Porous MgNiO2 Chrysanthemum Flower Nanostructure Electrode for Toxic Hg2+ Ion Monitoring in Aquatic Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MgNiO2 CFs
2.2. Characterization of MgNiO2 CFs
2.3. Electrochemical Sensing of Hg2+ Ions Using MgNiO2 CFs Electrode
3. Results
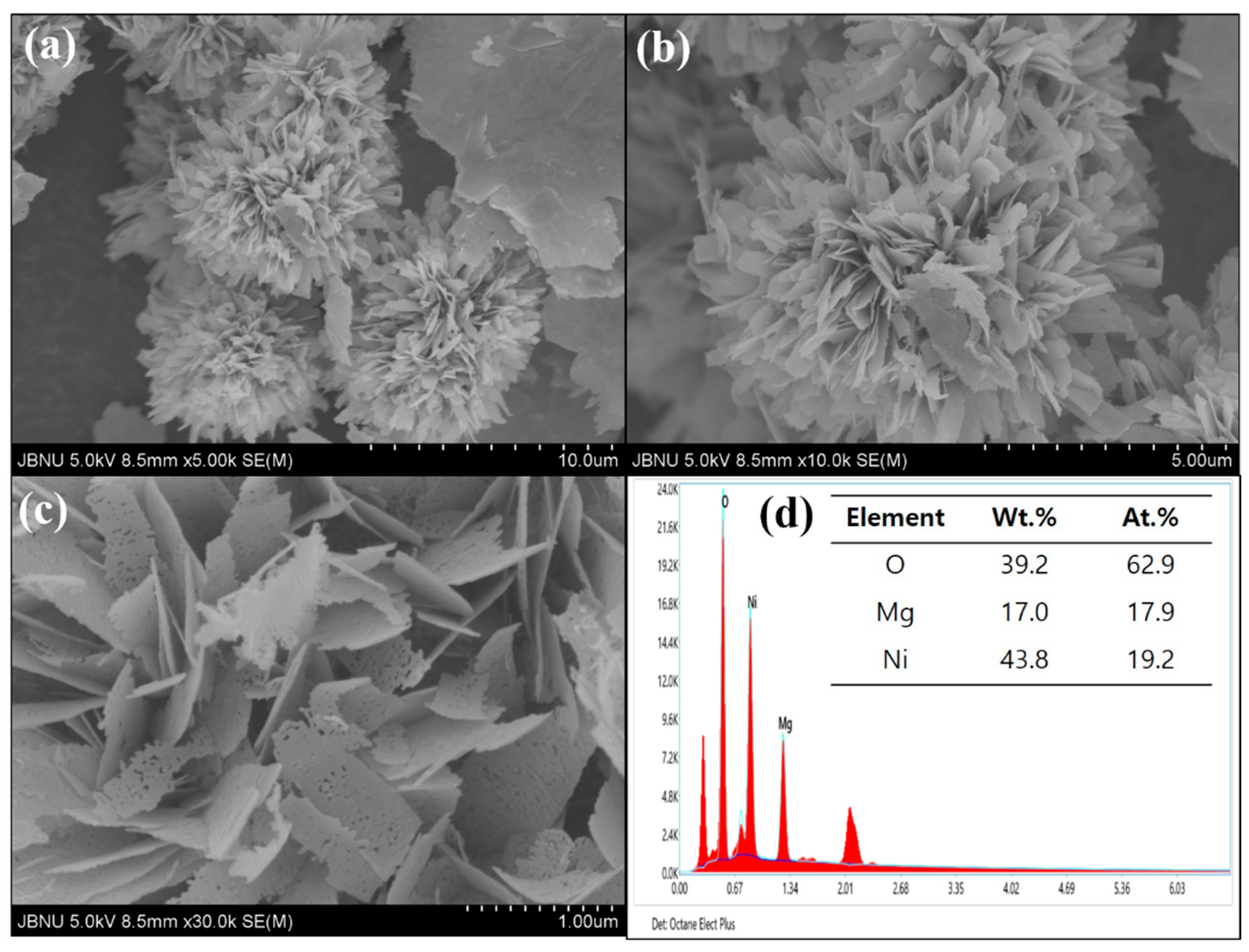
3.1. Morphological Properties and Element Analysis of MgNiO2 CFs
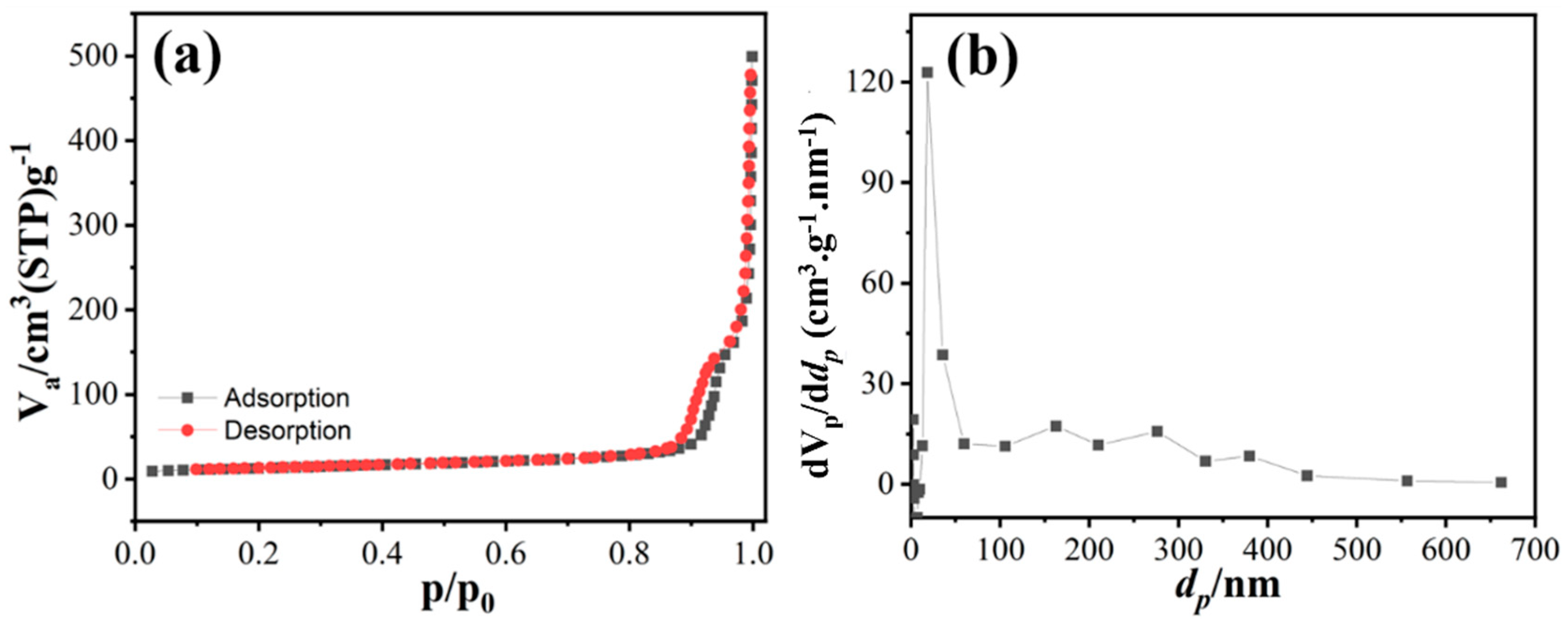
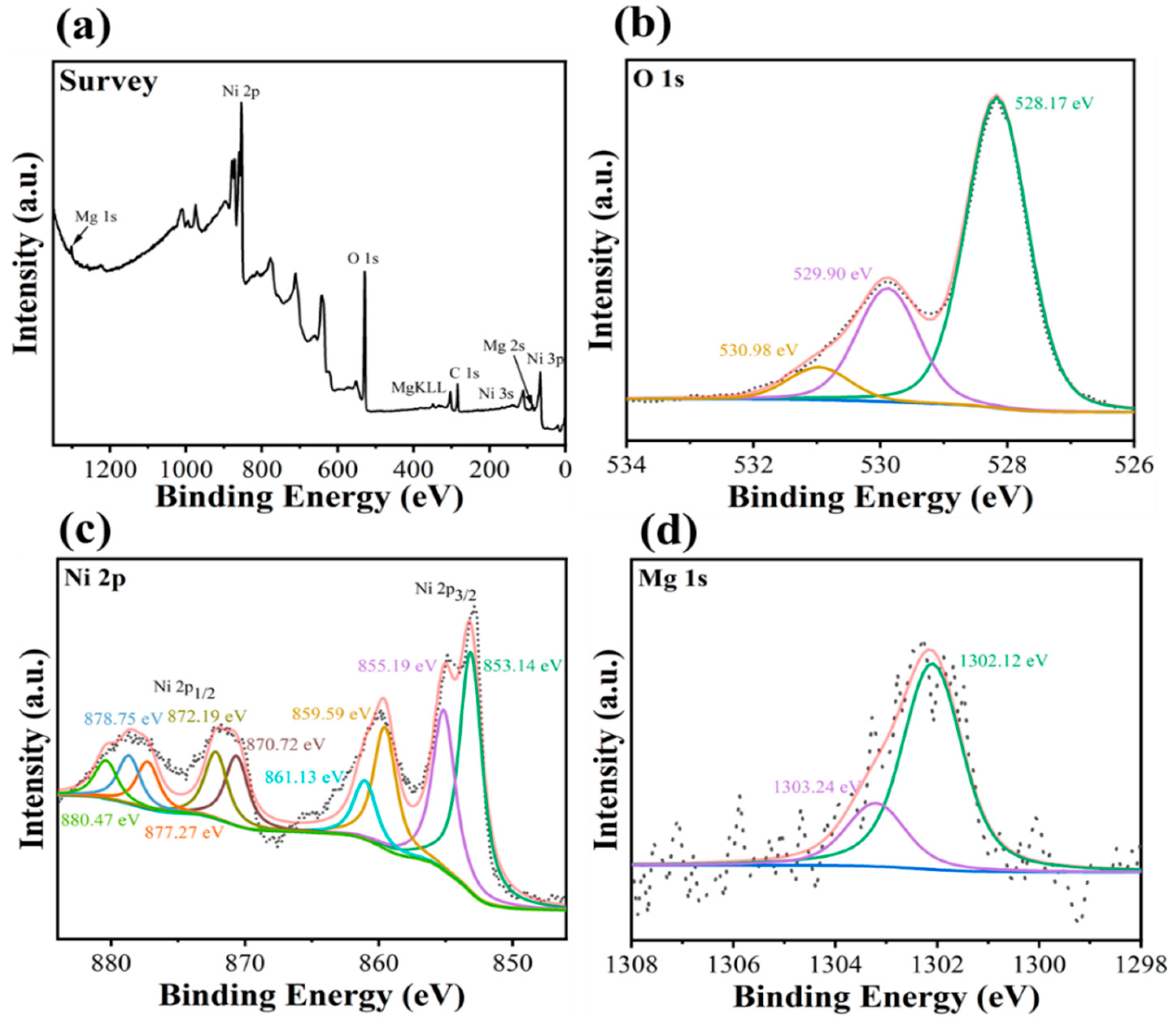
3.2. Structural and Surface Properties of MgNiO2 CFs
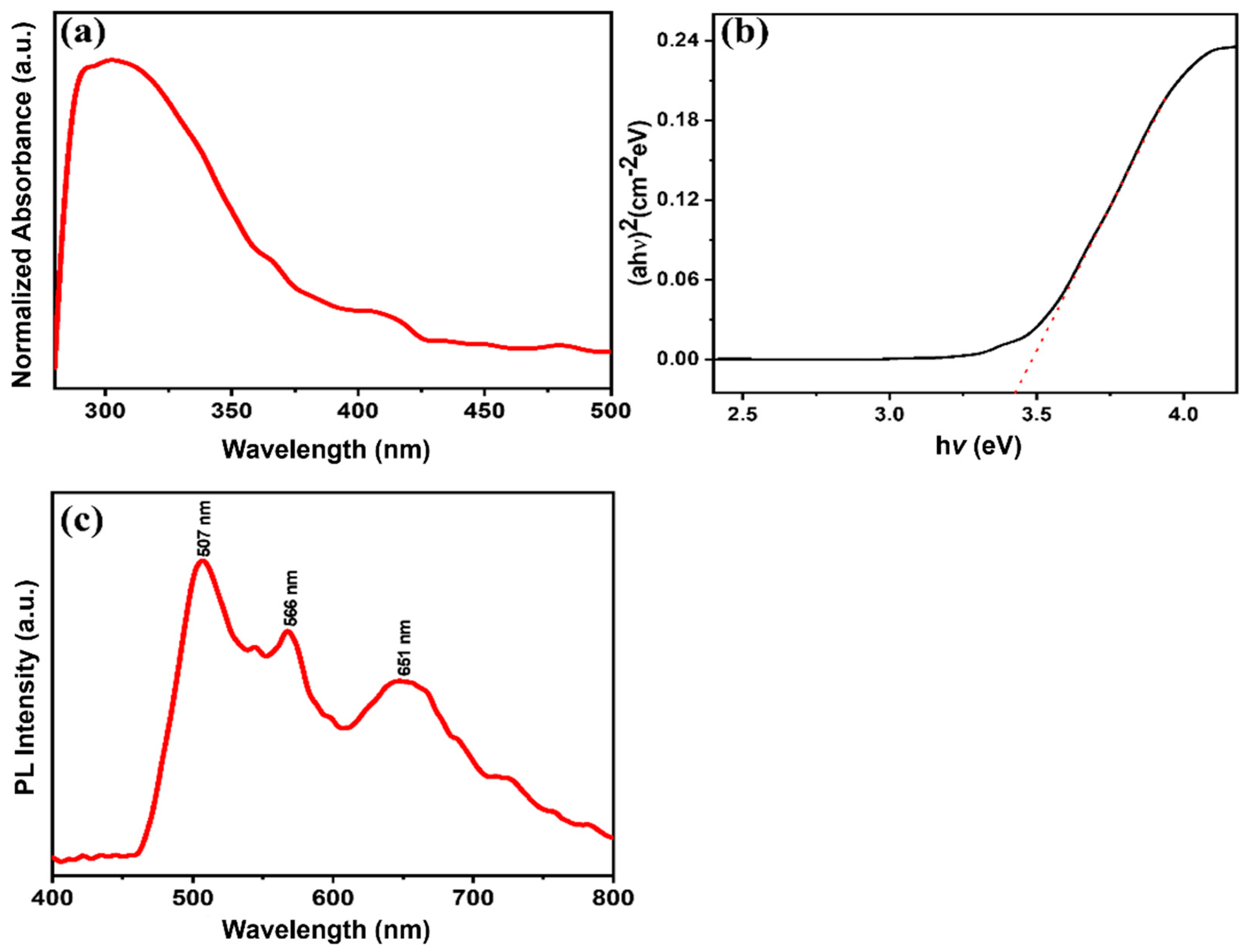
3.3. Optical Properties of MgNiO2 CFs
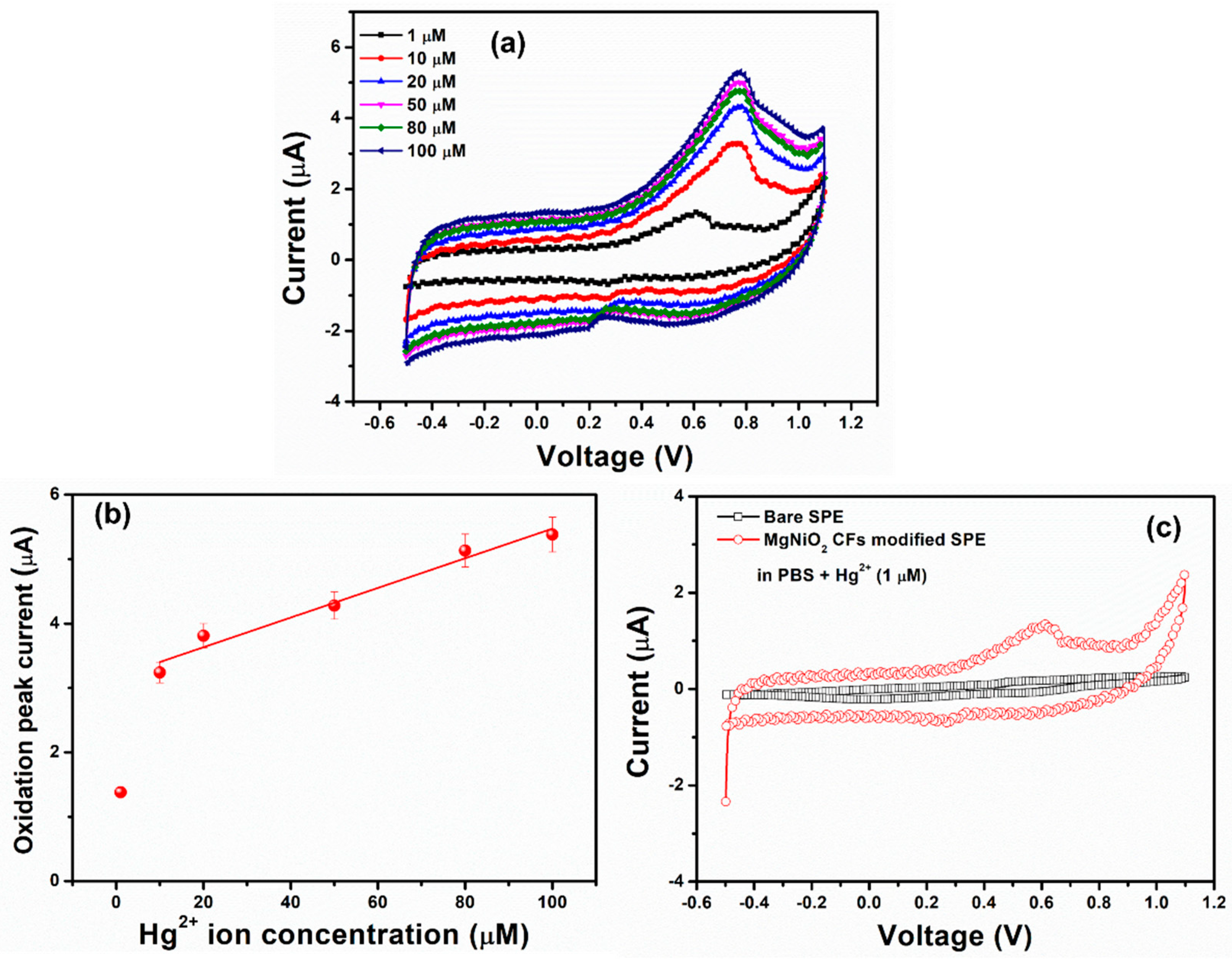
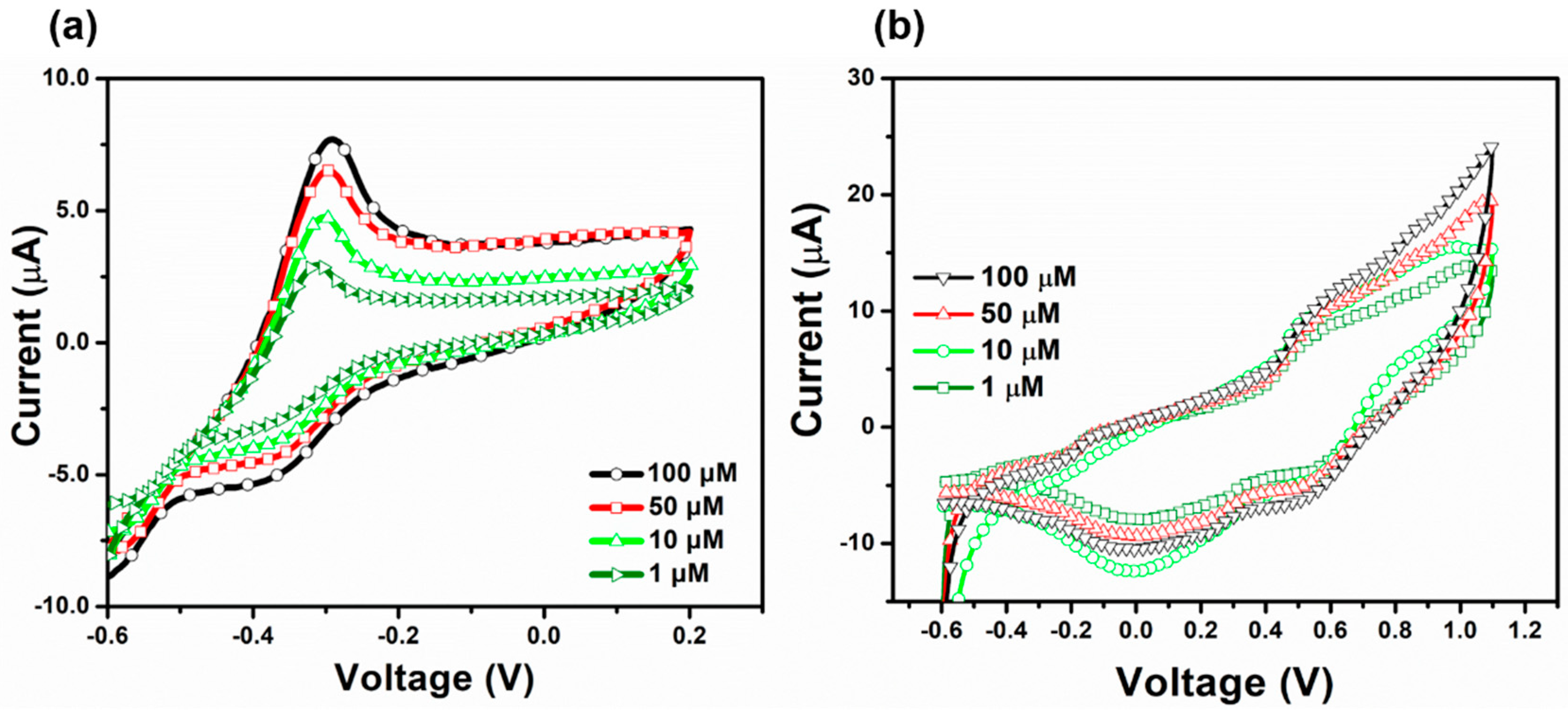
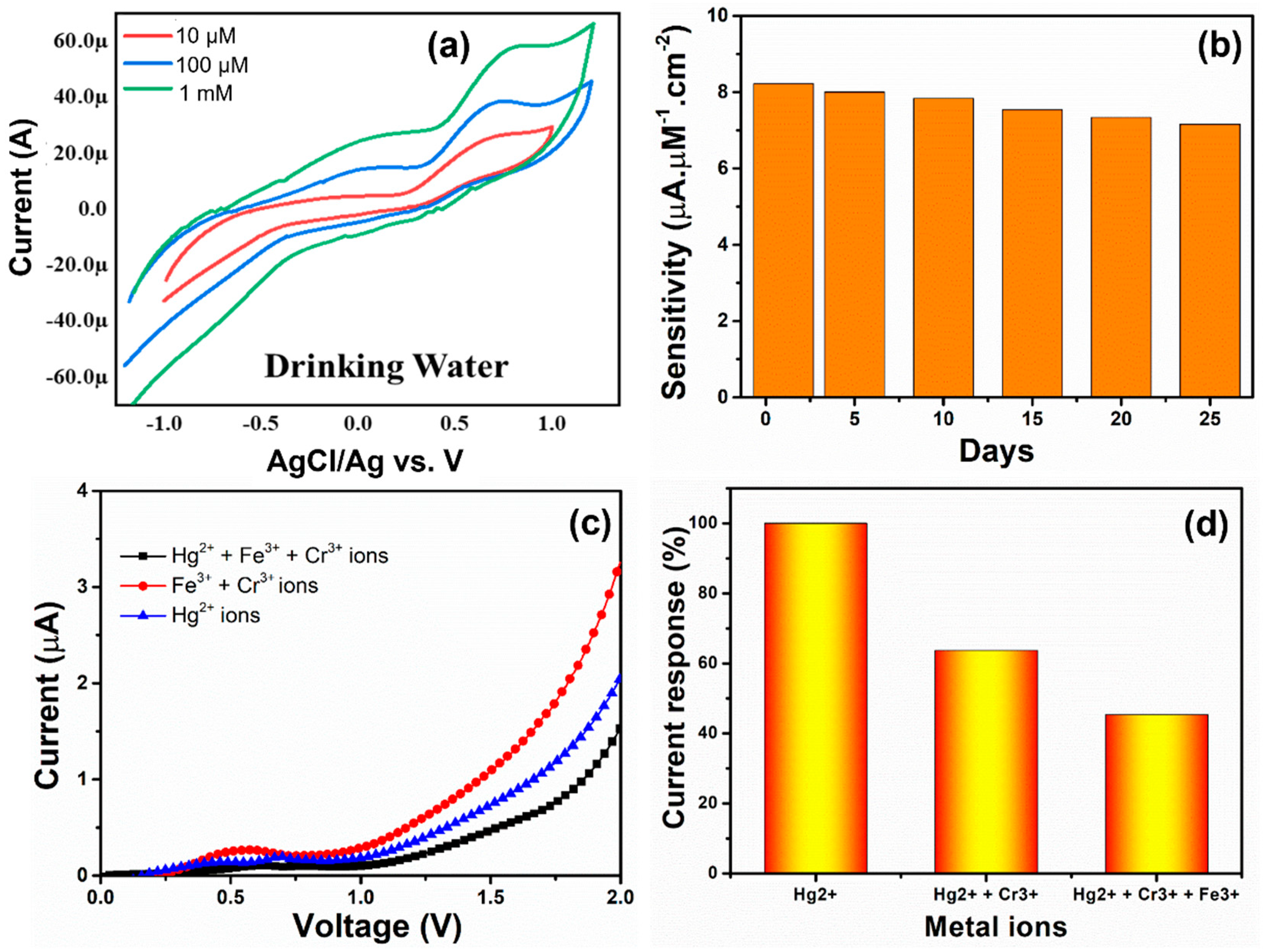
3.4. Sensing Performance, Selectivity, and Real Sample Performance of MgNiO2 CFs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dilek, D.Y.; Derya, A.D.; Hamdi, M. Colorimetric detection of mercury ion using chlorophyll functionalized green silver nanoparticles in aqueous medium. Surf. Inter. 2021, 22, 100840. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Wang, Z.; Su, S.; Yu, C.; Chen, Y.; Xia, D. Synthesis, Characterization, and Electrochemical Properties of Spherical-like LiFePO4 by Hydrothermal Method. J. Power Sour. 2008, 184, 633–636. [Google Scholar] [CrossRef]
- Annadhasan, M.; Rajendiran, N. Highly Selective and Sensitive Colorimetric Detection of Hg (II) Ions Using Green Synthesized Silver Nanoparticles. RSC Adv. 2015, 5, 94513–94518. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent Advances in the Design of Colorimetric Sensors for Environmental Monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Cyril, N.; George, J.B.; Joseph, L.; Sylas, V.P. Catalytic Degradation of Methyl Orange and Selective Sensing of Mercury Ion in Aqueous Solutions Using Green Synthesized Silver Nanoparticles from the Seeds of Derris trifoliata. J. Cluster Sci. 2019, 30, 459–468. [Google Scholar] [CrossRef]
- Luiz, J.; Nascimento, M.; Oliveira, K.R.M.; Crespolopez, M.E.; Barbarella, M.; Maués, L.A.L. Methylmercury Neurotoxicity & Antioxidant Defenses. Indian J. Med. Res. 2008, 128, 373–382. [Google Scholar]
- Dayan, A.D.; Paine, A.J. Human & Experimental Toxicology: Mechanisms of Chromium Toxicity, Carcinogenicity, and Allergenicity: Review of the Literature from 1985 to 2000. Hum. Exp. Toxicol. 2001, 20, 439–451. [Google Scholar]
- Graeme, K.A.; Charles, V.P. Toxicology Heavy Metal Toxicity. Part I: Arsenic and Mercury. J. Emerg. Med. 1998, 16, 45–56. [Google Scholar] [CrossRef]
- Barringer, J.L.; Szabo, Z.; Reilly, P.A. Occurrence and Mobility of Mercury in Groundwater. Curr. Perspect. Contam. Hydrol. Water Res. Sus. 2012, 1, 117–147. [Google Scholar]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic Exposure and Toxicology: A Historical Perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J. Zinc Toxicity in Humans. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 500–508. [Google Scholar]
- Patrick, L. Lead Toxicity. Part II: The Role of Free Radical Damage and the Use of Antioxidants in the Pathology and Treatment of Lead Toxicity. Altern. Med. Rev. 2006, 11, 114–127. [Google Scholar] [PubMed]
