Electrical Properties of Taste Sensors with Positively Charged Lipid Membranes Composed of Amines and Ammonium Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Solutions
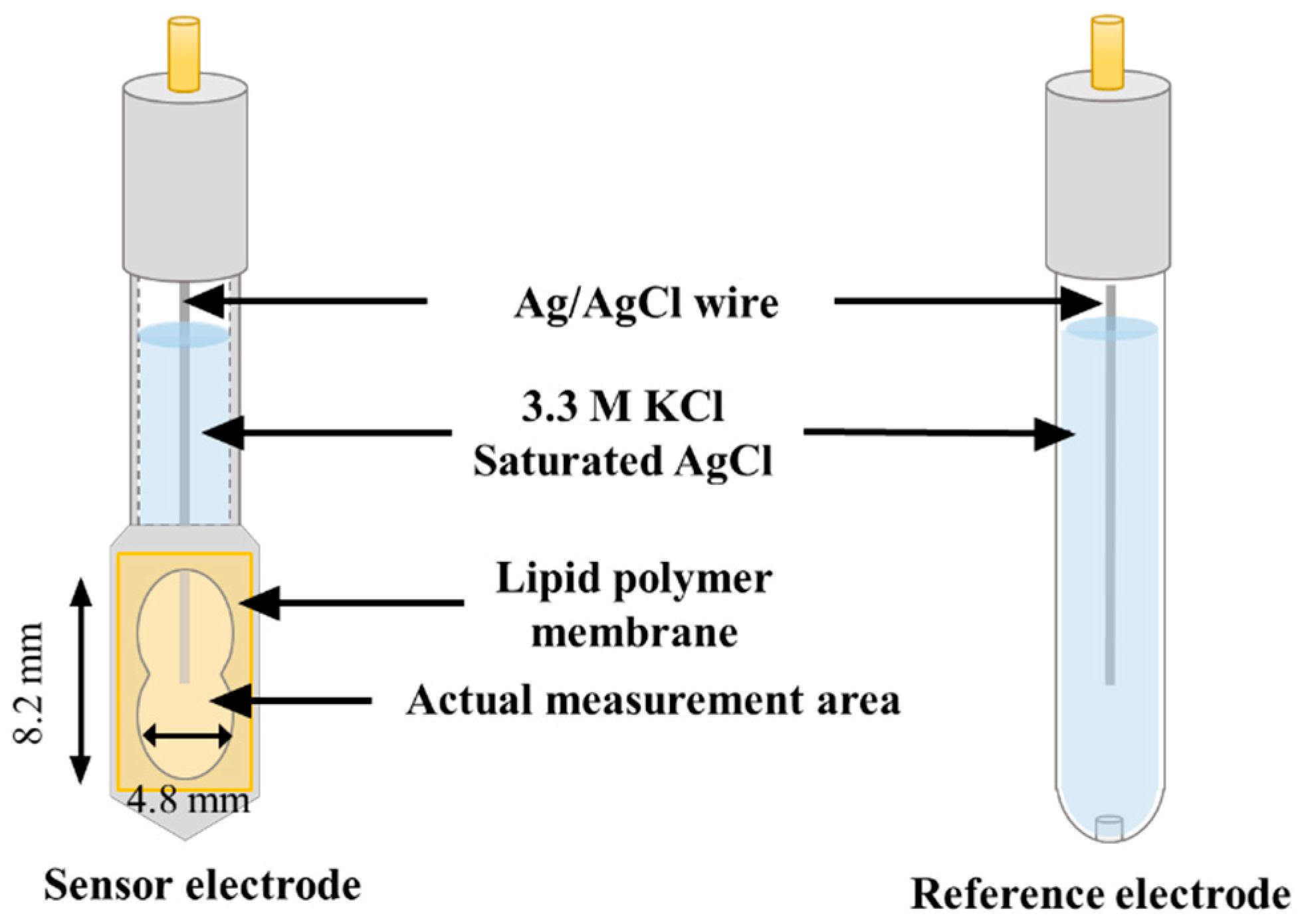
2.3. Formation of Lipid Polymer Membranes and Fabrication of Sensor Electrodes
2.4. Apparatus
2.5. Measuring Procedure of Electrochemical Potentials of Sensors and Calculation of Relative Values
2.6. Calculation of Selectivity Coefficient
2.7. Measurement of Membrane Resistance
3. Results and Discussion
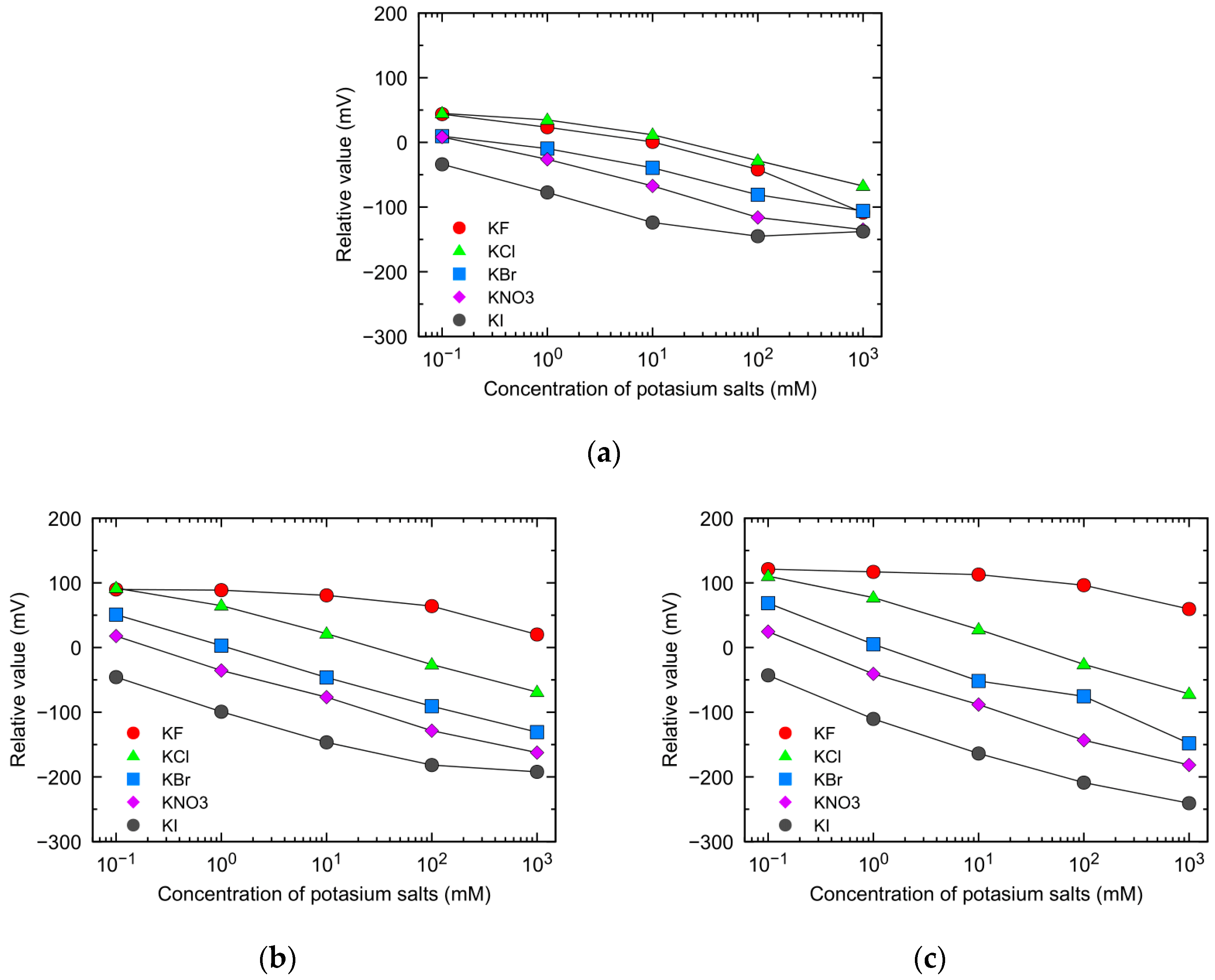
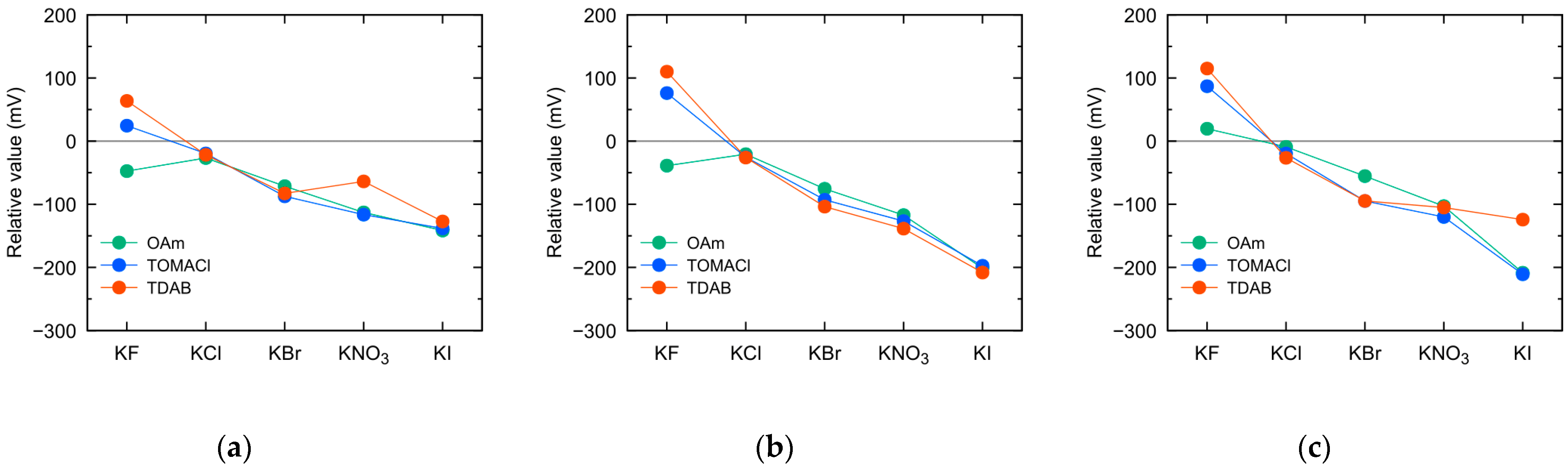
3.1. Sensor Response and Selectivity Coefficients to Monovalent Anions Using Different Positively Charged Lipid Membranes
3.2. Changes in Monovalent Anion Selectivity with Different Lipid Concentrations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A Comparative Study on Two Electronic Tongues for Pharmaceutical Formulation Development. J. Pharm. Biomed. Anal. 2011, 55, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.; Kataria, M.; Kukkar, V.; Saharan, V.; Choudhury, P.K. The Latest Trends in the Taste Assessment of Pharmaceuticals. Drug Discov. Today 2007, 12, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Riul, A., Jr.; Dantas, C.; Miyazaki, C.; Oliveira, O. Recent Advances in Electronic Tongues. Analyst 2010, 135, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Toko, K. Electronic tongues–A review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Toko, K. Research