoplethysmography for Mobile Cardiorespiratory Monitoring and Alarming. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015. [Google Scholar] [CrossRef]
- Passler, S.; Müller, N.; Senner, V. In-Ear Pulse Rate Measurement: A Valid Alternative to Heart Rate Derived from Electrocardiography? Sensors 2019, 19, 3641. [Google Scholar] [CrossRef] [Green Version]
- Pedrana, A.; Comotti, D.; Re, V.; Traversi, G. Development of a Wearable In-Ear PPG System for Continuous Monitoring. IEEE Sens. J. 2020, 20, 14482–14490. [Google Scholar] [CrossRef]
- Da He, D.; Winokur, E.S.; Sodini, C.G. An Ear-Worn Vital Signs Monitor. IEEE Trans. Biomed. Eng. 2015, 62, 2547–2552. [Google Scholar] [CrossRef]
- Tigges, T.; Rockstroh, J.; Pielmuş, A.; Klum, M.; Feldheiser, A.; Hunsicker, O.; Orglmeister, R. In-Ear Photoplethysmography for Central Pulse Waveform Analysis in Non-Invasive Hemodynamic Monitoring. Curr. Dir. Biomed. Eng. 2017, 3, 587–590. [Google Scholar] [CrossRef]
- Venema, B.; Schiefer, J.; Blazek, V.; Blanik, N.; Leonhardt, S. Evaluating Innovative In-Ear Pulse Oximetry for Unobtrusive Cardiovascular and Pulmonary Monitoring during Sleep. IEEE J. Transl. Eng. Health Med. 2013, 1, 2700208. [Google Scholar] [CrossRef]
- Hickey, M.; Samuels, N.; Randive, N.; Langford, R.M.; Kyriacou, P.A. Measurement of Splanchnic Photoplethysmographic Signals Using a New Reflectance Fiber Optic Sensor. J. Biomed. Opt. 2010, 15, 027012. [Google Scholar] [CrossRef] [Green Version]
- Xing, X.; Ma, Z.; Xu, S.; Zhang, M.; Zhao, W.; Song, M.; Dong, W.F. Blood Pressure Assessment with In-Ear Photoplethysmography. Physiol. Meas. 2021, 42, 105009. [Google Scholar] [CrossRef]