- Dey, S.K.; Roy, S. Effects of Chromium on Certain Aspects of Cellular Toxicity. Iran. J. Toxicol. 2009, 2, 260–267. [Google Scholar]
- Adrienne, S.E.; Anne, G.W. Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2010; pp. 5–27.
- Liu, C.; Bai, R.; Ly, Q.S. Selective Removal of Copper and Lead Ions by Diethylenetriamine-Functionalized Adsorbent: Behaviors and Mechanisms. Water Res. 2008, 42, 1511–1522. [Google Scholar] [CrossRef]
- Arino, C.; Serrano, N.; Díaz-Cruz, J.M.; Esteban, M. Voltammetric Determination of Metal Ions beyond Mercury Electrodes: A Review. Anal. Chim. Acta 2017, 990, 11–53. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Shin, H.S. Spindles Shaped ZnO Modified Glassy Carbon Electrode for the Selective Monitoring of Piperidine. Mater. Lett. 2015, 148, 188–191. [Google Scholar] [CrossRef]
- Zhang, M.; Igalavithana, A.D.; Xu, L.; Sarkar, B.; Hou, D.; Zhang, M.; Bhatnagar, A.; Cho, W.C.; Ok, Y.S. Engineered/Designer Hierarchical Porous Carbon Materials for Organic Pollutant Removal from Water and Wastewater: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 2295–2328. [Google Scholar] [CrossRef]
- Kim, E.B.; Imran, M.; Akhtar, M.S.; Shin, H.S.; Ameen, S. Enticing 3D Peony-like ZnGa2O4 Microstructures for Electrochemical Detection of N, N-Dimethylmethanamide Chemical. J. Hazard. Mater. 2021, 404, 124069. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, J.; Li, P.; Zhai, F.; Wan, Q.; Liu, Z.; Qu, X. Dehydrogenation mechanism of ball-milled MgH2 doped with ferrites (CoFe2O4, ZnFe2O4, MnFe2O4 and Mn0.5Zn0.5Fe2O4) nanoparticles. J. Alloys Compd. 2015, 643, 174–180. [Google Scholar] [CrossRef]
