Kinematic Gait Analysis in People with Mild-Disability Multiple Sclerosis Using Statistical Parametric Mapping: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Experimental Protocol
2.4. Outcome Measures
2.5. Data Analysis
3. Results
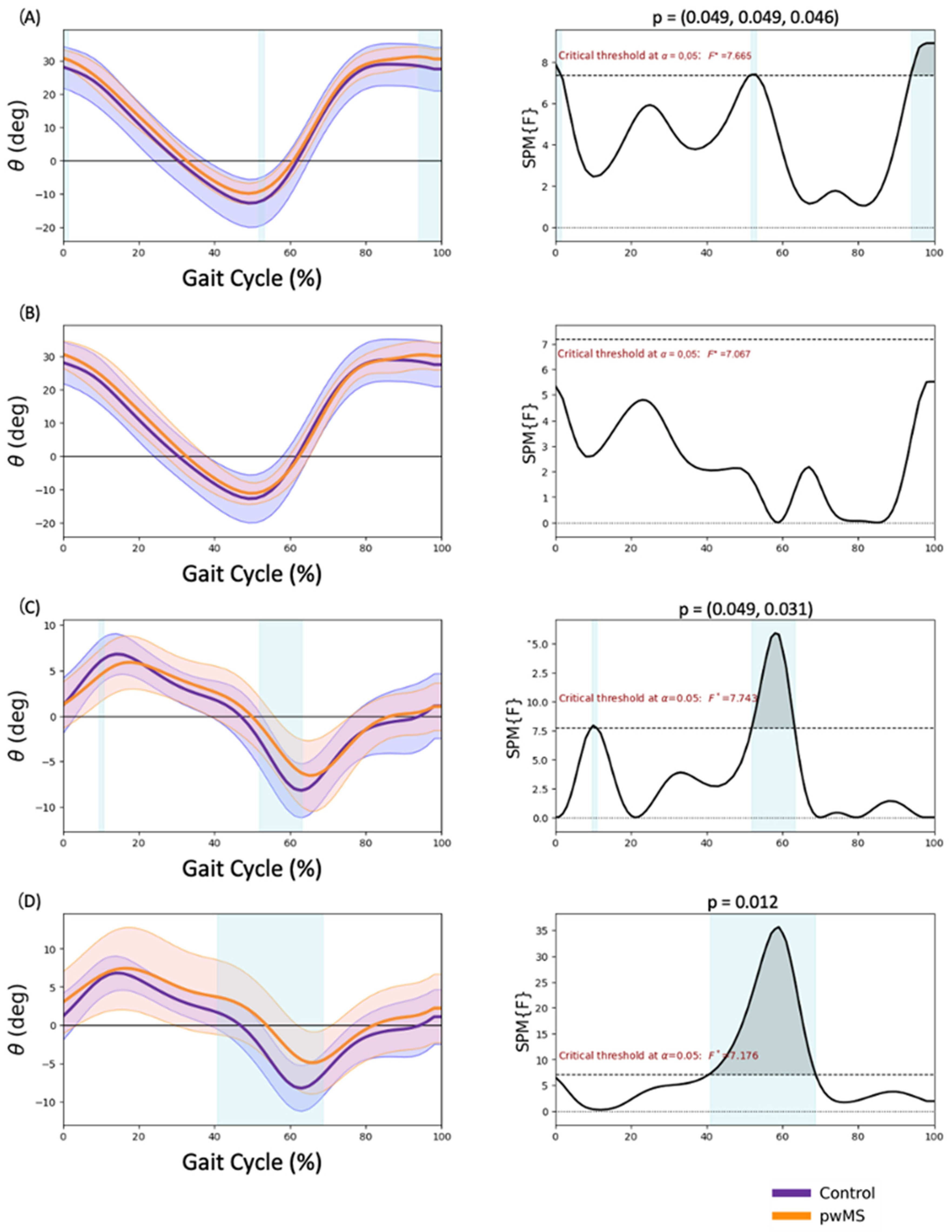
3.1. Pelvis Kinematics
3.2. Hip Kinematics
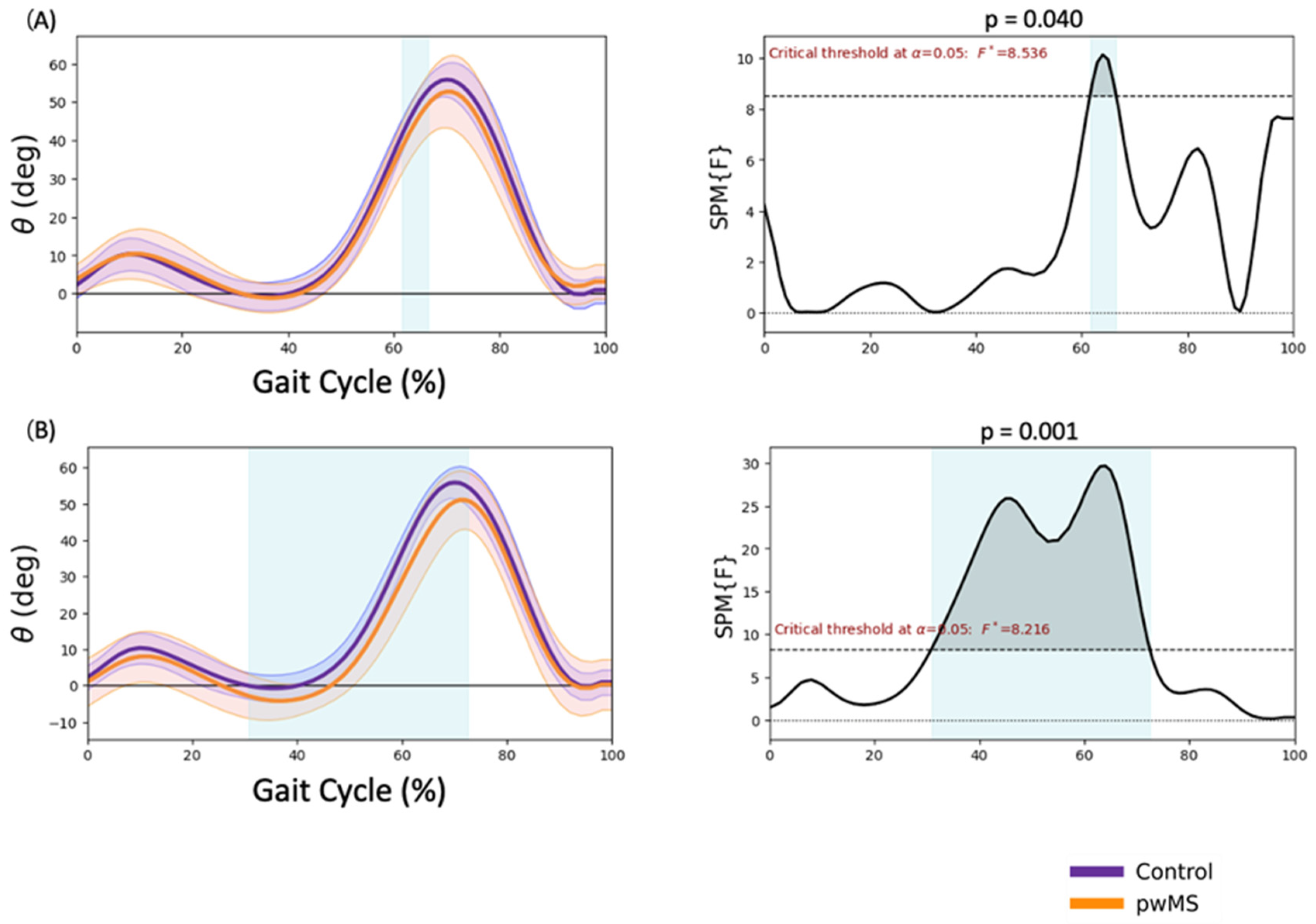
3.3. Knee Kinematics
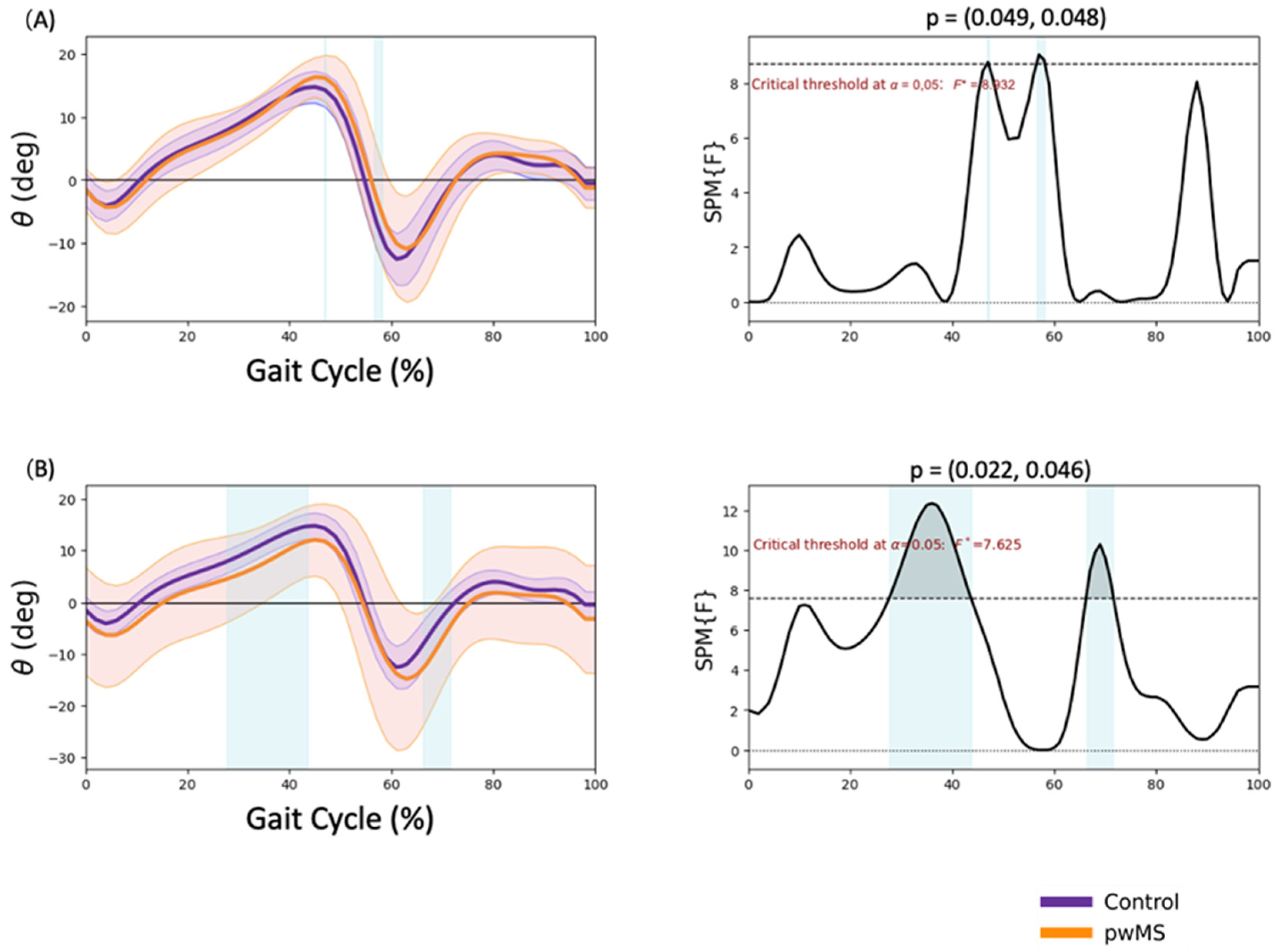
3.4. Ankle Kinematics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef]
- Nourbakhsh, B.; Mowry, E.M.; Solomon, A.J.; Kantarci, O.H.; Repovic, P.; Metz, L.M.; Bowen, J.D.; Gross, R.H.; Corboy, J.R.; Ontaneda, D.; et al. Multiple sclerosis and other CNS inflammatory diseases. Management 2019, 25, 636–654. [Google Scholar]
- Cameron, M.H.; Nilsagard, Y. Balance, gait, and falls in multiple sclerosis. Handb. Clin. Neurol. 2018, 159, 237–250. [Google Scholar] [CrossRef]
- Kasser, S.L.; Jacobs, J.V.; Foley, J.T.; Cardinal, B.J.; Maddalozzo, G.F. A prospective evaluation of balance, gait, and strength to predict falling in women with multiple sclerosis. Arch. Phys. Med. Rehabil. 2011, 92, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Greene, B.R.; Rutledge, S.; McGurgan, I.; McGuigan, C.; O’Connell, K.; Caulfield, B.; Tubridy, N. Assessment and classification of early-stage multiple sclerosis with inertial sensors: Comparison against clinical measures of disease state. IEEE J. Biomed. Health Inform. 2015, 19, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Sosnoff, J.J.; Sandroff, B.; Motl, R.W. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait Posture 2012, 36, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Coca-Tapia, M.; Cuesta-Gómez, A.; Molina-Rueda, F.; Carratalá-Tejada, M. Gait Pattern in People with Multiple Sclerosis: A Systematic Review. Diagnostics 2021, 11, 584. [Google Scholar] [CrossRef]