- Allen, J.; Murray, A. Age-Related Changes in the Characteristics of the Photoplethysmographic Pulse Shape at Various Body Sites. Physiol. Meas. 2003, 24, 297. [Google Scholar] [CrossRef]
- López-Beltrán, E.A.; Blackshear, P.L.; Finkelstein, S.M.; Cohn, J.N. Non-Invasive Studies of Peripheral Vascular Compliance Using a Non-Occluding Photoplethysmographic Method. Med. Biol. Eng. Comput. 1998, 36, 748–753. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and Its Application in Clinical Physiological Measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef] [Green Version]
- Poh, M.Z.; Swenson, N.C.; Picard, R.W. Motion-Tolerant Magnetic Earring Sensor and Wireless Earpiece for Wearable Photoplethysmography. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 786–794. [Google Scholar] [CrossRef]
- Wartzek, T.; Vogel, S.; Hennigy, T.; Broderseny, O.; Hülsbuschz, M.; Herzogz, M.; Leonhardt, S. Analysis of Heart Rate Variability with an In-Ear Micro-Optic Sensor in View of Motion Artifacts. In Proceedings of the 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 168–172. [Google Scholar] [CrossRef]
- Budidha, K.; Kyriacou, P.A. The Human Ear Canal: Investigation of Its Suitability for Monitoring Photoplethysmographs and Arterial Oxygen Saturation. Physiol. Meas. 2014, 35, 111. [Google Scholar] [CrossRef]
- Mahri, N.; Kok Beng, G.; Alauddin, M.; Ali, M.; Kejuruteraan, J.; Elektronik, E.; Kejuruteraan, F.; Bina, A. Best Pulse Selection of Photoplethysmography Signal Through Comparative Method. In Proceedings of the 2012 International Conference on Biomedical Engineering (ICoBE), Penang, Malaysia, 27–28 February 2012. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Chen, Z.; Herrero, J.; McKhann, G.M.; Sheth, S.A.; Mehta, A.D.; Mesgarani, N. Neural Decoding of Attentional Selection in Multi-Speaker Environments without Access to Clean Sources. J. Neural Eng. 2017, 14, 056001. [Google Scholar] [CrossRef] [Green Version]
