Breathable and Stretchable Organic Electrochemical Transistors with Laminated Porous Structures for Glucose Sensing
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fabrication of Porous SEBS Substrate
2.3. Fabrication of Active Film
2.4. Fabrication of the Stretchable and Breathable OECTs
2.5. Characterizations
2.5.1. Electrical Properties
2.5.2. Gas Permeability
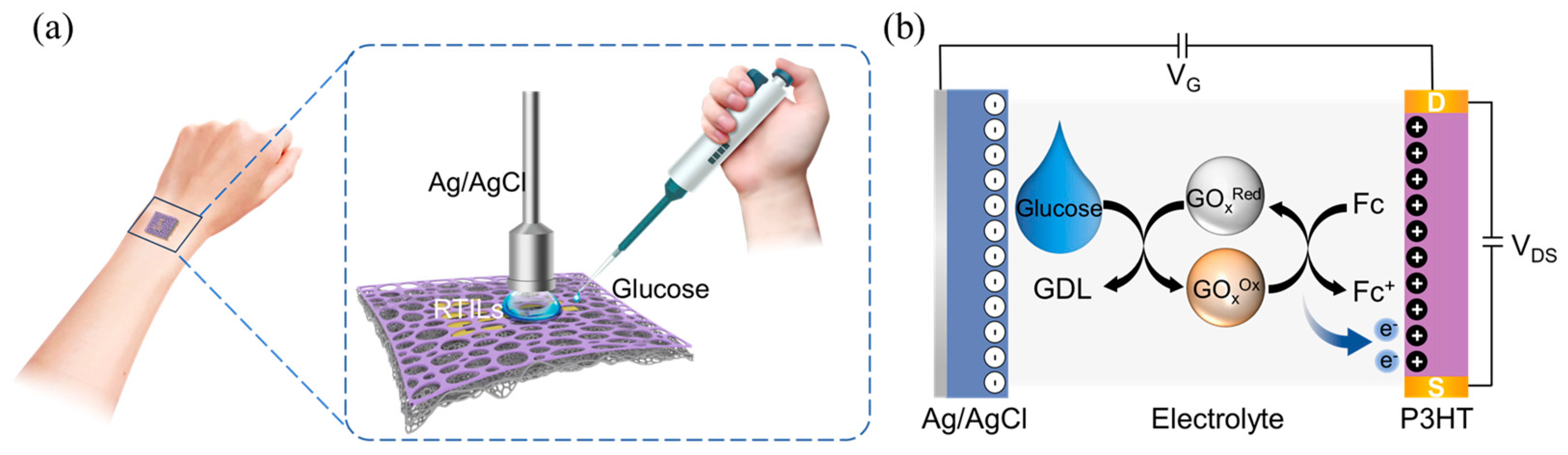
2.5.3. Glucose Detection
3. Results and Discussion
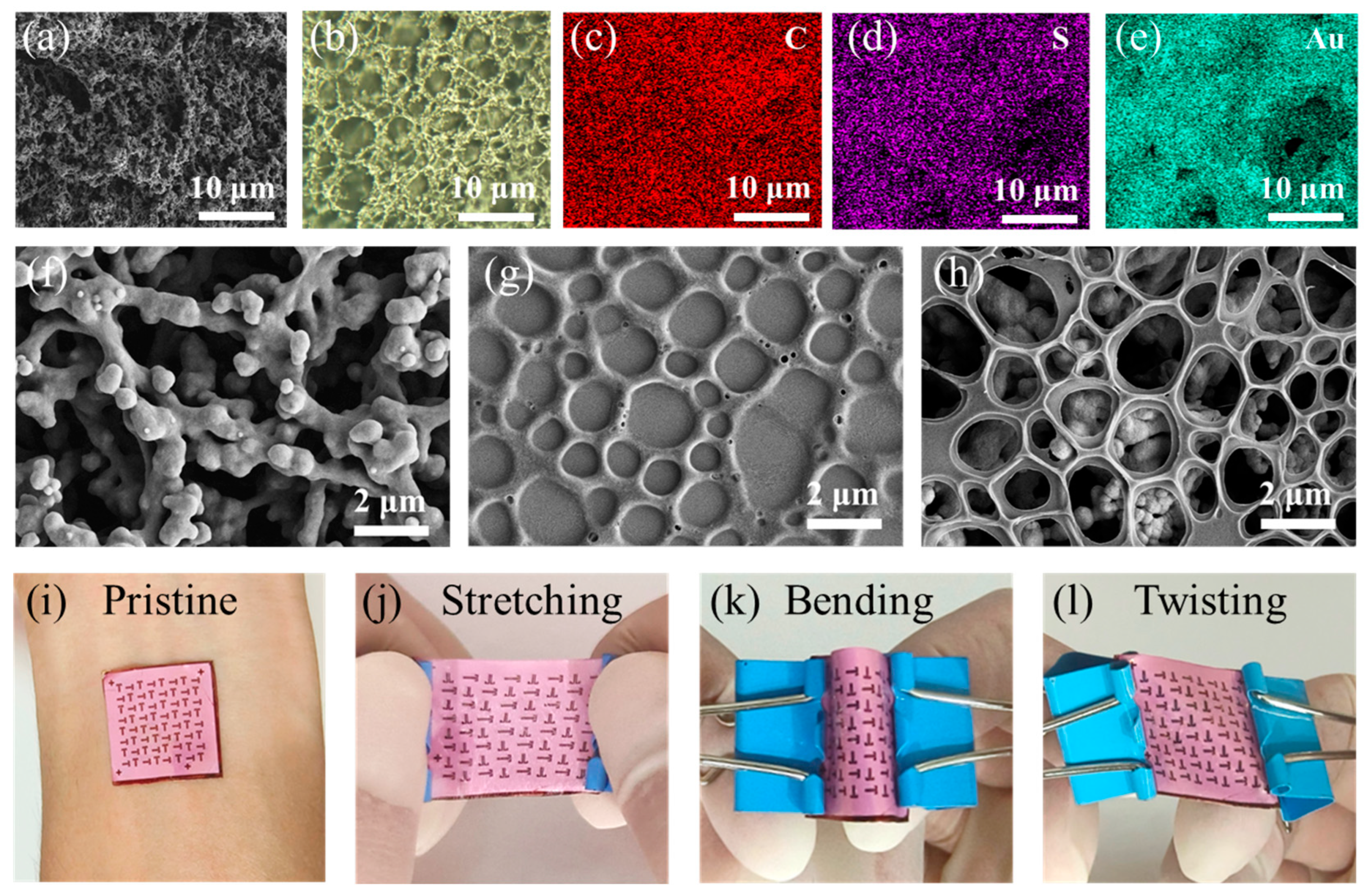
3.1. Fabrication and Characterization of the Breathable OECTs
3.2. Electrical and Mechanical Performance of Breathable OECTs
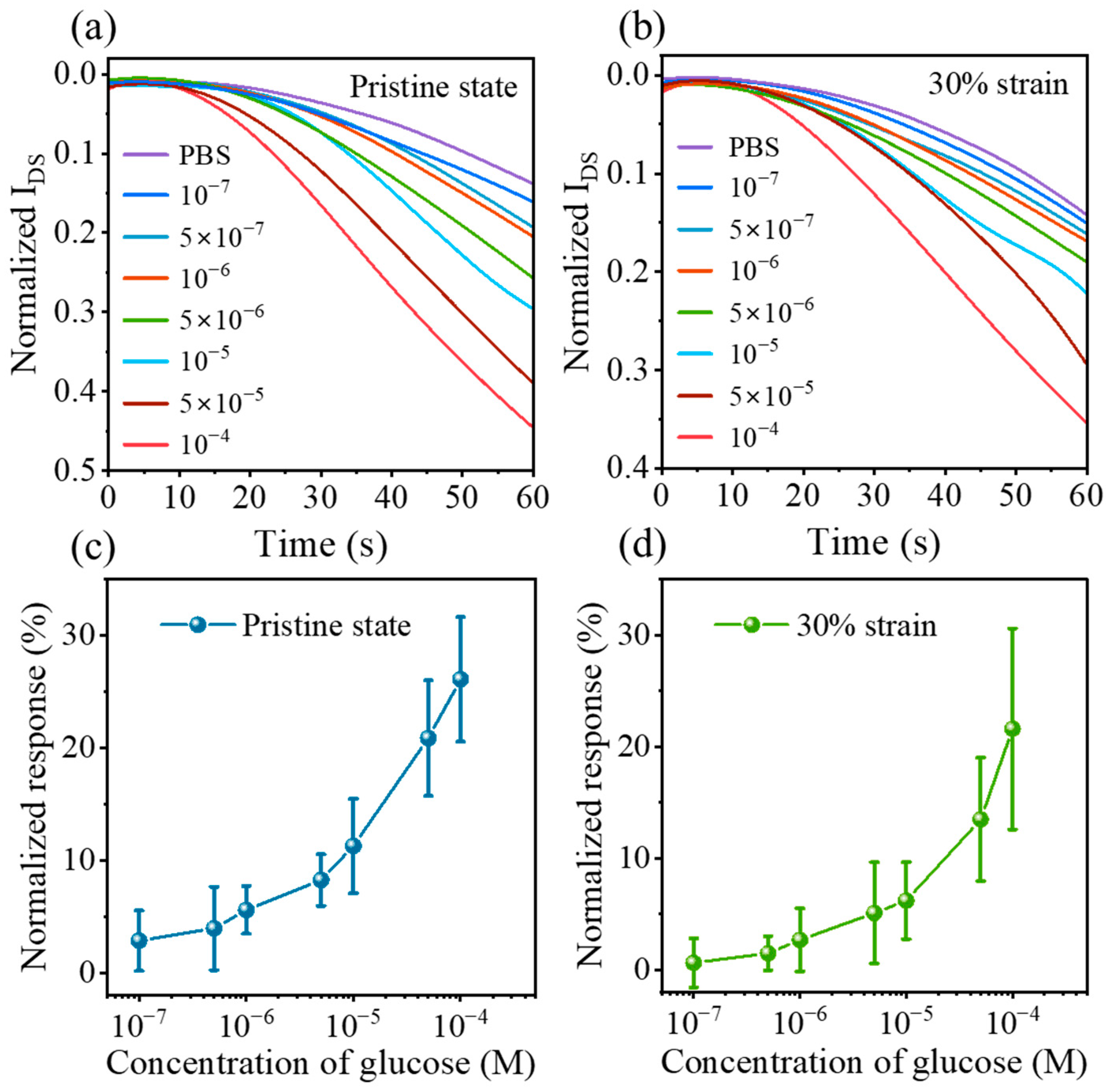
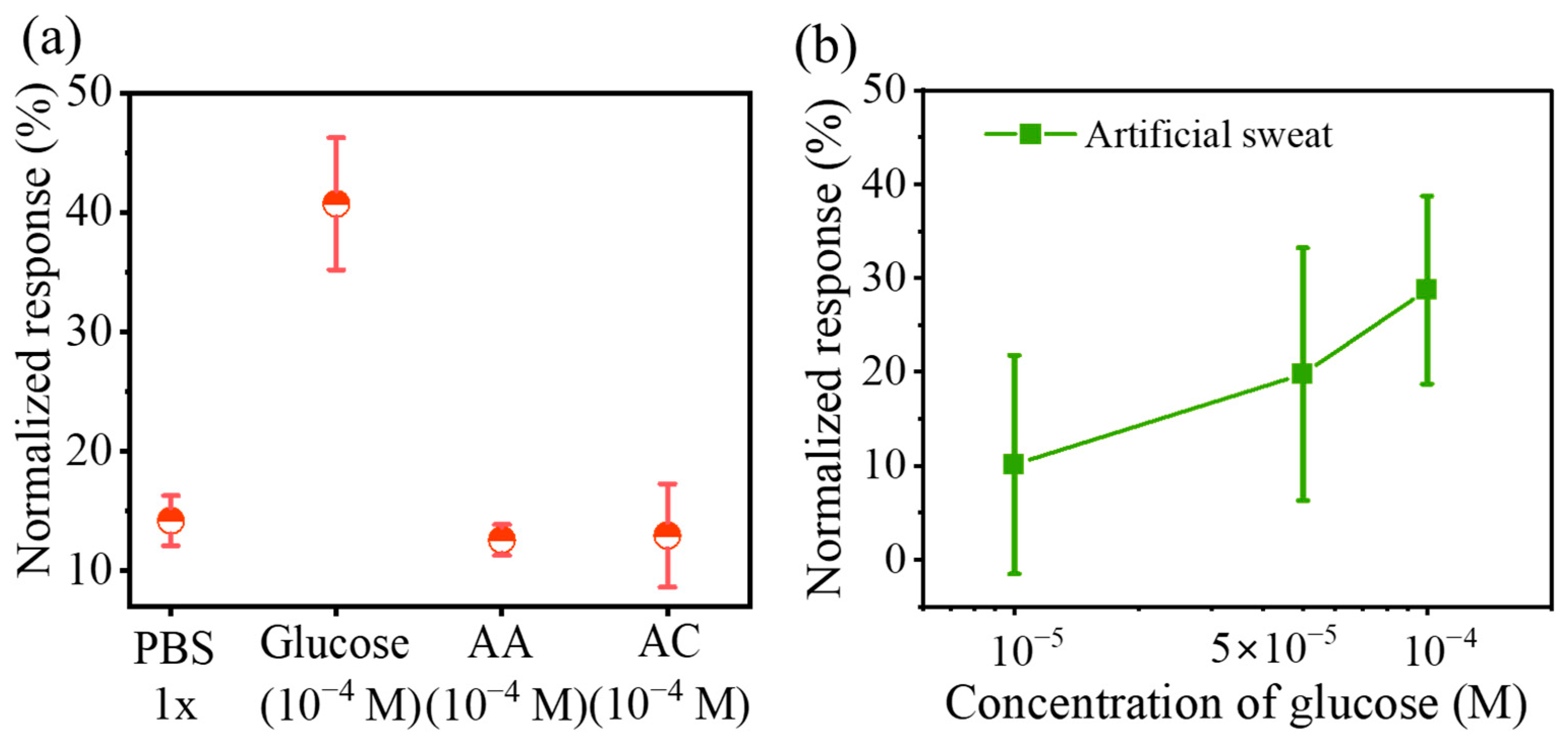
3.3. Sensitive Detection of Glucose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eslami, S.; Taherzadeh, Z.; Schultz, M.J.; Abu-Hanna, A. Glucose variability measures and their effect on mortality: A systematic review. Intensive Care Med. 2011, 37, 583–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Sarwar, M.; Zhu, J.-J.; Zhang, C.; Kaushik, A.; Li, C.-Z. Using a glucose meter to quantitatively detect disease biomarkers through a universal nanozyme integrated lateral fluidic sensing platform. Biosens. Bioelectron. 2019, 126, 690–696. [Google Scholar] [CrossRef]
- Jernelv, I.L.; Milenko, K.; Fuglerud, S.S.; Hjelme, D.R.; Ellingsen, R.; Aksnes, A. A review of optical methods for continuous glucose monitoring. Appl. Spectrosc. Rev. 2019, 54, 543–572. [Google Scholar] [CrossRef]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical bioadhesive interface for bioelec-tronics. Nat. Mater. 