- Jang, G.S.; Kim, E.B.; Akhtar, M.S.; Shin, H.S.; Ameen, S. An Exploration of 3-Methoxypropionitrile Chemical Sensor Based on Layered Hexagonal NiCo2O4 Nanoplates as Electrode Material. Ceram. Int. 2021, 47, 15357–15366. [Google Scholar] [CrossRef]
- Gineys, N.; Aouad, G.; Sorrentino, F.; Damidot, D. Incorporation of trace elements in Portland cement clinker: Thresholds limits for Cu, Ni, Sn or Zn. Cem. Concr. Res. 2011, 41, 1177–1184. [Google Scholar] [CrossRef]
- Franger, S.; Le Cras, F.; Bourbon, C.; Rouault, H. Comparison between different LiFePO4 synthesis routes and their influence on its physico-chemical properties. J. Power Sources 2003, 119–121, 252–257. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, P.; Dai, H.; Chen, L.; Ma, H.; Lin, M.; Shen, D. An Electrochemical Sensor Based on Co3O4 Nanosheets for Lead Ions Determination. RSC Adv. 2017, 7, 39611–39616. [Google Scholar] [CrossRef]
- Ali, N.A.; Idris, N.H.; Din, M.F.M.; Yahya, M.S.; Ismail, M. Nanoflakes MgNiO2 Synthesized via a Simple Hydrothermal Method and Its Catalytic Roles on the Hydrogen Sorption Performance of MgH2. J. Alloys Compd. 2019, 796, 279–286. [Google Scholar] [CrossRef]
- Kim, E.B.; Imran, M.; Lee, E.H.; Akhtar, M.S.; Ameen, S. Multiple Ions Detection by Field-Effect Transistor Sensors Based on ZnO@GO and ZnO@rGO Nanomaterials: Application to Trace Detection of Cr(III) and Cu(II). Chemosphere 2022, 286, 131695–131705. [Google Scholar] [CrossRef]
- Riman, D.; Jirovsky, D.; Hrbac, J.; Prodromidis, M.I. Green and Facile Electrode Modification by Spark Discharge: Bismuth Oxide-Screen Printed Electrodes for the Screening of Ultra-Trace Cd (II) and Pb (II). Electrochem. Comm. 2015, 50, 20–23. [Google Scholar] [CrossRef]