- Zörner, B.; Hostettler, P.; Meyer, C.; Killeen, T.; Gut, P.; Linnebank, M.; Weller, M.; Straumann, D.; Filli, L. Prognosis of walking function in multiple sclerosis supported by gait pattern analysis. Mult. Scler. Relat. Disord. 2022, 63, 103802. [Google Scholar] [CrossRef]
- Molina-Rueda, F.; Fernández-Vázquez, D.; Navarro-López, V.; López-González, R.; Carratalá-Tejada, M. Muscle Coactivation Index during Walking in People with Multiple Sclerosis with Mild Disability, a Cross-Sectional Study. Diagnostics 2023, 13, 2169. [Google Scholar] [CrossRef]
- Cofré Lizama, L.E.; Khan, F.; Lee, P.V.; Galea, M.P. The use of laboratory gait analysis for understanding gait deterioration in people with multiple sclerosis. Mult. Scler. J. 2016, 22, 1768–1776. [Google Scholar] [CrossRef]
- Molina-Rueda, F.; Fernández-Vázquez, D.; Navarro-López, V.; Miangolarra-Page, J.C.; Carratalá-Tejada, M. The Timing of Kinematic and Kinetic Parameters during Gait Cycle as a Marker of Early Gait Deterioration in Multiple Sclerosis Subjects with Mild Disability. J. Clin. Med. 2022, 11, 1892. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, R.; Vocenas, L. Identification of Gait-Cycle Phases for Prosthesis Control. Biomimetics 2021, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Burnfield, J.M. (Eds.) Basic Functions in Gait Analysis: Normal and Pathological Function, 2nd ed.; Slack Thorofare: New York, NY, USA, 2010. [Google Scholar]