2021, 20, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Dai, S.; Li, N.; Li, Y.; Moser, M.; Strzalka, J.; Prominski, A.; Liu, Y.; Zhang, Q.; Li, S.; et al. Stretchable Redox-Active Semiconducting Polymers for High-Performance Organic Electrochemical Transistors. Adv. Mater. 2022, 34, 2201178. [Google Scholar] [CrossRef]
- Nawaz, A.; Liu, Q.; Leong, W.L.; Fairfull-Smith, K.E.; Sonar, P. Organic Electrochemical Transistors for In Vivo Bioelectronics. Adv. Mater. 2021, 33, 2101874. [Google Scholar] [CrossRef]
- Xue, M.; Mackin, C.; Weng, W.-H.; Zhu, J.; Luo, Y.; Luo, S.-X.L.; Lu, A.-Y.; Hempel, M.; McVay, E.; Kong, J.; et al. Integrated biosensor platform based on graphene transistor arrays for real-time high-accuracy ion sensing. Nat. Commun. 2022, 13, 5064. [Google Scholar] [CrossRef]
- Han, S.; Yamamoto, S.; Polyravas, A.G.; Malliaras, G.G. Microfabricated Ion-Selective Transistors with Fast and Super-Nernstian Response. Adv. Mater. 2020, 32, 2004790. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Han, Y.; Sharma, A.; AlGhamdi, W.S.; Liu, C.-H.; Chang, T.-H.; Xiao, X.-W.; Lin, W.-Z.; Lu, P.-Y.; Seitkhan, A.; et al. A Tri-Channel Oxide Transistor Concept for the Rapid Detection of Biomolecules Including the SARS-CoV-2 Spike Protein. Adv. Mater. 2022, 34, 2104608. [Google Scholar] [CrossRef]
- Zhao, C.; Cheung, K.M.; Huang, I.-W.; Yang, H.; Nakatsuka, N.; Liu, W.; Cao, Y.; Man, T.; Weiss, P.S.; Monbouquette, H.G.; et al. Implantable aptamer–field-effect transistor neuroprobes for in vivo neurotransmitter monitoring. Sci. Adv. 2021, 7, eabj7422. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, C.; Peng, Y.; Deng, J.; Hou, S.; Cheng, Y.; Huang, W.; Yu, J. Ultrasensitive flexible NO2 gas sensors via multilayer porous polymer film. Sens. Actuators B Chem. 2022, 368, 132113. [Google Scholar] [CrossRef]