- Li, M.; Li, D.W.; Xiu, G.; Long, Y.T. Applications of Screen-Printed Electrodes in Current Environmental Analysis. Curr. Opin. Electrochem. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Fu, H. Synthesis of Bismuth-Nanoparticle-Enriched Nanoporous Carbon on Graphene for Efficient Electrochemical Analysis of Heavy-Metal Ions. Chem. Eur. J. 2015, 21, 11525–11530. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Shin, H.S. Low Temperature Grown ZnO Nanotubes as Smart Sensing Electrode for the Effective Detection of Ethanolamine Chemical. Mater. Lett. 2013, 106, 254–258. [Google Scholar] [CrossRef]
- Suppuraj, P.; Thirumalai, K.; Parthiban, S.; Swaminathan, M.; Muthuvel, I. Novel Ag-TiO2/ZnFe2O4 Nanocomposites for Effective Photocatalytic, Electrocatalytic, and Cytotoxicity Applications. J. Nanosci. Nanotechnol. 2020, 20, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Suvith, V.S.; Devu, V.S.; Philip, D. Tannic Acid Mediated Synthesis of Nanostructured NiO and SnO2 for Catalytic Degradation of Methylene Blue. Opt. Quant. Electron. 2020, 52, 1–17. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Allaedini, G.; Aminayi, P.; Tasirin, S.M. Structural properties and optical characterization of flower-like Mg doped NiO. AIP Adv. 2015, 5, 077161. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, X.; Ren, H.; Huang, Z. Preparation of MgO nanocrystals and catalytic mechanism on phenol ozonation. RSC Adv. 2017, 7, 43464–43473. [Google Scholar] [CrossRef]
