Transcranial Magnetic Stimulation for the Treatment of Chemo Brain
Abstract
:1. Background
2. Case Presentation
3. Procedures and Data Analyses
4. Results
4.1. Stimulation Feasibility
4.2. Neuropsychological Testing
4.3. Subjective Reporting
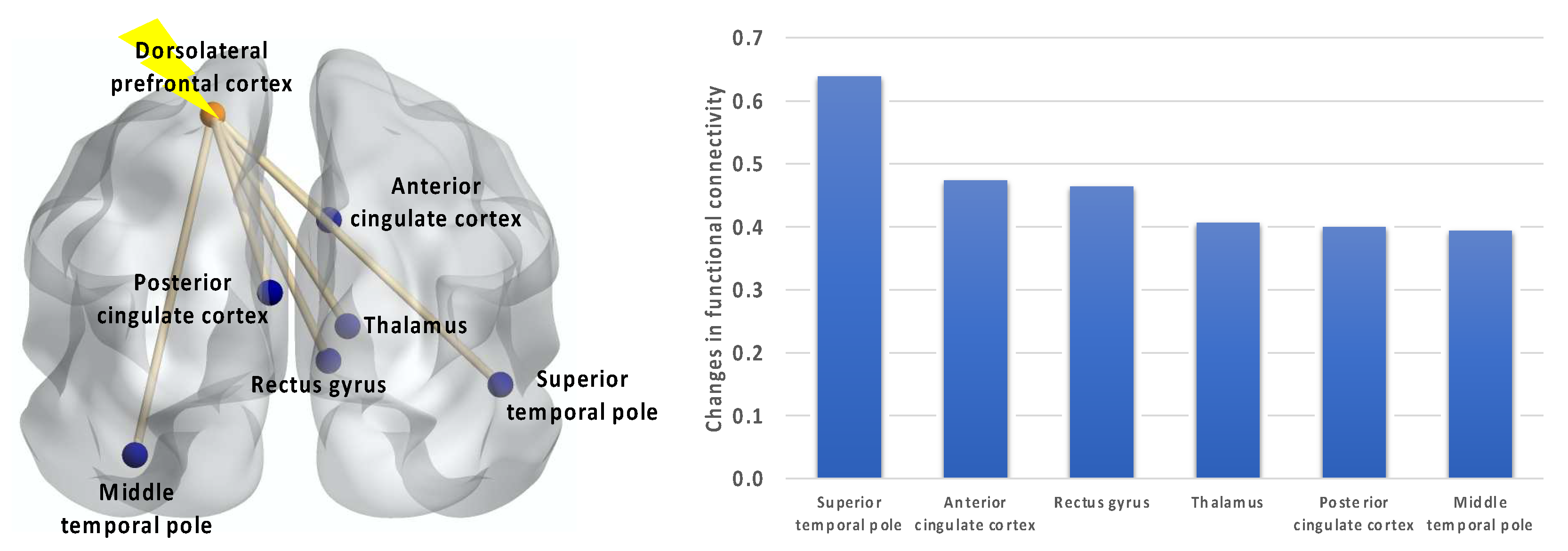
4.4. Resting-State Functional Connectivity
5. Discussion and Conclusions
6. Future Directions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Argyriou, A.A.; Assimakopoulos, K.; Iconomou, G.; Giannakopoulou, F.; Kalofonos, H.P. Either called “chemobrain” or “chemofog”, the long-term chemotherapy-induced cognitive decline in cancer survivors is real. J. Pain Symptom. Manag. 2011, 41, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Saykin, A.J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer 2007, 7, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Saykin, A.; Ahles, T.; McDonald, B. Mechanisms of Chemotherapy-Induced Cognitive Disorders: Neuropsychological, Pathophysiological, and Neuroimaging Perspectives. Semin. Clin. Neuropsychiatry 2003, 8, 201–216. [Google Scholar] [PubMed]
- Cimprich, B.; Reuter-Lorenz, P.; Nelson, J.; Clark, P.M.; Therrien, B.; Normolle, D.; Berman, M.G.; Hayes, D.F.; Noll, D.C.; Peltier, S.; et al. Prechemotherapy alterations in brain function in women with breast cancer. J. Clin. Exp. Neuropsychol. 2010, 32, 324–331. [Google Scholar] [CrossRef]
- Dietrich, J.; Monje, M.; Wefel, J.; Meyers, C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist 2008, 13, 1285–1295. [Google Scholar] [CrossRef]
- Donovan, K.A.; Jacobsen, P.B.; Andrykowski, M.A.; Winters, E.M.; Balducci, L.; Malik, U.; Kenady, D.; McGrath, P. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J. Pain Symptom. Manag. 2004, 28, 373–380. [Google Scholar] [CrossRef]
- Vearncombe, K.J.; Rolfe, M.; Wright, M.; Pachana, N.A.; Andrew, B.; Beadle, G. Predictors of cognitive decline after chemotherapy in breast cancer patients. J. Int. Neuropsychol. Soc. 2009, 15, 951–962. [Google Scholar] [CrossRef]
- Wefel, J.S.; Lenzi, R.; Theriault, R.; Buzdar, A.U.; Cruickshank, S.; Meyers, C.A. ‘Chemobrain’ in breast carcinoma? A prologue. Cancer 2004, 101, 466–475. [Google Scholar] [CrossRef]
- Wefel, J.S.; Schagen, S.B. Chemotherapy-Related Cognitive Dysfunction. Curr. Neurol. Neurosci. Rep. 2012, 12, 267–275. [Google Scholar] [CrossRef]