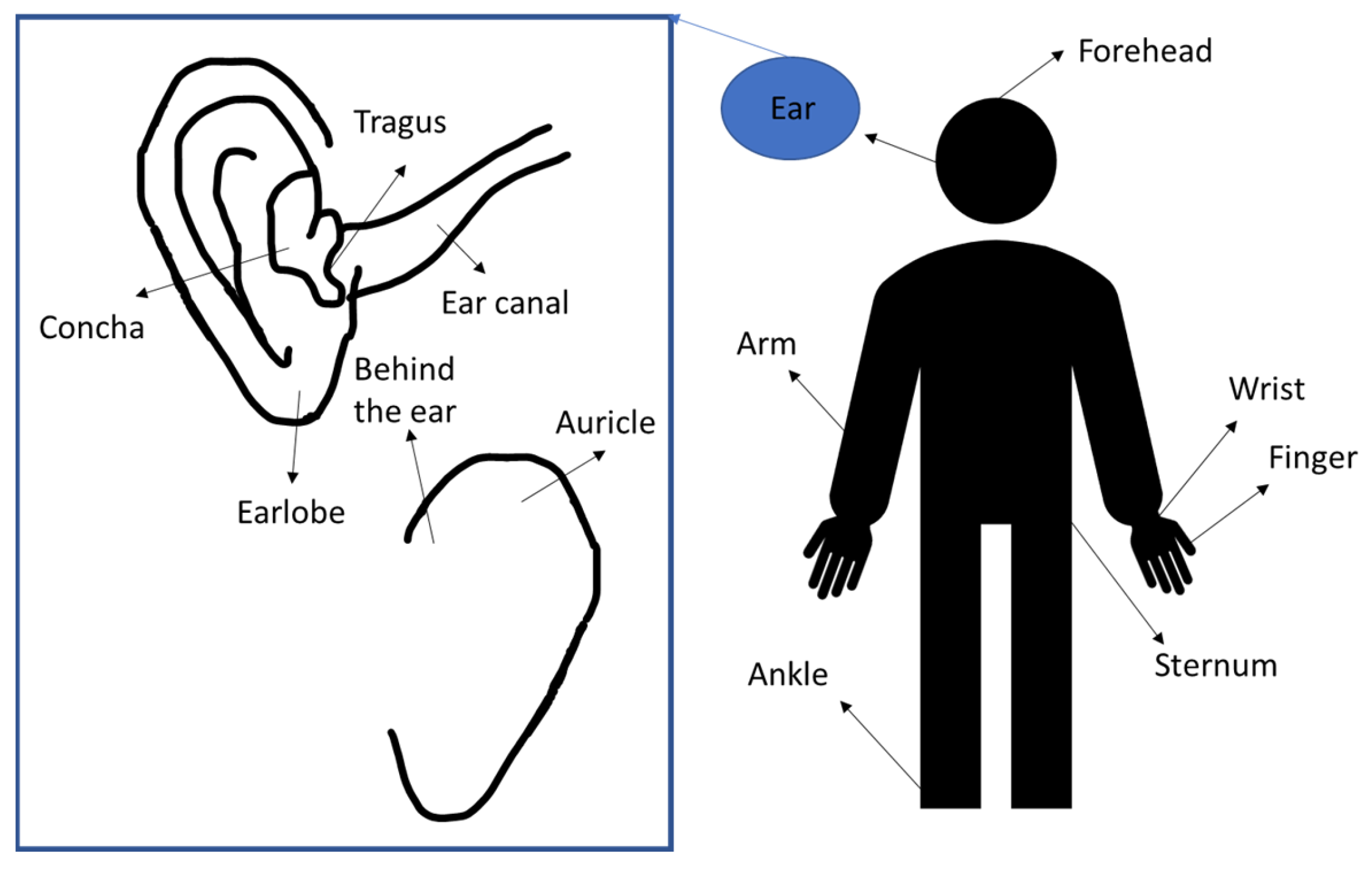
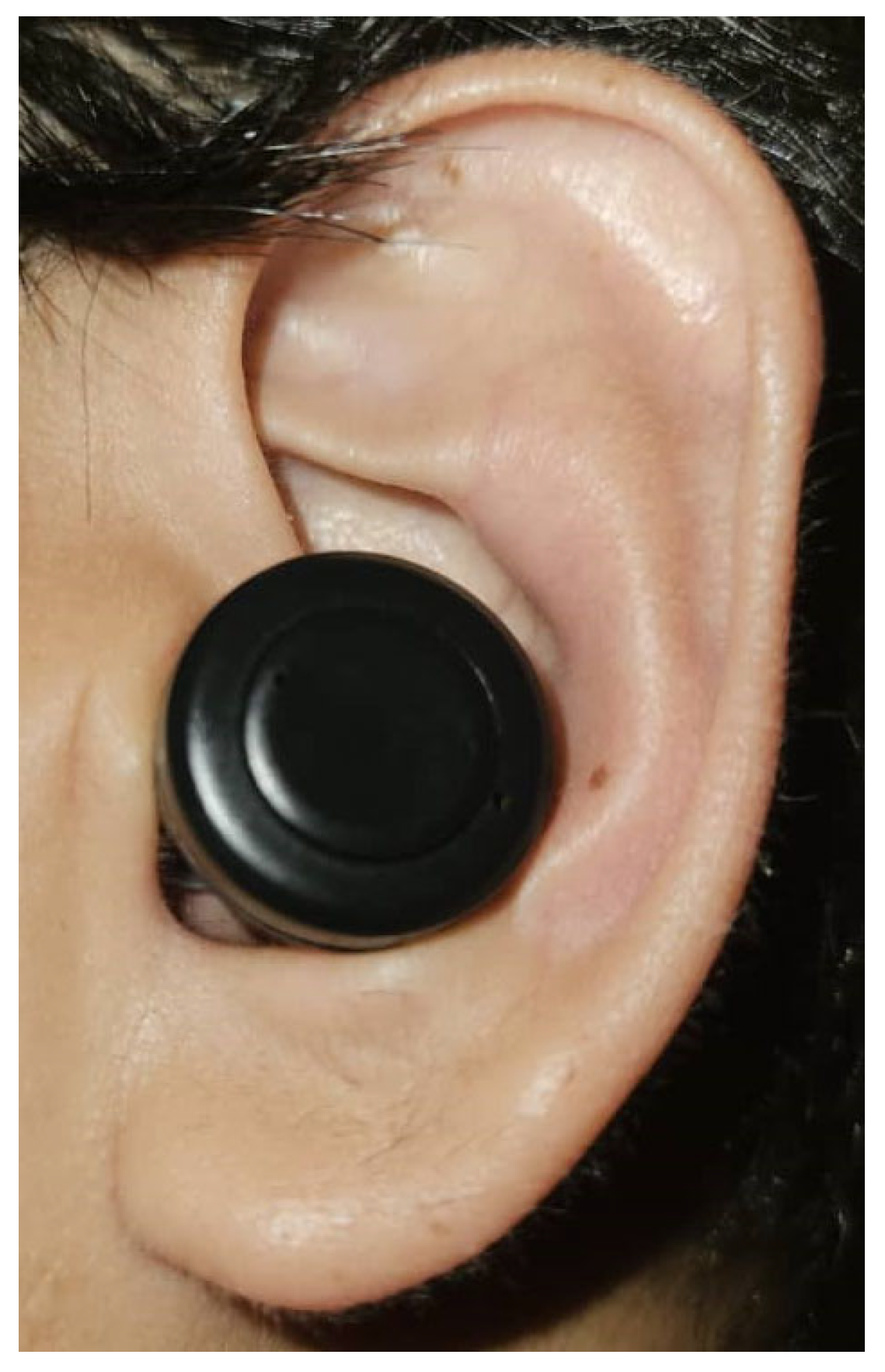
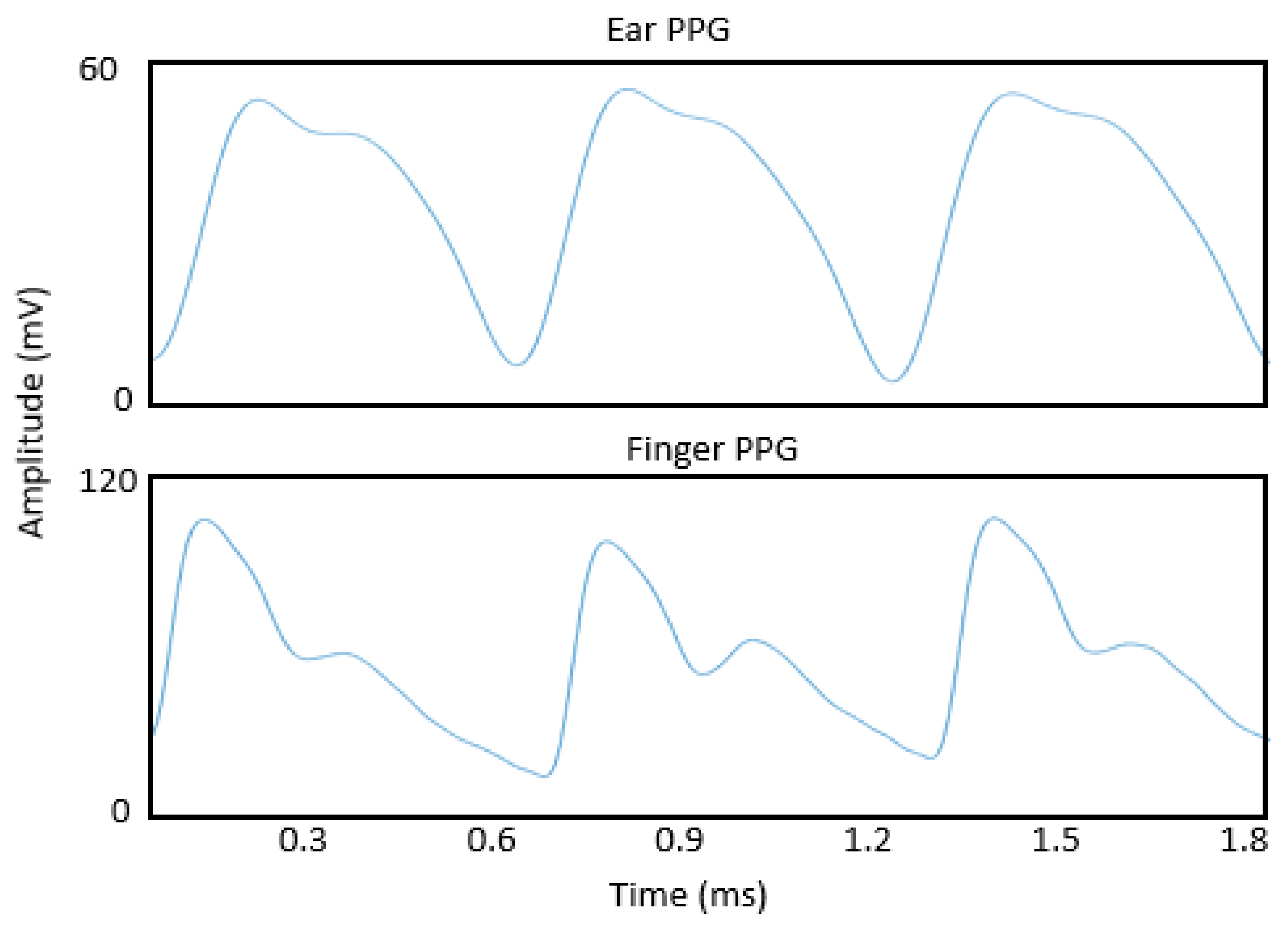

| Study | Sensor Position |
|---|---|
| Vogel et al., 2009 [20] | Near tragus and deeper inside the auditory canal |
| Poh et al., 2012 [12] | Tragus |
| Venema et al., 2012, 2013 [21,43] | PPG on tragus and measuring system behind the auricle |
| Venema et al., 2014 [13] | Inner part of tragus |
| Tigges et al., 2017 [42] | Ear canal (Sensors in two positions: radially oriented and axially oriented) |
| Budidha and Kyricou, 2018 [16] | Concha of the ear with an ear hook hang on top of the helix |
| Pedrana et al., 2020 [40] | Thumb (R/L), forefinger (R/L), middle finger (R/L), posterior wrist (R/L), anterior wrist (R/L), forehead, nasal bridge, ankle (R/L), ear canal (R/L), manubrium and xiphoid process (sternum area) |
| Ferlini et al., 2022 [14] | Behind the auricle, front of the auricle and the ear canal |
| Davies et al., 2022 [18] | Ear canal |
| Pre-Processing Method | Details | Purpose |
|---|---|---|
| Frequency filtering | Linear FIR [16] Butterworth FIR [40] Bandpass and notch filter (0.5 Hz–10 Hz) [14] Bandpass 0.9–30 Hz [18,19] | Trend reduction Reduction for high-frequency noise |
| Empirical mode decomposition | Noise-assisted multivariate empirical mode decomposition (NA-MEMD) [18] | Low frequency reduction Noise reduction Baseline reduction |
