Peptide-Functionalized Carbon Nanotube Chemiresistors: The Effect of Nanotube Density on Gas Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemiresistor Platform Fabrication
2.3. Peptide-Functionalized CNT Solution and Assembly
2.4. Gas Exposure Setup and Measurement Protocols
3. Results and Discussion
3.1. Assembly of the Nanotube with Diverse Densities on the Chemiresistors
3.2. Characterization of Chemiresistor Signals
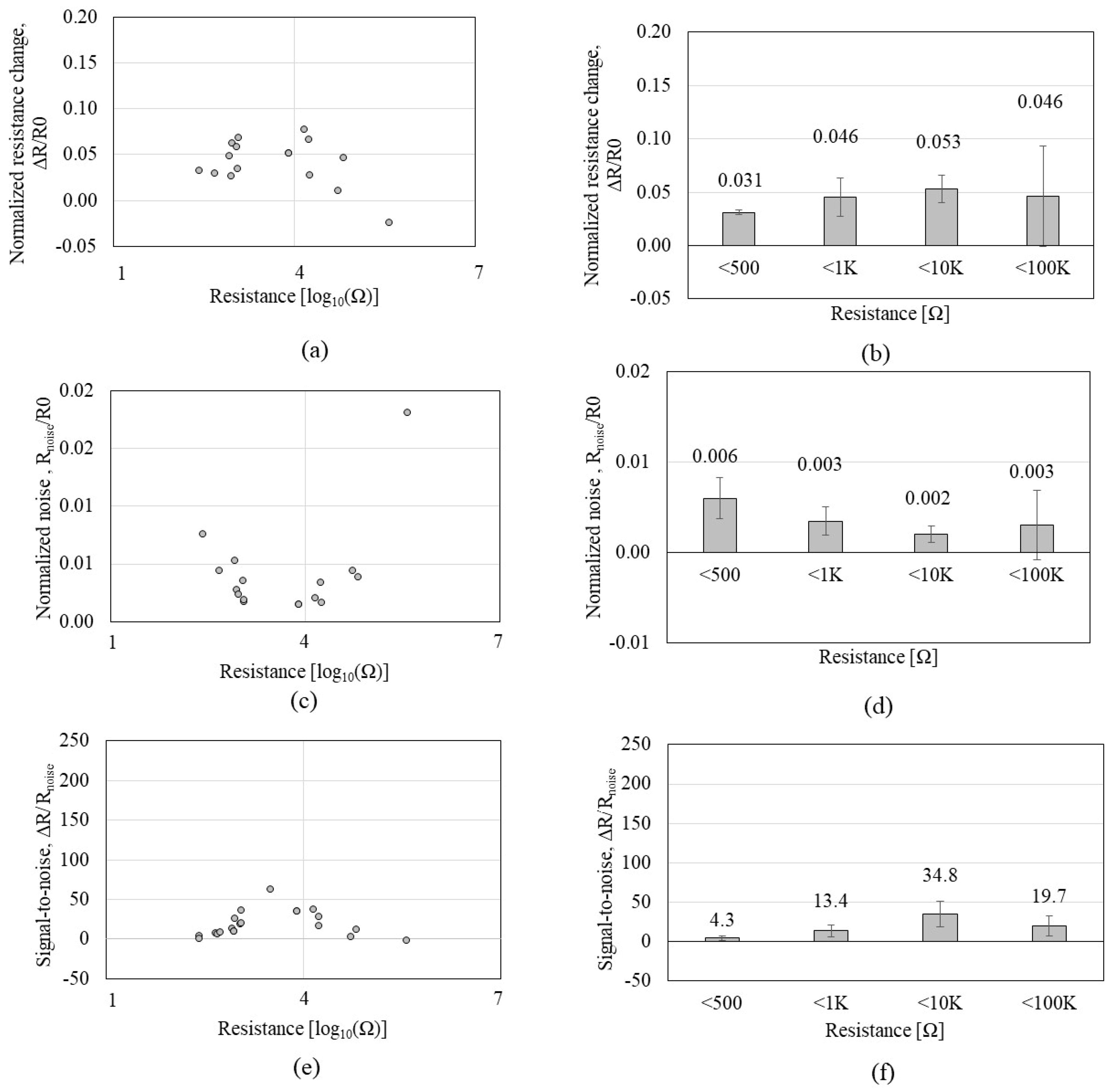
3.3. Effect of Nanotube Density on IPA Sensing of CCR1s
3.4. Effect of Nanotube Density on IPA Sensing of CCR2s
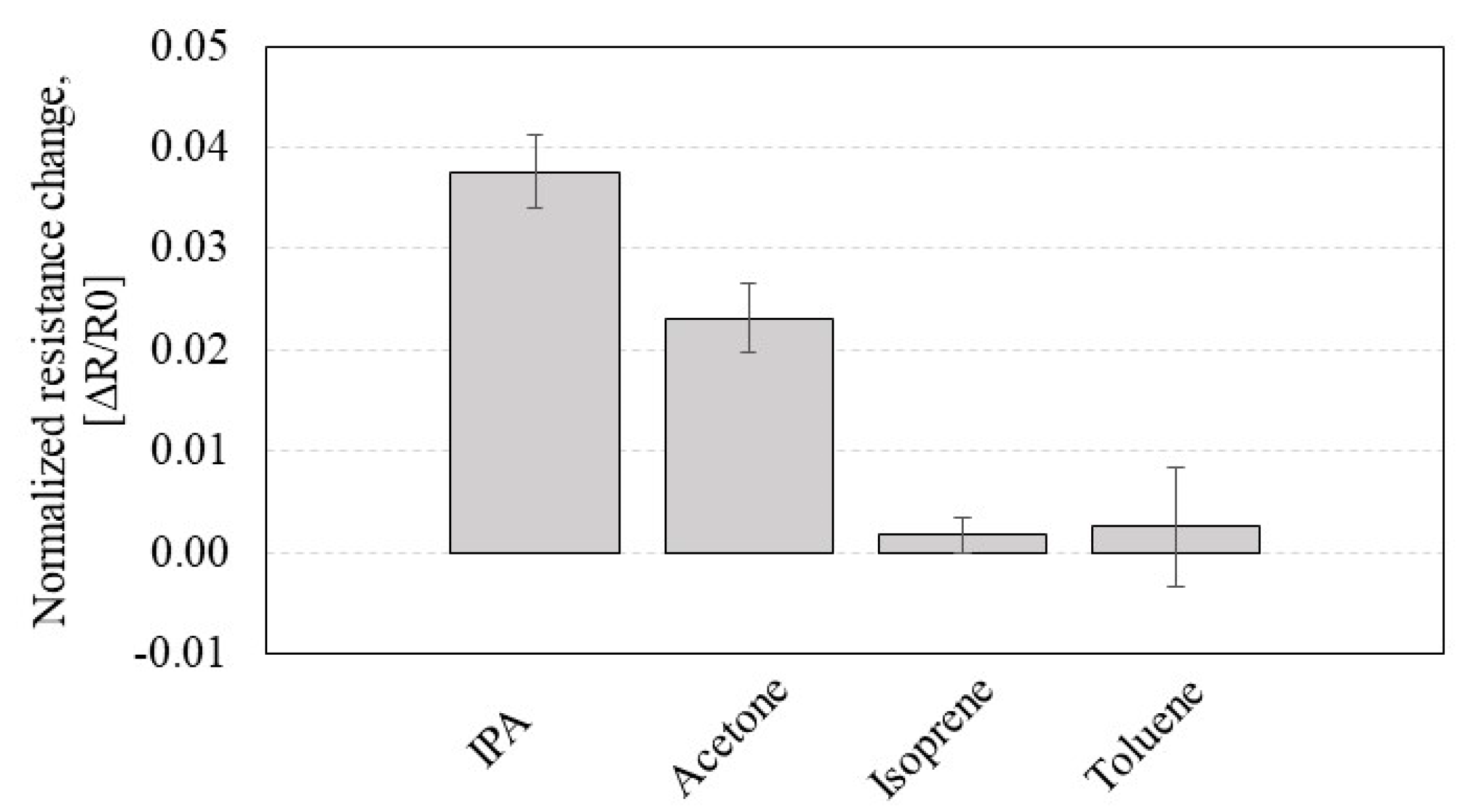
3.5. Effect of Nanotube Density on Acetone Sensing of CCR2
3.6. Effect of Nanotube Density on IPA Sensing of Bare CNT Chemiresistors
3.7. SEM Investigations for Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tricoli, A.; Nasiri, N.; De, S. Wearable and Miniaturized Sensor Technologies for Personalized and Preventive Medicine. Adv. Sci. News 2017, 27, 1605271. [Google Scholar] [CrossRef]
- Kyoung, N.; Sun, T.; La, D.; Hoon, J.; Woo, Y.; Shin, Y. Chemical Evaluation of the limit-of-detection capability of carbon black-polymer composite sensors for volatile breath biomarkers. Sens. Actuators B Chem. 2010, 147, 55–60. [Google Scholar] [CrossRef]
- Park, C.H.; Schroeder, V.; Kim, B.J.; Swager, T.M. Ionic Liquid-Carbon Nanotube Sensor Arrays for Human Breath Related Volatile Organic Compounds. ACS Sens. 2018, 3, 2432–2437. [Google Scholar] [CrossRef]
- Ju, S.; Lee, K.-Y.; Min, S.-J.; Yoo, Y.K.; Hwang, K.S.; Kim, S.K.; Yi, H. Single-carbon discrimination by selected peptides for individual detection of volatile organic compounds. Sci. Rep. 2015, 5, 9196. [Google Scholar] [CrossRef] [PubMed]
- Kolk, A.H.J.; Van Berkel, J.J.B.N.; Claassens, M.M.; Walters, E.; Kuijper, S.; Dallinga, J.W.; Van Schooten, F.J. Breath analysis as a potential diagnostic tool for tuberculosis. Int. J. Tuberc. Lung Dis. 2012, 16, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Fens, N.; van der Schee, M.P.; Brinkman, P.; Sterk, P.J. Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin. Exp. Allergy 2013, 43, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Harshman, S.W.; Geier, B.A.; Fan, M.; Rinehardt, S.; Watts, B.S.; Drummond, L.A.; Preti, G.; Phillips, J.B.; Ott, D.K.; Grigsby, C.C. The identification of hypoxia biomarkers from exhaled breath under normobaric conditions. J. Breath Res. 2015, 9, 47103. [Google Scholar] [CrossRef]