and Development of Taste Sensors as a Novel Analytical Tool. Proc. Jpn. Acad. Ser. B 2023, 99, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, F.; Ienaga, T.; Wu, X.; Tahara, Y.; Ikezaki, H.; Sano, H.; Muto, Y.; Kaneda, Y.; Toko, K. Development of a sensor with a lipid/polymer membrane comprising Na+ ion ionophores to evaluate the saltiness enhancement effect. Sensors 2019, 19, 5251. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Watanabe, K.; Watanabe, T.; Kimura, S.; Toko, K. Development and Optimization of a Highly Sensitive Sensor to Quinine-Based Saltiness Enhancement Effect. Sensors 2023, 23, 3178. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Jing, Y.; Ikezaki, H.; Toko, K. Electrical properties of two types of membrane component used in taste sensors. Sensors 2021, 21, 8343. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shiino, T.; Tahara, Y.; Ikezaki, H.; Toko, K. Quantification of pharmaceutical bitterness using a membrane electrode based on a hydrophobic tetrakis[3,5-bis(trifluoromethyl)phenyl] borate. Chemosensors 2021, 9, 28. [Google Scholar] [CrossRef]
- Noda, H.; Makuda, T. Effect of the nitrate ion contained in brassica rapa var. perviridis and spinacia oleracea on their taste values obtained by taste sensor. Stud. Sci. Technol. 2015, 4, 177–181. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, Y.; Tahara, Y.; Habara, M.; Ikezaki, H.; Toko, K. Reusability enhancement of taste sensor using lipid polymer membranes by surfactant cleaning treatment. IEEE Sens. J. 2020, 20, 4579–4586. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ionselective electrodes. Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Kamo, N.; Oikawa, M.; Kobatake, Y. Effective fixed charge density governing membrane phenomena. V. A reduced expression of permselectivity. J. Phys. Chem. 1973, 77, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Jyo, A.; Torikai, M.; Ishibashi, N. The Selectivity Evaluation of an Ion-Selective Electrode with a Liquid Membrane by Electrode Response to a Foreign Ion. Bull. Chem. Soc. Jpn. 1974, 47, 2862–2868. [Google Scholar] [CrossRef]
- Helfferich, F. Equilibria. In Ion Exchange; Dover Publications: Mineola, NY, USA, 1995; p. 624. [Google Scholar]
- Wegmann, D.; Weiss, H.; Ammann, D.; Morf, W.E.; Pretsch, E.; Sugahara, K.; Simon, W. Anion-Selective Liquid Membrane Electrodes Based on Lipophilic Quaternary Ammonium Compounds. Microchim. Acta 1984, 84, 1–16. [Google Scholar] [CrossRef]
- Goddard, E.D.; Kao, O.; Kung, H.C. Counterion Effects in Charged Monolayers. J. Colloid Interface Sci. 1968, 27, 616–624. [Google Scholar] [CrossRef]
- Del Bene, J.E. Proton Affinities of Ammonia, Water, and Hydrogen Fluoride and Their Anions: A Quest for the Basis-Set Limit Using the Dunning Augmented Correlation-Consistent Basis Sets. J. Phys. Chem. 1993, 97, 107–110. [Google Scholar] [CrossRef]