| Multi Gaussian decomposition | Each pulse was decomposed into five Gaussian components: the two Gaussian function models the main forward pulse, the three Gaussian function represents different pulse reflections in the system [45] | Each component has a physiological meaning Noise reduction Highlight analytical features |
| Moving difference filter | Sixth-order FIR differentiator filter [40] Calculating the sample difference within a window size of a moving window | Eliminate high frequency noise Highlight PPG slope |
| Noise reduction | ||
| Moving average filter | Calculating the sample average within a window size [21] | Noise reduction |
| Features | Vogel et al. (2009) [17,20] | Pedrana et al. (2020) [40] | Ferlini et al. (2022) [14] | Poh et al. (2012) [12] | Poh et al. (2010) [49] | Passler et al. (2019) [39] | Xing et al. (2021) [45] | Venema et al. (2012, 2013) [13,21,43] | Davies et al. (2020) | Budidha et al. [16,51] |
|---|---|---|---|---|---|---|---|---|---|---|
| Ear location | Tragus near auditory canal | Ear canal | (1) Behind the ear, (2) Front of the auricle (3) Ear canal | Tragus | Ear Lobe | External auditory canal | Ear canal | Tragus | Ear canal | Ear canal |
| Attachment to the ear | Otoplastic insertion | Inserted inside the ear | Hook on the ear | Inserted inside the ear | Magnetic earring | Inserted inside the ear and hook | Inserted inside the ear | Inserted inside the ear | Inserted inside the ear | Inserted inside the ear |
| Wireless/Bluetooth | Yes | Bluetooth | NA | Bluetooth | Bluetooth | wireless | No | No | No | No |
| Motion analysis | No | Yes Adaptive noise cancellation | Yes | No | Yes Adaptive noise cancellation | No | No | No | No | No |