- Su, X.; Wu, X.; Chen, S.; Nedumaran, A.M.; Stephen, M.; Hou, K.; Czarny, B.; Leong, W.L. A Highly Conducting Polymer for Self-Healable, Printable, and Stretchable Organic Electrochemical Transistor Arrays and Near Hysteresis-Free Soft Tactile Sensors. Adv. Mater. 2022, 34, 2200682. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huang, W.; Chen, J.; Liu, X.; Bai, L.; Chen, W.; Cheng, Y.; Ping, J.; Marks, T.J.; Facchetti, A. Flexible and Stretchable Organic Electrochemical Transistors for Physiological Sensing Devices. Adv. Mater. 2023, 2209906. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, W.; Zheng, D.; Xie, Z.; Zhuang, X.; Zhao, D.; Chen, Y.; Su, N.; Chen, H.; Pankow, R.M.; et al. Highly stretchable organic electrochemical transistors with strain-resistant performance. Nat. Mater. 2022, 21, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Demuru, S.; Kim, J.; Chazli, M.; Bruce, S.; Dupertuis, M.; Binz, P.-A.; Saubade, M.; Lafaye, C.; Briand, D. Antibody-Coated Wearable Organic Electrochemical Transistors for Cortisol Detection in Human Sweat. ACS Sens. 2022, 7, 2721–2731. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, Q.; Xu, Q.; Zhuang, Q.; Zhao, X.; Yang, Y.; Qiu, H.; Yang, Z.; Wang, C.; Chai, Y.; et al. Permeable superelastic liquid-metal fibre mat enables biocompatible and monolithic stretchable electronics. Nat. Mater. 2021, 20, 859–868. [Google Scholar] [CrossRef]
