Torque Regulation Is Influenced by the Nature of the Isometric Contraction
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Design and Protocol
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Reliability Analysis
3.2. Mean Torque
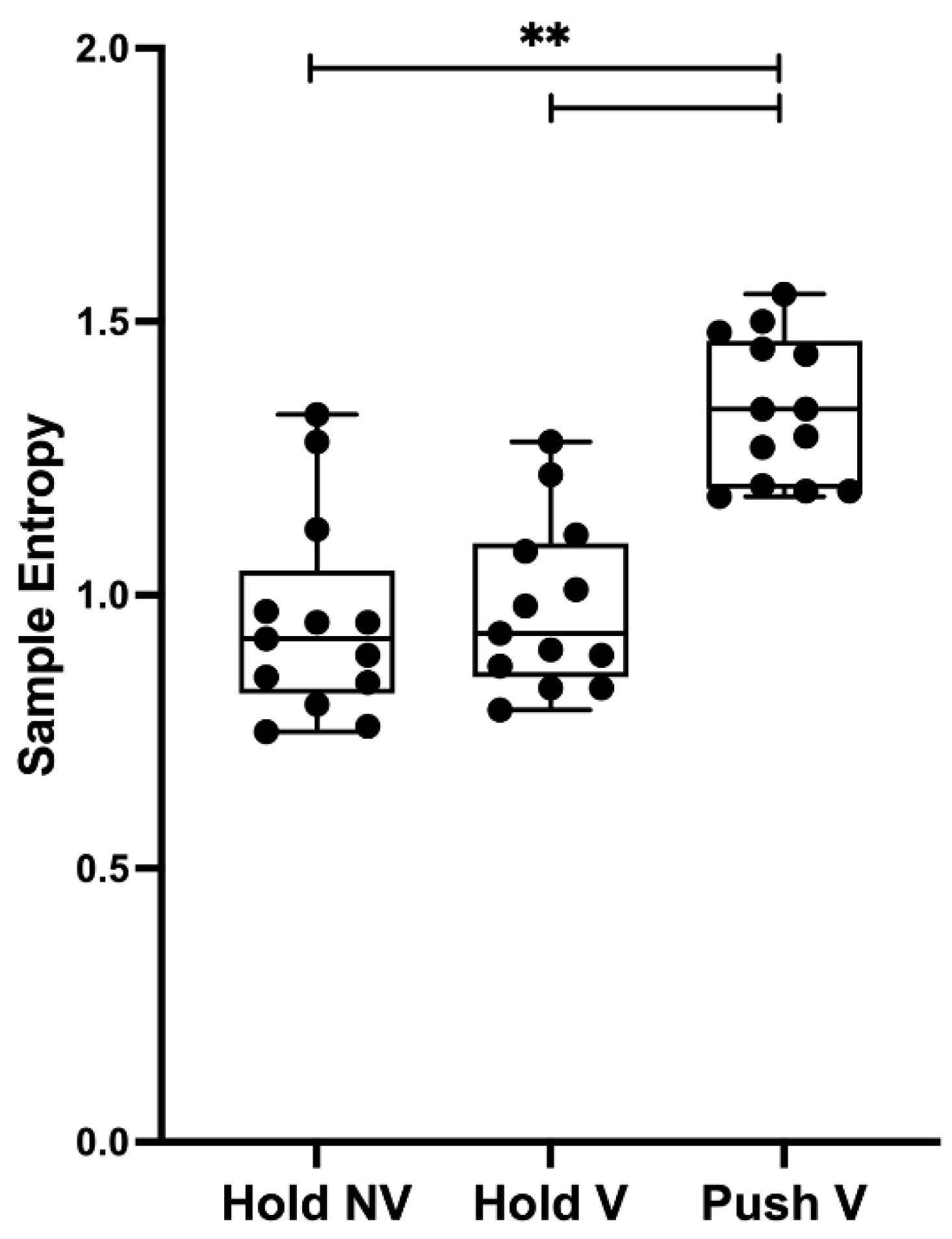
3.3. Torque’s Sample Entropy (SampEn)
3.4. Torque’s Coefficient of Variation (CV)
3.5. Mean Knee Joint Angle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, X.; Loncharich, M.; Newell, K.M. Visual information interacts with neuromuscular factors in the coordination of bimanual isometric force. Exp. Brain Res. 2011, 209, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Tracy, B.L.; Dienenno, D.V.; Jorgensen, B.; Welsh, S.J. Aging, visuomotor correction, and force fluctuations in large muscles. Med. Sci. Sport. Exerc. 2007, 39, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, L.; Yang, S.N.; Wang, H.; Yao, J.; Dai, Q.; Chang, S. Contributions of visuo-oculomotor abilities to interceptive skills in sports. Optom. Vis. Sci. 2015, 92, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Ellmers, T.J.; Cocks, A.J.; Kal, E.C.; Young, W.R. Conscious movement processing, fall-related anxiety, and the visuomotor control of locomotion in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2020, 75, 1911–1920. [Google Scholar] [CrossRef]
- Baweja, H.S.; Patel, B.K.; Neto, O.P.; Christou, E.A. The interaction of respiration and visual feedback on the control of force and neural activation of the agonist muscle. Hum. Mov. Sci. 2011, 30, 1022–1038. [Google Scholar] [CrossRef] [Green Version]
- Davis, N.J. Memory and coordination in bimanual isometric finger force production. Exp. Brain Res. 2007, 182, 137–142. [Google Scholar] [CrossRef]
- Baweija, H.S.; Patel, B.K.; Martinkewiz, J.D.; Vu, J.; Christou, E.A. Removal of visual feedback alters muscle activity and reduces force variability during constant isometric contractions. Exp. Brain Res. 2009, 197, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Newell, K.M. Adaptation to bimanual asymmetric weights in isometric force coordination. Neurosci. Lett. 2011, 490, 121–125. [Google Scholar] [CrossRef]
- Prodoehl, J.; Corcos, D.M.; Vaillancourt, D.E. Effects of focal hand dystonia on visually guided and internally guided force control. J. Neurol. Neurosurg. Psychiatry 2006, 77, 909–914. [Google Scholar] [CrossRef]
- Hong, L.S.; Brown, A.J.; Newell, K.M. Compensatory properties of visual information in the control of isometric force. Percept. Pyschophys. 2008, 70, 306–313. [Google Scholar] [CrossRef]
- Kuznetsov, N.A.; Riley, M.A. Spatial resolution of visual feedback affects variability and structure of isometric force. Neurosci. Lett. 2010, 470, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Riemann, B.; Lephart, S. The sensorimotor system, part I: The physiologic basis of functional joint stability. J. Athl. Train. 2002, 37, 71–79. [Google Scholar] [PubMed]
- Enoka, R.M.; Farina, D. Force Steadiness: From Motor Units to Voluntary Actions. Physiology 2021, 36, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Bittmann, F.N. Are there two forms of isometric muscle action? Results of the experimental study support a distinction between a holding and a pushing isometric muscle function. BMC Sport. Sci. Med. Rehabil. 2017, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Hunter, S.K.; Ryan, D.L.; Ortega, J.D.; Enoka, R.M. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J. Neurophysiol. 2002, 88, 3087–3096. [Google Scholar] [CrossRef]