- Serrien, B.; Clijsen, R.; Blondeel, J.; Goossens, M.; Baeyens, J.-P. Differences in ball speed and three-dimensional kinematics between male and female handball players during a standing throw with run-up. BMC Sports Sci. Med. Rehabil. 2015, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Vijayan, V.; Reissman, M.E.; Kinney, A.L.; Reissman, T. How Do Joint Kinematics and Kinetics Change When Walking Overground with Added Mass on the Lower Body? Sensors 2022, 22, 9177. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Umehara, J.; Pataky, T.C.; Yagi, M.; Hirono, T.; Ueda, Y.; Ichihashi, N. Application of statistical parametric mapping for comparison of scapular kinematics and EMG. J. Biomech. 2022, 145, 111357. [Google Scholar] [CrossRef]
- Mestanza Mattos, F.G.; Luciano, F.; Lencioni, T.; Gervasoni, E.; Jonsdottir, J.; Anastasi, D.; Pavei, G.; Clerici, M.; Cattaneo, D. Complementary use of statistical parametric mapping and gait profile score to describe walking alterations in multiple sclerosis: A cross-sectional study. Sci. Rep. 2023, 13, 10465. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Pataky, T.C. Power1D: A Python toolbox for numerical power estimates in experiments involving one-dimensional continua. PeerJ Comput. Sci. 2017, 3, e125. [Google Scholar] [CrossRef]
- Van Rossum, G.; Drake, F., Jr. The Python Language Reference Manual; Network Theory Limited: Godalming, UK, 2011. [Google Scholar]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Mañago, M.M.; Kline, P.W.; Alvarez, E.; Christiansen, C.L. Trunk and pelvis movement compensation in people with multiple sclerosis: Relationships to muscle function and gait performance outcomes. Gait Posture 2020, 78, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Piperno, R.; Simoncini, L.; Bonato, P.; Tonini, A.; Giannini, S. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult. Scler. J. 1999, 5, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Coghe, G.; Corona, F.; Marrosu, M.G.; Cocco, E. Effect of spasticity on kinematics of gait and muscular activation in people with Multiple Sclerosis. J. Neurol. Sci. 2015, 358, 339–344. [Google Scholar] [CrossRef]