- Ye, Z.; Ling, Y.; Yang, M.; Xu, Y.; Zhu, L.; Yan, Z.; Chen, P.-Y. A Breathable, Reusable, and Zero-Power Smart Face Mask for Wireless Cough and Mask-Wearing Monitoring. ACS Nano. 2022, 16, 5874–5884. [Google Scholar] [CrossRef]
- Chen, S.; Gao, S.; Jing, J.; Lu, Q. Designing 3D Biological Surfaces via the Breath-Figure Method. Adv. Healthc. Mater. 2018, 7, 1701043. [Google Scholar] [CrossRef]
- Liu, C.; Wu, M.; Gao, L.; Liu, H.; Yu, J. Nanoporous polymer films based on breath figure method for stretchable chemiresistive NO2 gas sensors. Sens. Actuators B Chem. 2022, 371, 132540. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, B.; Ling, Y.; Fei, Q.; Chen, Z.; Li, X.; Guo, P.; Jeon, N.; Goswami, S.; Liao, Y.; et al. Multiscale porous elastomer substrates for multifunctional on-skin electronics with passive-cooling capabilities. Proc. Natl. Acad. Sci. USA 2020, 117, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, W.; Luo, G.; Li, Z.; Zhao, C.; Zhang, H.; Zhu, M.; Xu, Q.; Wang, X.; Zhao, C.; et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ. Sci. 2018, 11, 3375–3379. [Google Scholar] [CrossRef]
- Diacci, C.; Lee, J.W.; Janson, P.; Dufil, G.; Méhes, G.; Berggren, M.; Simon, D.T.; Stavrinidou, E.J.A.M.T. Real-Time Monitoring of Glucose Export from Isolated Chloroplasts Using an Organic Electrochemical Transistor. Adv. Mater. Technol. 2019, 5, 1900262. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.-S.; Ershad, F.; Rao, Z.; Ke, Z.; da Costa, E.C.; Xiang, Q.; Lu, Y.; Wang, X.; Mei, J.; Vanderslice, P.; et al. Elastic electronics based on micromesh-structured rubbery semiconductor films. Nat. Electron. 2022, 5, 881–892. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Huang, L.; Huang, W.; Wang, Z.; Zhu, W.; Chen, Y.; Mao, Y.; Facchetti, A.; Marks, T.J. Breath figure–derived porous semiconducting films for organic electronics. Sci. Adv. 2020, 6, eaaz1042. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Deng, J.; Gao, L.; Cheng, J.; Peng, Y.; Zeng, H.; Huang, W.; Feng, L.-W.; Yu, J. Multilayer Porous Polymer Films for High-Performance Stretchable Organic Electrochemical Transistors. Adv. Electron. Mater. 2023, 2300119. [Google Scholar] [CrossRef]
- Peng, Y.; Gao, L.; Liu, C.; Deng, J.; Xie, M.; Bai, L.; Wang, G.; Cheng, Y.; Huang, W.; Yu, J. Stretchable organic electrochemical transistors via three-dimensional porous elastic semiconducting films for artificial synaptic applications. Nano Res. 2023, 16, 10206–10214. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, D.; Lin, Z.; Wang, P.; Cao, B.; Ren, H.; Song, F.; Wan, C.; Wang, L.; Zhou, J.; et al. Highly stretchable van der Waals thin films for adaptable and breathable electronic membranes. Science 2022, 375, 852–859. [Google Scholar] [CrossRef]
- Gogurla, N.; Roy, B.; Park, J.-Y.; Kim, S. Skin-contact actuated single-electrode protein triboelectric nanogenerator and strain sensor for biomechanical energy harvesting and motion sensing. Nano Energy 2019, 62, 674–681. [Google Scholar] [CrossRef]
- Boda, U.; Petsagkourakis, I.; Beni, V.; Andersson Ersman, P.; Tybrandt, K. Fully Screen-Printed Stretchable Organic Electrochemical Transistors. Adv. Mater. Technol. 2023, 2300247. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Rastak, R.; Ochiai, Y.; Niu, S.; Jiang, Y.; Arunachala, P.K.; Zheng, Y.; Xu, J.; Matsuhisa, N.; et al. Strain-insensitive intrinsically stretchable transistors and circuits. Nat. Electron. 2021, 4, 143–150. [Google Scholar] [CrossRef]