- Rudroff, T.; Christou, E.A.; Poston, B.; Bojsen-Møller, J.; Enoka, R.M. Time to failure of a sustained contraction is predicted by target torque and initial electromyographic bursts in elbow flexor muscles. Muscle Nerve 2007, 35, 657–666. [Google Scholar] [CrossRef]
- Rudroff, T.; Justice, J.N.; Matthews, S.; Zuo, R.; Enoka, R.M. Muscle activity differs with load compliance during fatiguing contractions with the knee extensor muscles. Exp. Brain Res. 2010, 203, 307–316. [Google Scholar] [CrossRef]
- Pethick, J.; Winter, S.L.; Burnley, M. Loss of knee extensor torque complexity during fatiguing isometric muscle contractions occurs exclusively above the critical torque. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1144–R1153. [Google Scholar] [CrossRef] [Green Version]
- Pethick, J.; Winter, S.L.; Burnley, M. Effects of ipsilateral and contralateral fatigue and muscle blood flow occlusion on the complexity of knee-extensor torque output in humans. Exp. Physiol. 2018, 103, 956–967. [Google Scholar] [CrossRef] [Green Version]
- Fiogbé, E.; Vassimon-Barroso, V.; Catai, A.M.; de Melo, R.C.; Quitério, R.J.; Porta, A.; de Medeiros Takahashi, A.C. Complexity of knee extensor torque: Effect of aging and contraction intensity. J. Strength Cond. Res. 2018, 35, 1050–1057. [Google Scholar] [CrossRef]
- Forrest, S.M.; Challis, J.H.; Winter, S.L. The effect of signal acquisition and processing choices on ApEn values: Towards a “gold standard” for distinguishing effort levels from isometric force records. Med. Eng. Phys. 2014, 36, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Slifkin, A.B.; Newell, K.M. Noise, information transmission, and force variability. J. Exp. Psychol. Hum. Percept. Perform. 1999, 25, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.E.; Newell, K.M. Aging and the time and frequency structure of force output variability. J. Appl. Physiol. 2003, 94, 903–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaillancourt, D.E.; Slifkin, A.B.; Newell, K.M. Regularity of force tremor in Parkinson’s disease. Clin. Neurophysiol. 2001, 112, 1594–1603. [Google Scholar] [CrossRef]
- Pethick, J.; Winter, S.L.; Burnley, M. Fatigue reduces the complexity of knee extensor torque fluctuations during maximal and submaximal intermittent isometric contractions in man. J. Physiol. 2015, 593, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.M.; Luchies, C.W.; Piscitelle, L.; Hasselquist, L.; Gregorczyk, K.N. Discrete bandwidth visual feedback increases structure of output as compared to continuous visual feedback in isometric force control tasks. Clin. Biomech. 2006, 21, 1042–1050. [Google Scholar] [CrossRef]
- Chow, J.W.; Stokic, D.S. Improvements in force variability and structure from vision-to memory-guided submaximal isometric knee extension in subacute stroke. J. Appl. Physiol. 2018, 124, 592–603. [Google Scholar] [CrossRef]