- Das, A.; Ranadive, N.; Kinra, M.; Nampoothiri, M.; Arora, D.; Mudgal, J. An Overview on Chemotherapy-induced Cognitive Impairment and Potential Role of Antidepressants. Curr. Neuropharmacol. 2020, 18, 838–851. [Google Scholar] [CrossRef]
- Artherholt, S.B.; Fann, J.R. Psychosocial care in cancer. Curr. Psychiatry Rep. 2012, 14, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Boykoff, N.; Moieni, M.; Subramanian, S.K. Confronting chemobrain: An in-depth look at survivors’ reports of impact on work, social networks, and health care response. J. Cancer Surviv. 2009, 3, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.R. The impact of chemo brain on the patient with a high-grade glioma. Adv. Exp. Med. Biol. 2010, 678, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics, 2012. CA A Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, A.I. The economic burden. Adv. Exp. Med. Biol. 2010, 678, 33–36. [Google Scholar] [CrossRef]
- Was, H.; Borkowska, A.; Bagues, A.; Tu, L.; Liu, J.Y.H.; Lu, Z.; Rudd, J.A.; Nurgali, K.; Abalo, R. Mechanisms of Chemotherapy-Induced Neurotoxicity. Front. Pharmacol. 2022, 13, 750507. [Google Scholar] [CrossRef]
- John, J.; Kinra, M.; Mudgal, J.; Viswanatha, G.L.; Nandakumar, K. Animal models of chemotherapy-induced cognitive decline in preclinical drug development. Psychopharmacology 2021, 238, 3025–3053. [Google Scholar] [CrossRef]
- Skurlova, M.; Holubova, K.; Kleteckova, L.; Kozak, T.; Kubova, H.; Horacek, J.; Vales, K. Chemobrain in blood cancers: How chemotherapeutics interfere with the brain’s structure and functionality, immune system, and metabolic functions. Med. Res. Rev. 2023. [Google Scholar] [CrossRef]
- McDonald, B.C.; Conroy, S.K.; Ahles, T.A.; West, J.D.; Saykin, A.J. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast. Cancer Res. Treat. 2010, 123, 819–828. [Google Scholar] [CrossRef]
- McDonald, B.C.; Conroy, S.K.; Ahles, T.A.; West, J.D.; Saykin, A.J. Alterations in brain activation during working memory processing associated with breast cancer and treatment: A prospective functional magnetic resonance imaging study. J. Clin. Oncol. 2012, 30, 2500–2508. [Google Scholar] [CrossRef]
- Silverman, D.H.; Dy, C.J.; Castellon, S.A.; Lai, J.; Pio, B.S.; Abraham, L.; Waddell, K.; Petersen, L.; Phelps, M.E.; Ganz, P.A. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast. Cancer Res. Treat. 2007, 103, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Tatti, E.; Rossi, S.; Innocenti, I.; Rossi, A.; Santarnecchi, E. Non-invasive brain stimulation of the aging brain: State of the art and future perspectives. Ageing Res. Rev. 2016, 29, 66–89. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorff, J.; Göke, K.; Kiebs, M.; Kasten, F.H.; Herrmann, C.S.; Witt, K.; Hurlemann, R. Evidence of Neuroplastic Changes after Transcranial Magnetic, Electric, and Deep Brain Stimulation. Brain Sci. 2022, 12, 929. [Google Scholar] [CrossRef]