- Pyo, S.; Lee, K.; Noh, T.; Jo, E.; Kim, J. Sensitivity enhancement in photoionization detector using microelectrodes with integrated 1D nanostructures. Sens. Actuators B Chem. 2019, 288, 618–624. [Google Scholar] [CrossRef]
- Peng, F.M.; Xie, P.H.; Shi, Y.G.; Wang, J.D.; Liu, W.Q.; Li, H.Y. Photoionization detector for portable rapid GC. Chromatographia 2007, 65, 331–336. [Google Scholar] [CrossRef]
- Ngo, Y.H.; Brothers, M.; Martin, J.A.; Grigsby, C.C.; Fullerton, K.; Naik, R.R.; Kim, S.S. Chemically Enhanced Polymer-Coated Carbon Nanotube Electronic Gas Sensor for Isopropyl Alcohol Detection. ACS Omega 2018, 3, 6230–6236. [Google Scholar] [CrossRef]
- Akbar, M.; Shakeel, H.; Agah, M. GC-on-chip: Integrated column and photoionization detector. Lab Chip 2015, 15, 1748–1758. [Google Scholar] [CrossRef]
- Martin, J.A.; Chushak, Y.; Chávez, J.L.; Hagen, J.A.; Kelley-loughnane, N. Microarrays as Model Biosensor Platforms to Investigate the Structure and Affinity of Aptamers. J. Nucleic Acids 2016, 2016, 9718612. [Google Scholar] [CrossRef]
- Hagen, J.; Lyon, W.; Chushak, Y.; Tomczak, M.; Naik, R.; Stone, M.; Kelley-loughnane, N. Detection of Orexin A Neuropeptide in Biological Fluids Using a Zinc Oxide Field E ff ect Transistor. ACS Chem. Neurosci. 2013, 4, 444–453. [Google Scholar] [CrossRef]
- Xiao, X.; Kuang, Z.; Slocik, J.M.; Tadepalli, S.; Brothers, M.; Kim, S.; Mirau, P.A.; Butkus, C.; Farmer, B.L.; Singamaneni, S.; et al. Advancing Peptide-Based Biorecognition Elements for Biosensors Using in-Silico Evolution. ACS Sens. 2018, 3, 1024–1031. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Yang, K.A.; Abendroth, J.M.; Cheung, K.M.; Xu, X.; Yang, H.; Zhao, C.; Zhu, B.; Rim, Y.S.; Yang, Y.; et al. Aptamer-field-effect transistors overcome Debye length limitations for small-molecule sensing. Science 2018, 362, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.A.; Halpern, J.M. Guide to Selecting a Biorecognition Element for Biosensors. Bioconjug. Chem. 2018, 29, 3231–3239. [Google Scholar] [CrossRef]