- Li, K.; Wei, N. Fingertip force variability on the left and right hand during low-level sustained precision pinch. In Proceedings of the IEEE 2014 7th International Conference on Biomedical Engineering and Informatics, Dalian, China, 14–16 October 2014; pp. 302–306. [Google Scholar]
- Skurvydas, A.; Masiulis, N.; Gudas, R.; Dargevičiūtė, G.; Parulytė, D.; Trumpickas, V.; Kalesinskas, J.R. Extension and flexion torque variability in ACL deficiency. Knee Surg. Sport. Traumatol. Arthrosc. 2011, 19, 1307–1313. [Google Scholar] [CrossRef]
- Stergiou, N. Innovative Analyses of Human Movement; Human Kinetics: Champaign, IL, USA, 2003; ISBN 9780736044677. [Google Scholar]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. American journal of physiology. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [Green Version]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef]
- Cavanaugh, J.T.; Guskiewicz, K.M.; Stergiou, N. A nonlinear dynamic approach for evaluating postural control: New directions for the management of sport-related cerebral concussion. Sport. Med. 2005, 35, 935–950. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi (Version 1.6). 2021. Available online: https://www.jamovi.org (accessed on 10 September 2022).
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pincivero, D.M.; Salfetnikov, Y.; Campy, R.M.; Coelho, A.J. Angle-and gender-specific quadriceps femoris muscle recruitment and knee extensor torque. J. Biomech. 2004, 37, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Pethick, J.; Winter, S.L.; Burnley, M. Fatigue-induced changes in knee-extensor torque complexity and muscle metabolic rate are dependent on joint angle. Eur. J. Appl. Physiol. 2021, 121, 3117–3131. [Google Scholar] [CrossRef] [PubMed]
- Grabiner, M.D.; Owings, T.M. EMG differences between concentric and eccentric maximum voluntary contractions are evident prior to movement onset. Exp. Brain Res. 2022, 145, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Westing, S.H.; Cresswell, A.G.; Thorstensson, A. Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 62, 104–108. [Google Scholar] [CrossRef]
- Grabiner, M.D.; Owings, T.M.; George, M.R.; Enoka, R.M. Eccentric Contractions are Specified a Priori by the CNS. In Proceedings of the 50th Congress of the International Society of Biomechanics, Jyvaskyla, Finland, 2–6 July 1995; pp. 338–339. [Google Scholar]
- Hernandez, L.R.; Camic, C.L. Fatigue-Mediated Loss of Complexity is Contraction-Type Dependent in Vastus Lateralis Electromyographic Signals. Sports 2019, 7, 78. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, P.; Gomes, J.S.; Oliveira, J.; Santos, P.; Pezarat-Correia, P.; Vaz, J.R. Torque Regulation Is Influenced by the Nature of the Isometric Contraction. Sensors 2023, 23, 726. https://doi.org/10.3390/s23020726
Bauer P, Gomes JS, Oliveira J, Santos P, Pezarat-Correia P, Vaz JR. Torque Regulation Is Influenced by the Nature of the Isometric Contraction. Sensors. 2023; 23(2):726. https://doi.org/10.3390/s23020726
Chicago/Turabian StyleBauer, Philipp, João Sá Gomes, João Oliveira, Paulo Santos, Pedro Pezarat-Correia, and João R. Vaz. 2023. "Torque Regulation Is Influenced by the Nature of the Isometric Contraction" Sensors 23, no. 2: 726. https://doi.org/10.3390/s23020726