- Schulz, R.; Gerloff, C.; Hummel, F.C. Non-invasive brain stimulation in neurological diseases. Neuropharmacology 2013, 64, 579–587. [Google Scholar] [CrossRef]
- Chou, Y.-H.; Ton That, V.; Sundman, M. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2020, 86, 1–10. [Google Scholar] [CrossRef]
- Chou, Y.H.; Hickey, P.T.; Sundman, M.; Song, A.W.; Chen, N.K. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2015, 72, 432–440. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Ni, H.-C.; Chen, Y.-L.; Chao, Y.-P.; Wu, C.-T.; Wu, Y.-Y.; Liang, S.H.-Y.; Chin, W.-C.; Chou, T.-L.; Gau, S.S.-F.; Huang, Y.-Z.; et al. Intermittent theta burst stimulation over the posterior superior temporal sulcus for children with autism spectrum disorder: A 4-week randomized blinded controlled trial followed by another 4-week open-label intervention. Autism 2021, 25, 1279–1294. [Google Scholar] [CrossRef]
- Bai, L.; Yu, E. A narrative review of risk factors and interventions for cancer-related cognitive impairment. Ann. Transl. Med. 2021, 9, 72. [Google Scholar] [CrossRef]
- Chung, S.W.; Sullivan, C.M.; Rogasch, N.C.; Hoy, K.E.; Bailey, N.W.; Cash, R.F.H.; Fitzgerald, P.B. The effects of individualised intermittent theta burst stimulation in the prefrontal cortex: A TMS-EEG study. Hum. Brain Mapp. 2019, 40, 608–627. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.T.; Cheng, C.M.; Liang, C.S.; Chang, W.H.; Juan, C.H.; Huang, Y.Z.; Jeng, J.S.; Bai, Y.M.; Tsai, S.J.; Chen, M.H.; et al. Efficacy and tolerability of theta-burst stimulation for major depression: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110168. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.E.; Ivnik, R.J.; Smith, G.E. Mayo’s Older Americans Normative Studies: Expanded AVLT Recognition Trial Norms for Ages 57 to 98. J. Clin. Exp. Neuropsychol. 2002, 24, 214–220. [Google Scholar] [CrossRef]
- Schmidt, M. Rey Auditory Verbal Learning Test: A Handbook; Western Psychological Services: Los Angeles, CA, USA, 1996; Volume 17. [Google Scholar]