- Maitra, S.; Mitra, R.; Nath, T.K. Investigation of electrochemical performance of MgNiO2 prepared by sol-gel synthesis route for aqueous-based supercapacitor application. Curr. Appl. Phys. 2020, 20, 628–637. [Google Scholar] [CrossRef]
- Kotta, A.; Kim, E.B.; Ameen, S.; Shin, H.S.; Seo, H.K. Ultra-Small NiO Nanoparticles Grown by Low-Temperature Process for Electrochemical Application. J. Electrochem. Soc. 2020, 167, 167517. [Google Scholar] [CrossRef]
- Wu, P.Y.; Jiang, Y.-P.; Zhang, Q.Y.; Jia, Y.; Peng, D.Y.; Xu, W. Comparative Study on Arsenate Removal Mechanism of MgO and MgO/TiO2 Composites: FTIR and XPS Analysis. New J. Chem. 2016, 40, 2878–2885. [Google Scholar] [CrossRef]
- Huang, W.; Ding, S.; Chen, Y.; Hao, W.; Lai, X.; Peng, J.; Tu, J.; Cao, Y.; Li, X. 3D NiO Hollow Sphere/Reduced Graphene Oxide Composite for High-Performance Glucose Biosensor. Nat. Sci. Rep. 2017, 7, 5220. [Google Scholar] [CrossRef]
- Yang, B.; Bai, X.; Wang, J.; Fang, M.; Wu, X.; Liu, Y.; Huang, Z.; Lao, C.Y.; Min, X. Photocatalytic Performance of NiO/NiTiO3 Composite Nanofiber Films. Catalysts 2019, 9, 561. [Google Scholar] [CrossRef]
- Han, B.; Yang, Y.; Huang, Z.; You, L.; Huang, H.; Wang, K. A Composite Anodic Coating Containing Graphene on AZ31 Magnesium Alloy. Int. J. Electrochem. Sci. 2017, 12, 9829–9843. [Google Scholar] [CrossRef]
- El-Kemary, M.; Nagy, N.; El-Mehasseb, I. Nickel oxide nanoparticles: Synthesis and spectral studies of interactions with glucose. Mat. Sci. Semicon. Proc. 2013, 16, 1747–1752. [Google Scholar] [CrossRef]