- Chambers, J.P.; Arulanandam, B.P.; Matta, L.L.; Weis, A.; Valdes, J.J. Biosensor Recognition Elements. Curr. Issues Mol. Biol. 2002, 10, 1–12. [Google Scholar]
- You, A.; Be, M.A.Y.; In, I. Selective gas detection using a carbon nanotube sensor. Appl. Phys. Lett. 2010, 2280, 9–12. [Google Scholar] [CrossRef]
- Liu, J.; Casavant, M.J.; Cox, M.; Walters, D.A.; Boul, P.; Lu, W.; Rimberg, A.J.; Smith, K.A.; Colbert, D.T.; Smalley, R.E. Controlled deposition of individual single-walled carbon nanotubes on chemically functionalized templates. Chem. Phys. Lett. 1999, 303, 125–129. [Google Scholar] [CrossRef]
- Allen, B.L.; Kichambare, P.D.; Star, A. Field-Effect-Transistor-Based Biosensors. Adv. Mater. 2007, 19, 1439–1451. [Google Scholar] [CrossRef]
- Kuang, Z.; Kim, S.N.; Crookes-goodson, W.J.; Farmer, B.L.; Naik, R.R. Biomimetic Chemosensor: Designing Peptide Recognition Elements for Surface Functionalization of Carbon Nanotube Field Effect Transistors. ACS Nano 2010, 4, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.T.; Tue, P.T.; Thi, T.; Lien, N.; Ohno, Y. Peptide aptamer-modified single- walled carbon nanotube-based transistors for high-performance biosensors. Sci. Rep. 2017, 7, 17881. [Google Scholar] [CrossRef] [PubMed]
- Meskher, H.; Ragdi, T.; Thakur, A.K.; Ha, S.; Khelfaoui, I.; Sathyamurthy, R.; Sharshir, S.W.; Pandey, A.K.; Saidur, R.; Singh, P.; et al. A Review on CNTs-Based Electrochemical Sensors and Biosensors: Unique Properties and Potential Applications. Crit. Rev. Anal. Chem. 2023, 1–24. [Google Scholar] [CrossRef]
- Machín, A.; Cotto, M.; Duconge, J.; Morant, C.; Petrescu, F.I.; Márquez, F. Sensitive and Reversible Ammonia Gas Sensor Based on Single-Walled Carbon Nanotubes. Chemosensors 2023, 11, 247. [Google Scholar] [CrossRef]
- Dai, B.; Zhou, R.; Ping, J.; Ying, Y.; Xie, L. Recent advances in carbon nanotube-based biosensors for biomolecular detection. TrAC Trends Anal. Chem. 2022, 154, 116658. [Google Scholar] [CrossRef]
- Guo, S.Y.; Hou, P.X.; Zhang, F.; Liu, C.; Cheng, H.M. Gas Sensors Based on Single-Wall Carbon Nanotubes. Molecules 2022, 27, 5381. [Google Scholar] [CrossRef]