- Severini, G.; Manca, M.; Ferraresi, G.; Caniatti, L.M.; Cosma, M.; Baldasso, F.; Straudi, S.; Morelli, M.; Basaglia, N. Evaluation of Clinical Gait Analysis Parameters in Patients Affected by Multiple Sclerosis: Analysis of Kinematics. Clin. Biomech. 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Filli, L.; Sutter, T.; Easthope, C.S.; Killeen, T.; Meyer, C.; Reuter, K.; Lörincz, L.; Bolliger, M.; Weller, M.; Curt, A.; et al. Profiling walking dysfunction in multiple sclerosis: Characterisation, classification and progression over time. Sci. Rep. 2018, 8, 4984. [Google Scholar] [CrossRef]
- Massot, C.; Guyot, M.-A.; Donze, C.; Simoneau, E.; Gillet, C.; Leteneur, S. Ankle dysfunction in multiple sclerosis and the effects on walking. Disabil. Rehabil. 2021, 43, 2454–2463. [Google Scholar] [CrossRef]
- van der Linden, M.L.; Scott, S.M.; Hooper, J.E.; Cowan, P.; Mercer, T.H. Gait kinematics of people with multiple sclerosis and the acute application of functional electrical stimulation. Gait Posture 2014, 39, 1092–1096. [Google Scholar] [CrossRef]
- Morel, E.; Allali, G.; Laidet, M.; Assal, F.; Lalive, P.H.; Armand, S. Gait Profile Score in multiple sclerosis patients with low disability. Gait Posture 2017, 51, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Galea, M.P.; Cofré Lizama, L.E.; Butzkueven, H.; Kilpatrick, T.J. Gait and Balance Deterioration over a 12-Month Period in Multiple Sclerosis Patients with EDSS Scores ≤ 3.0. NeuroRehabilitation 2017, 40, 277–284. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Multiple Sclerosis | Controls | p |