- Zhu, L.P.; Liao, G.H.; Yang, Y.; Xiao, H.M.; Wang, J.F.; Fu, S.Y. Self-Assembled 3D Flower-Like Hierarchical b-Ni(OH)2 Hollow Architectures and their In Situ Thermal Conversion to NiO. Nanoscale Res. Lett. 2009, 4, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Musevi, S.J.; Aslani, A.; Motahari, H.; Salimi, H. A Novel Method for Size Appraisal of NiO Nanoparticles by PL Analysis: Synthesis by Sonochemical Method. J. Saudi. Chem. Soc. 2016, 20, 245–252. [Google Scholar] [CrossRef]
- Kumar, S.A.; Shankar, J.S.; Periyasamy, B.K.; Nayak, S.K. Role of Defective States in MgO Nanoparticles on the Photophysical Properties and Photostability of MEH-PPV/MgO Nanocomposite. Phys. Chem. Chem. Phys. 2021, 23, 22804–22816. [Google Scholar] [CrossRef]
- Anandan, K.; Rajendran, V. Morphological and Size Effects of NiO Nanoparticles via Solvothermal Process and Their Optical Properties. Mat. Sci. Semicon. Proc. 2011, 14, 43–47. [Google Scholar] [CrossRef]
- Taşer, A.; Güldüren, M.E.; Güney, H. Tuning PL Emission Energy and Bandgap with Ni Dopant of MgO Thin Films. Ceram. Int. 2021, 47, 15792–15800. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman. Microwave-assisted MgO NP catalyzed one-pot multicomponent synthesis of polysubstituted steroidal pyridines. New J. Chem. 2018, 42, 184–197. [Google Scholar] [CrossRef]
- Gao, X.H.; Wei, W.Z.; Yang, L.; Yin, T.J. Simultaneous Determination of Lead, Copper, and Mercury Free from Macromolecule Contaminants by Square Wave Stripping Voltammetry. Mat. Lett. 2005, 38, 2327–2343. [Google Scholar] [CrossRef]