- Ivnik, R.J.; Malec, J.F.; Smith, G.E.; Tangalos, E.G.; Petersen, R.C.; Kokmen, E.; Kurland, L.T. Mayo’s older americans normative studies: Updated AVLT norms for ages 56 to 97. Clin. Neuropsychol. 1992, 6, 83–104. [Google Scholar] [CrossRef]
- Jak, A.J.; Bondi, M.W.; Delano-Wood, L.; Wierenga, C.; Corey-Bloom, J.; Salmon, D.P.; Delis, D.C. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am. J. Geriatr. Psychiatry 2009, 17, 368–375. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta Burst Stimulation of the Human Motor Cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef]
- Borckardt, J.J.; Nahas, Z.; Koola, J.; George, M.S. Estimating resting motor thresholds in transcranial magnetic stimulation research and practice: A computer simulation evaluation of best methods. J. Ect. 2006, 22, 169–175. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2020, 132, 269–306. [Google Scholar] [CrossRef] [PubMed]
- Fried, P.; Jannati, A.; Morris, T.; Buss, S.; Santarnecchi, E.; Shafi, M.; Pascual-Leone, A. Relationship of Active to Resting Motor Threshold Influences the Aftereffects of Theta-Burst Stimulation. Brain Stimul. 2019, 12, 465. [Google Scholar] [CrossRef]
- Mir-Moghtadaei, A.; Caballero, R.; Fried, P.; Fox, M.D.; Lee, K.; Giacobbe, P.; Daskalakis, Z.J.; Blumberger, D.M.; Downar, J. Concordance Between BeamF3 and MRI-neuronavigated Target Sites for Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex. Brain Stimul. 2015, 8, 965–973. [Google Scholar] [CrossRef]
- Field, A.; Birn, R. Two for the Price of One? Analyzing Task-based fMRI Data with Resting-State fMRI Methods. Radiology 2021, 301, 185–186. [Google Scholar] [CrossRef]
- Smitha, K.A.; Akhil Raja, K.; Arun, K.M.; Rajesh, P.G.; Thomas, B.; Kapilamoorthy, T.R.; Kesavadas, C. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol. J. 2017, 30, 305–317. [Google Scholar] [CrossRef]
- Feng, Y.; Tuluhong, D.; Shi, Z.; Zheng, L.J.; Chen, T.; Lu, G.M.; Wang, S.; Zhang, L.J. Postchemotherapy hippocampal functional connectivity patterns in patients with breast cancer: A longitudinal resting state functional MR imaging study. Brain Imaging Behav. 2020, 14, 1456–1467. [Google Scholar] [CrossRef]
- Bruno, J.; Hosseini, S.M.H.; Kesler, S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol. Dis. 2012, 48, 329–338. [Google Scholar] [CrossRef]
- Yao, S.; Zhu, Q.; Zhang, Q.; Cai, Y.; Liu, S.; Pang, L.; Jing, Y.; Yin, X.; Cheng, H. Managing Cancer and Living Meaningfully (CALM) alleviates chemotherapy related cognitive impairment (CRCI) in breast cancer survivors: A pilot study based on resting-state fMRI. Cancer Med. 2023, 12, 16231–16242. [Google Scholar] [CrossRef]