- Islam, M.R.; Khondaker, S.I. Recent progress in parallel fabrication of individual single walled carbon nanotube devices using dielectrophoresis. Mater. Express 2014, 4, 263–278. [Google Scholar] [CrossRef]
- Islam, A.E.; Rogers, J.A.; Alam, M.A. Recent Progress in Obtaining Semiconducting Single- Walled Carbon Nanotubes for Transistor Applications. Adv. Mater. 2015, 27, 7908–7937. [Google Scholar] [CrossRef]
- Ishikawa, F.N.; Curreli, M.; Olson, C.A.; Liao, H.I.; Sun, R.; Roberts, R.W.; Cote, R.J.; Thompson, M.E.; Zhou, C. Importance of controlling nanotube density for highly sensitive and reliable biosensors functional in physiological conditions. ACS Nano 2010, 4, 6914–6922. [Google Scholar] [CrossRef]
- Suehiro, J.; Zhou, G.; Imakiire, H.; Ding, W.; Hara, M. Controlled fabrication of carbon nanotube NO2 gas sensor using dielectrophoretic impedance measurement. Sens. Actuators B Chem. 2005, 108, 398–403. [Google Scholar] [CrossRef]
- Sim, D.; Huang, T.; Naik, R.R.; Kim, S.S. Influence of the Carbon Nanotube Density on Building Sensitive and Noise-Free Volatile Organic Compound Sensors. In Proceedings of the 2021 21st International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Orlando, FL, USA, 20–24 June 2021; pp. 847–850. [Google Scholar] [CrossRef]
- Sim, D.; Kuang, Z.; Sant’Anna, G.; Krabacher, R.M.; Brothers, M.C.; Chávez, J.L.; Martin, J.A.; Islam, A.E.; Maruyama, B.; Naik, R.R.; et al. Rational Design of Peptide Biorecognition Elements on Carbon Nanotubes for Sensing Volatile Organic Compounds. Adv. Mater. Interfaces 2022, 10, 2201707. [Google Scholar] [CrossRef]
- Sim, D.; Brothers, M.C.; Slocik, J.M.; Islam, A.E.; Maruyama, B.; Grigsby, C.C.; Naik, R.R.; Kim, S.S. Biomarkers and Detection Platforms for Human Health and Performance Monitoring: A Review. Adv. Sci. 2022, 9, 2104426. [Google Scholar] [CrossRef] [PubMed]
- Krabacher, R.; Kim, S.; Ngo, Y.; Slocik, J.; Harsch, C.; Naik, R. Identification of Chiral-Specific Carbon Nanotube Binding Peptides Using a Modified Biopanning Method. Chemosensors 2021, 9, 245. [Google Scholar] [CrossRef]
- Chikkadi, K.; Muoth, M.; Liu, W.; Maiwald, V.; Hierold, C. Enhanced signal-to-noise ratio in pristine, suspended carbon nanotube gas sensors. Sens. Actuators B Chem. 2014, 196, 682–690. [Google Scholar] [CrossRef]