|---|---|---|---|
| Gender | 7 female/3 male | 7 female/3 male | |
| Age | 35.8 (9.1) | 35.7 (9.3) | 0.981 |
| Height (cm) | 1.7 (0.08) | 1.73 (0.07) | 0.432 |
| Weight (kg) | 68.6 (8.6) | 66.2 (14.1) | 0.65 |
| Years since diagnosis | 9.12 (8.82) | ||
| EDSS (Median IQR) | 2.25 (1.9) | ||
| Gait speed (m/s) | 1.16 (0.19) | 1.24 (0.1) | 0.098 |
| Stride length (m) | 1.27 (0.13) | 1.3 (0.08) | 0.248 |
| Steep width (m) | 0.09 (0.02) | 0.07 (0.03) | 0.105 |
| Cadence (m) | 110.91 (12.03) | 113.8 (6.65) | 0.353 |
| Foot-off (%) | 61.12 (2.57) | 60.58 (1.76) | 0.441 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Vázquez, D.; Calvo-Malón, G.; Molina-Rueda, F.; López-González, R.; Carratalá-Tejada, M.; Navarro-López, V.; Miangolarra-Page, J.C. Kinematic Gait Analysis in People with Mild-Disability Multiple Sclerosis Using Statistical Parametric Mapping: A Cross-Sectional Study. Sensors 2023, 23, 7671. https://doi.org/10.3390/s23187671
Fernández-Vázquez D, Calvo-Malón G, Molina-Rueda F, López-González R, Carratalá-Tejada M, Navarro-López V, Miangolarra-Page JC. Kinematic Gait Analysis in People with Mild-Disability Multiple Sclerosis Using Statistical Parametric Mapping: A Cross-Sectional Study. Sensors. 2023; 23(18):7671. https://doi.org/10.3390/s23187671
Chicago/Turabian StyleFernández-Vázquez, Diego, Gabriela Calvo-Malón, Francisco Molina-Rueda, Raúl López-González, María Carratalá-Tejada, Víctor Navarro-López, and Juan Carlos Miangolarra-Page. 2023. "Kinematic Gait Analysis in People with Mild-Disability Multiple Sclerosis Using Statistical Parametric Mapping: A Cross-Sectional Study" Sensors 23, no. 18: 7671. https://doi.org/10.3390/s23187671