- Granado, E.; Gervais, E.; Gotti, G.S.; Meireles, M.; Gros, P.; Evrard, D. Mercury (II) Trace Detection Using a Glassy Carbon Electrode Functionalized by Chemically Prepared Gold Nanoparticles. Influence of Coating Process on Surface Reactivity and Analytical Performances. Int. J. Electrochem. Sci. 2017, 12, 6092–6107. [Google Scholar] [CrossRef]
- Zhou, L.; Xiong, W.; Liu, S. Preparation of a gold electrode modified with Au–TiO2 nanoparticles as an electrochemical sensor for the detection of mercury (II) ions. J. Mater. Sci. 2015, 50, 769–776. [Google Scholar] [CrossRef]


| Material and Method | LOD (μM) | Sensitivity (μA.μM−1cm−2) | Linear Range (μM) | R2 | Ref. |
|---|---|---|---|---|---|
| AuNPs-GCE (Electrodeposition method) | 0.64 | 0.274 | - | - | [51] |
| AuNPs (Turkevich method) | 1.0 | 0.269 | 0.36–10 | - | [52] |
| Au-TiO2 (Sol-gel method) | 1.0 | - | 0.05–4 | - | [53] |
| MgNiO2 (Hydrothermal synthesis) | 11.7 | 8.22 | 10–100 | 0.9721 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.; Kim, E.-B.; Kwak, D.-H.; Ameen, S. Porous MgNiO2 Chrysanthemum Flower Nanostructure Electrode for Toxic Hg2+ Ion Monitoring in Aquatic Media. Sensors 2023, 23, 7910. https://doi.org/10.3390/s23187910
Imran M, Kim E-B, Kwak D-H, Ameen S. Porous MgNiO2 Chrysanthemum Flower Nanostructure Electrode for Toxic Hg2+ Ion Monitoring in Aquatic Media. Sensors. 2023; 23(18):7910. https://doi.org/10.3390/s23187910
Chicago/Turabian StyleImran, Mohammad, Eun-Bi Kim, Dong-Heui Kwak, and Sadia Ameen. 2023. "Porous MgNiO2 Chrysanthemum Flower Nanostructure Electrode for Toxic Hg2+ Ion Monitoring in Aquatic Media" Sensors 23, no. 18: 7910. https://doi.org/10.3390/s23187910