| Physical testing | Walking, jaw motion | Walking | Speaking, walking, running | Standing, walking, cycling, exercise | Walking, running | Cycling | No | Surgery in operational room | No | Cold exposure, ice water immersion |
| Physiological measurement | HR, HRV | HR | HR, HRV, SPO2, RR | HR | HR | HR | Blood pressure | HR, RR, SPO2, hypoxia | SPO2, COPD | SPO2, body temperature, hypothermia |
| LED colours/wavelength | Red and infrared | Green, red, and infrared | Infrared and red | Infrared | Infrared (940 nm) | Infrared and green | Green (550 nm) | Red and infrared | Green, red, and infrared | Infrared, red |
| Accelerometer | 2D and 3D accelerometer fixed on head | 3D accelerometer | None | No | 3D accelerometer | No | No | 3D accelerometer | No | No |
| Reference | Finger PPG, ECG | ECG | Finger PPG, ECG | ECG | ECG | ECG-Bodyguard 2 | spygmomanometer | Finger PPG, ECG | Finger PPG | Laser Doppler flowmeter, tympanic thermometer, ECG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azudin, K.; Gan, K.B.; Jaafar, R.; Ja’afar, M.H. The Principles of Hearable Photoplethysmography Analysis and Applications in Physiological Monitoring–A Review. Sensors 2023, 23, 6484. https://doi.org/10.3390/s23146484
Azudin K, Gan KB, Jaafar R, Ja’afar MH. The Principles of Hearable Photoplethysmography Analysis and Applications in Physiological Monitoring–A Review. Sensors. 2023; 23(14):6484. https://doi.org/10.3390/s23146484
Chicago/Turabian StyleAzudin, Khalida, Kok Beng Gan, Rosmina Jaafar, and Mohd Hasni Ja’afar. 2023. "The Principles of Hearable Photoplethysmography Analysis and Applications in Physiological Monitoring–A Review" Sensors 23, no. 14: 6484. https://doi.org/10.3390/s23146484