- Rolls, E.T.; Huang, C.-C.; Lin, C.-P.; Feng, J.; Joliot, M. Automated anatomical labelling atlas 3. NeuroImage 2020, 206, 116189. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Fisher, R.A. On the “Probable Error” of a Coefficient of Correlation Deduced from a Small Sample. Metron 1921, 10, 507–521. [Google Scholar]
- Van Der Weijst, L.; Surmont, V.; Schrauwen, W.; Lievens, Y. Real Life Data on Patient-Reported Outcomes and Neuro-Cognitive Functioning of Lung Cancer Patients: The PRO-Long Study. Front. Oncol. 2021, 11, 685605. [Google Scholar] [CrossRef]
- Stapleton, S.; Darlington, A.E.; de Bono, J.S.; Wiseman, T. What is the impact of targeted therapies given within phase I trials on the cognitive function of patients with advanced cancer: A mixed-methods exploratory study conducted in an early clinical trials unit. BMJ Open 2022, 12, e050590. [Google Scholar] [CrossRef]
- Hawkins, K.; Dean, D.; Pearlson, G. Alternative Forms of the Rey Auditory Verbal Learning Test: A Review. Behav. Neurol. 2004, 15, 99–107. [Google Scholar] [CrossRef]
- Kardan, O.; Reuter-Lorenz, P.A.; Peltier, S.; Churchill, N.W.; Misic, B.; Askren, M.K.; Jung, M.S.; Cimprich, B.; Berman, M.G. Brain connectivity tracks effects of chemotherapy separately from behavioral measures. NeuroImage Clin. 2019, 21, 101654. [Google Scholar] [CrossRef]
- Corcoles-Parada, M.; Ubero-Martinez, M.; Morris, R.G.M.; Insausti, R.; Mishkin, M.; Munoz-Lopez, M. Frontal and Insular Input to the Dorsolateral Temporal Pole in Primates: Implications for Auditory Memory. Front. Neurosci. 2019, 13, 1099. [Google Scholar] [CrossRef]
- Olson, I.R.; Plotzker, A.; Ezzyat, Y. The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain 2007, 130, 1718–1731. [Google Scholar] [CrossRef]
- Ray, R.D.; Zald, D.H. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 2012, 36, 479–501. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef]
- Dumas, J.A.; Makarewicz, J.; Schaubhut, G.J.; Devins, R.; Albert, K.; Dittus, K.; Newhouse, P.A. Chemotherapy altered brain functional connectivity in women with breast cancer: A pilot study. Brain Imaging Behav. 2013, 7, 524–532. [Google Scholar] [CrossRef]
- Brown, M.J.N.; Goldenkoff, E.R.; Chen, R.; Gunraj, C.; Vesia, M. Using Dual-Site Transcranial Magnetic Stimulation to Probe Connectivity between the Dorsolateral Prefrontal Cortex and Ipsilateral Primary Motor Cortex in Humans. Brain Sci. 2019, 9, 177. [Google Scholar] [CrossRef]