- Kar, M.; Tutusaus, O.; MacFarlane, D.R.; Mohtadi, R. Novel and versatile room temperature ionic liquids for energy storage. Energy Environ. Sci. 2019, 12, 566–571. [Google Scholar] [CrossRef]
- Husson, E.; Hadad, C.; Huet, G.; Laclef, S.; Lesur, D.; Lambertyn, V.; Jamali, A.; Gottis, S.; Sarazin, C.; Nguyen Van Nhien, A. The effect of room temperature ionic liquids on the selective biocatalytic hydrolysis of chitin via sequential or simultaneous strategies. Green. Chem. 2017, 19, 4122–4131. [Google Scholar] [CrossRef]
- Yang, Z. Hofmeister effects: An explanation for the impact of ionic liquids on biocatalysis. J. Biotechnol. 2009, 144, 12–22. [Google Scholar] [CrossRef]
- Bian, W.; Yan, B.; Shi, N.; Qiu, F.; Lou, L.-L.; Qi, B.; Liu, S. Room temperature ionic liquid (RTIL)-decorated mesoporous silica SBA-15 for papain immobilization: RTIL increased the amount and activity of immobilized enzyme. Mater. Sci. Eng. C 2012, 32, 364–368. [Google Scholar] [CrossRef]
- De María, P.D. “Nonsolvent” Applications of Ionic Liquids in Biotransformations and Organocatalysis. Angew. Chem. Int. Ed. 2008, 47, 6960–6968. [Google Scholar] [CrossRef]
- Zhang, L.; Khayour, S.; Ren, G.; He, S.; Wang, J.; Yu, L.; Song, Y.; Zhu, C.; Kang, X.; Zhang, Y.; et al. Proton-penetrable Nafion-induced phase separation in organic semiconductors for high-performance organic electrochemical transistors. J. Mater. Chem. C 2023, 11, 7272–7282. [Google Scholar] [CrossRef]


| Direction of Strain | Range of Strain (%) | gm, p (mS) | Ion (mA) | Vth-for (V) | Vth-back (V) |
|---|---|---|---|---|---|