- Toko, K.; Hara, D.; Tahara, Y.; Yasuura, M.; Ikezaki, H. Relationship between the Amount of Bitter Substances Adsorbed onto Lipid/Polymer Membrane and the Electric Response of Taste Sensors. Sensors 2014, 14, 16274–16286. [Google Scholar] [CrossRef] [PubMed]
- Kobatake, Y.; Kamo, N. Transport processes in charged membranes. In Progress in Polymer Science Japan; Imahori, K., Murahashi, S., Eds.; Kodansha LTD: Tokyo, Japan, 1973; pp. 257–302. [Google Scholar]



| Membrane Component | ||||
|---|---|---|---|---|
| OAm (10 mM) | TOMACl (10 mM) | TDAB (10 mM) | ||
| Vr (mV) | 48.0 | 74.5 | 115.8 | |
| Selectivity Coefficients ) | F− | −0.61 | −7.01 | −10.64 |
| Cl− | −1.34 | −1.52 | −1.61 | |
| Br− | 0 | 0 | 0 | |
| NO3− | 0.74 | 0.68 | 0.66 | |
| I− | 2.33 | 2.08 | 2.06 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, K.; Watanabe, T.; Kimura, S.; Ikezaki, H.; Toko, K. Electrical Properties of Taste Sensors with Positively Charged Lipid Membranes Composed of Amines and Ammonium Salts. Sensors 2023, 23, 8145. https://doi.org/10.3390/s23198145
Watanabe K, Watanabe T, Kimura S, Ikezaki H, Toko K. Electrical Properties of Taste Sensors with Positively Charged Lipid Membranes Composed of Amines and Ammonium Salts. Sensors. 2023; 23(19):8145. https://doi.org/10.3390/s23198145
Chicago/Turabian StyleWatanabe, Kentaro, Tatsukichi Watanabe, Shunsuke Kimura, Hidekazu Ikezaki, and Kiyoshi Toko. 2023. "Electrical Properties of Taste Sensors with Positively Charged Lipid Membranes Composed of Amines and Ammonium Salts" Sensors 23, no. 19: 8145. https://doi.org/10.3390/s23198145