- Wolff, M.; Vann, S.D. The Cognitive Thalamus as a Gateway to Mental Representations. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 3–14. [Google Scholar] [CrossRef]
- Gent, T.C.; Bandarabadi, M.; Herrera, C.G.; Adamantidis, A.R. Thalamic dual control of sleep and wakefulness. Nat. Neurosci. 2018, 21, 974–984. [Google Scholar] [CrossRef]
- Kirkovski, M.; Donaldson, P.H.; Do, M.; Speranza, B.E.; Albein-Urios, N.; Oberman, L.M.; Enticott, P.G. A systematic review of the neurobiological effects of theta-burst stimulation (TBS) as measured using functional magnetic resonance imaging (fMRI). Brain Struct. Funct. 2023, 228, 717–749. [Google Scholar] [CrossRef] [PubMed]
- Oken, B.S. Placebo effects: Clinical aspects and neurobiology. Brain 2008, 131, 2812–2823. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. Brain imaging before and after COVID-19 in UK Biobank. medRxiv 2021. [Google Scholar] [CrossRef]
- Sasaki, N.; Yamatoku, M.; Tsuchida, T.; Sato, H.; Yamaguchi, K. Effect of Repetitive Transcranial Magnetic Stimulation on Long Coronavirus Disease 2019 with Fatigue and Cognitive Dysfunction. Prog. Rehabil. Med. 2023, 8, 20230004. [Google Scholar] [CrossRef] [PubMed]
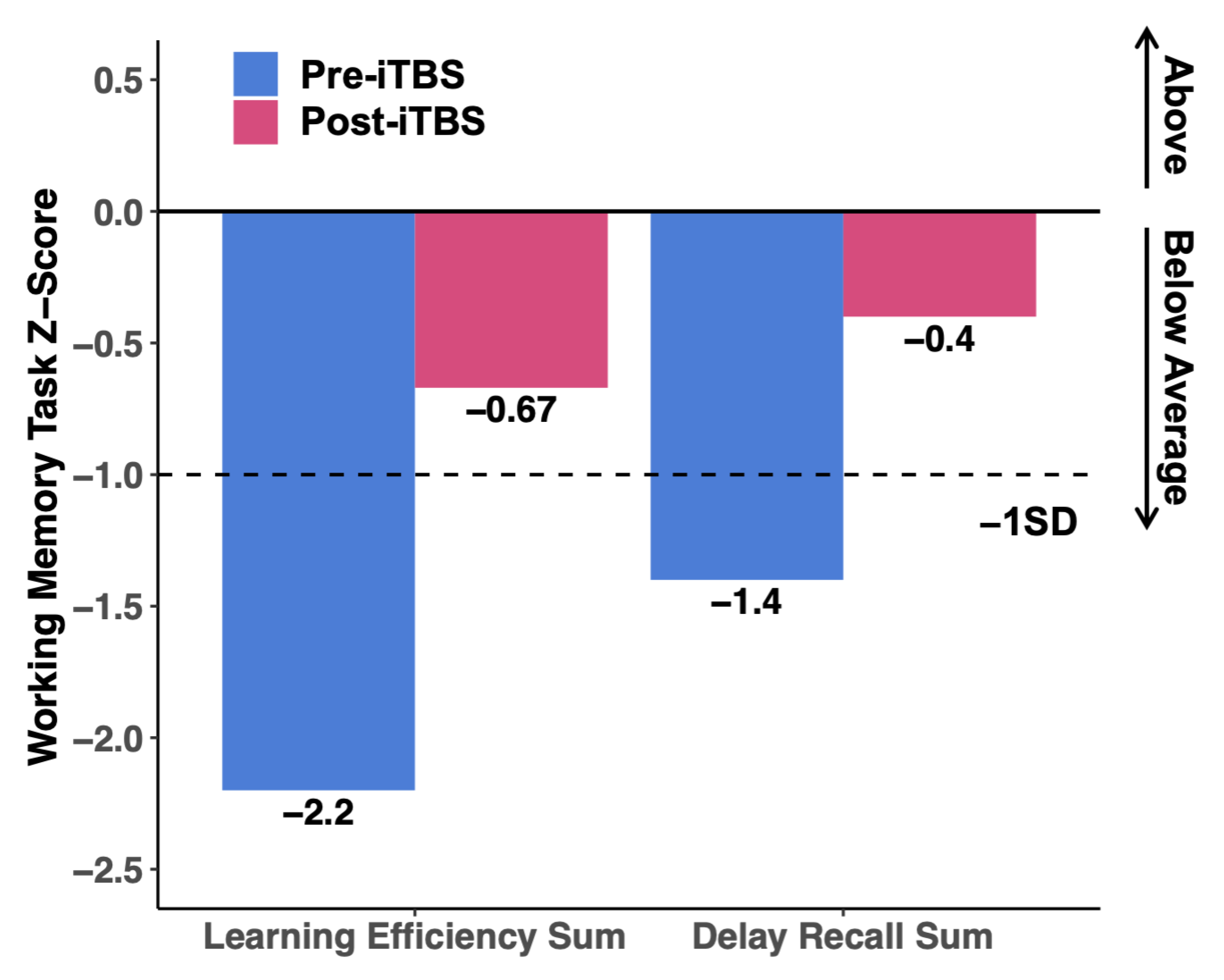
| Cognitive Domains | Tasks | Pre-iTBS * | Post-iTBS * |
|---|---|---|---|
| Working Memory | Forward Digit Span | 7 | 8 |
| Backward Digit Span | 8 | 8 | |
| Verbal Memory | RAVLT ** | 49 | 66 |
| Semantic Memory | Verbal Fluency Task | 33 | 32 |
| Cognitive Flexibility, Response Inhibition | Stroop Color-Word Interference Task | 33 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, P.H.; Chen, A.Y.-C.; Rodriguez, R.J.; Stuehm, C.; Chalasani, P.; Chen, N.-K.; Chou, Y.-H. Transcranial Magnetic Stimulation for the Treatment of Chemo Brain. Sensors 2023, 23, 8017. https://doi.org/10.3390/s23198017
Kuo PH, Chen AY-C, Rodriguez RJ, Stuehm C, Chalasani P, Chen N-K, Chou Y-H. Transcranial Magnetic Stimulation for the Treatment of Chemo Brain. Sensors. 2023; 23(19):8017. https://doi.org/10.3390/s23198017
Chicago/Turabian StyleKuo, Phillip H., Allison Yu-Chin Chen, Rudolph J. Rodriguez, Carol Stuehm, Pavani Chalasani, Nan-Kuei Chen, and Ying-Hui Chou. 2023. "Transcranial Magnetic Stimulation for the Treatment of Chemo Brain" Sensors 23, no. 19: 8017. https://doi.org/10.3390/s23198017