| L-direction | 0 | 1.710 ± 0.458 | 0.393 ± 0.099 | −0.711 ± 0.008 | −0.642 ± 0.010 |
| 10 | 0.984 ± 0.075 | 0.239 ± 0.011 | −0.703 ± 0.002 | −0.635 ± 0.017 | |
| 20 | 0.539 ± 0.164 | 0.123 ± 0.036 | −0.726 ± 0.017 | −0.639 ± 0.015 | |
| 30 | 0.512 ± 0.177 | 0.115 ± 0.042 | −0.736 ± 0.005 | −0.659 ± 0.015 | |
| 40 | 0.394 ± 0.295 | 0.090 ± 0.072 | −0.725 ± 0.013 | −0.644 ± 0.004 | |
| 50 | 0.352 ± 0.281 | 0.081 ± 0.065 | −0.729 ± 0.006 | −0.660 ± 0.058 | |
| W-direction | 0 | 1.860 ± 0.289 | 0.454 ± 0.069 | −0.711 ± 0.007 | −0.636 ± 0.004 |
| 10 | 1.670 ± 0.311 | 0.407 ± 0.073 | −0.718 ± 0.004 | −0.635 ± 0.008 | |
| 20 | 1.500 ± 0.315 | 0.368 ± 0.076 | −0.710 ± 0.005 | −0.640 ± 0.011 | |
| 30 | 0.825 ± 0.631 | 0.267 ± 0.103 | −0.712 ± 0.006 | −0.640 ± 0.010 | |
| 40 | 0.632 ± 0.330 | 0.151 ± 0.087 | −0.712 ± 0.023 | −0.647 ± 0.028 | |
| 50 | 0.362 ± 0.287 | 0.083 ± 0.071 | −0.731 ± 0.008 | −0.650 ± 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Liu, C.; Peng, Y.; Gao, L.; Yu, J. Breathable and Stretchable Organic Electrochemical Transistors with Laminated Porous Structures for Glucose Sensing. Sensors 2023, 23, 6910. https://doi.org/10.3390/s23156910
Guo H, Liu C, Peng Y, Gao L, Yu J. Breathable and Stretchable Organic Electrochemical Transistors with Laminated Porous Structures for Glucose Sensing. Sensors. 2023; 23(15):6910. https://doi.org/10.3390/s23156910
Chicago/Turabian StyleGuo, Haihong, Changjian Liu, Yujie Peng, Lin Gao, and Junsheng Yu. 2023. "Breathable and Stretchable Organic Electrochemical Transistors with Laminated Porous Structures for Glucose Sensing" Sensors 23, no. 15: 6910. https://doi.org/10.3390/s23156910