- Shin, W.; Hong, S.; Jung, G.; Jeong, Y.; Park, J.; Kim, D.; Jang, D.; Park, B.G.; Lee, J.H. Improved signal-to-noise-ratio of FET-type gas sensors using body bias control and embedded micro-heater. Sens. Actuators B Chem. 2021, 329, 129166. [Google Scholar] [CrossRef]
- Kumar, D.; Chaturvedi, P.; Saho, P.; Jha, P.; Chouksey, A.; Lal, M.; Rawat, J.S.B.S.; Tandon, R.P.; Chaudhury, P.K. Effect of single wall carbon nanotube networks on gas sensor response and detection limit. Sens. Actuators B Chem. 2017, 240, 1134–1140. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef] [PubMed]
- Dube, I.; Jiménez, D.; Fedorov, G.; Boyd, A.; Gayduchenko, I.; Paranjape, M.; Barbara, P. Understanding the electrical response and sensing mechanism of carbon-nanotube-based gas sensors. Carbon 2015, 87, 330–337. [Google Scholar] [CrossRef]
- Niu, L.; Luo, Y.; Li, Z. A highly selective chemical gas sensor based on functionalization of multi-walled carbon nanotubes with poly (ethylene glycol). Sens. Actuators B Chem. 2007, 126, 361–367. [Google Scholar] [CrossRef]
- Salehi-Khojin, A.; Khalili-Araghi, F.; Kuroda, M.A.; Lin, K.Y.; Leburton, J.P.; Masel, R.I. On the sensing mechanism in carbon nanotube chemiresistors. ACS Nano 2011, 5, 153–158. [Google Scholar] [CrossRef]
- Boyd, A.; Dube, I.; Fedorov, G.; Barbara, P. Gas sensing mechanism of carbon nanotubes: From single tubes to high-density networks. Carbon 2013, 69, 417–423. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, B.Y.; Jaworski, J.; Yokoyama, K.; Chung, W.; Wang, E.; Hong, S.; Majumdar, A.; Lee, S.; Al, K.I.M.E.T. Selective and Sensitive TNT Sensors Using Biomimetic Polydiacetylene- Coated CNT-FETs. ACS Nano 2011, 5, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Park, T.H. Bioelectronic Nose Using Odorant Binding Protein-Derived Peptide and Carbon Nanotube Field-E ff ect Transistor for the Assessment of Salmonella Contamination in Food. Anal. Chem. 2016, 88, 11283–11287. [Google Scholar] [CrossRef]
- Kerman, K.; Morita, Y.; Takamura, Y.; Tamiya, E.; Maehashi, K.; Matsumoto, K. Peptide Nucleic Acid–Modified Carbon Nanotube Field-Effect Transistor for Ultra-Sensitive Real-Time Detection of DNA Hybridization. Nanobiotechnology 2005, 1, 65–70. [Google Scholar] [CrossRef]


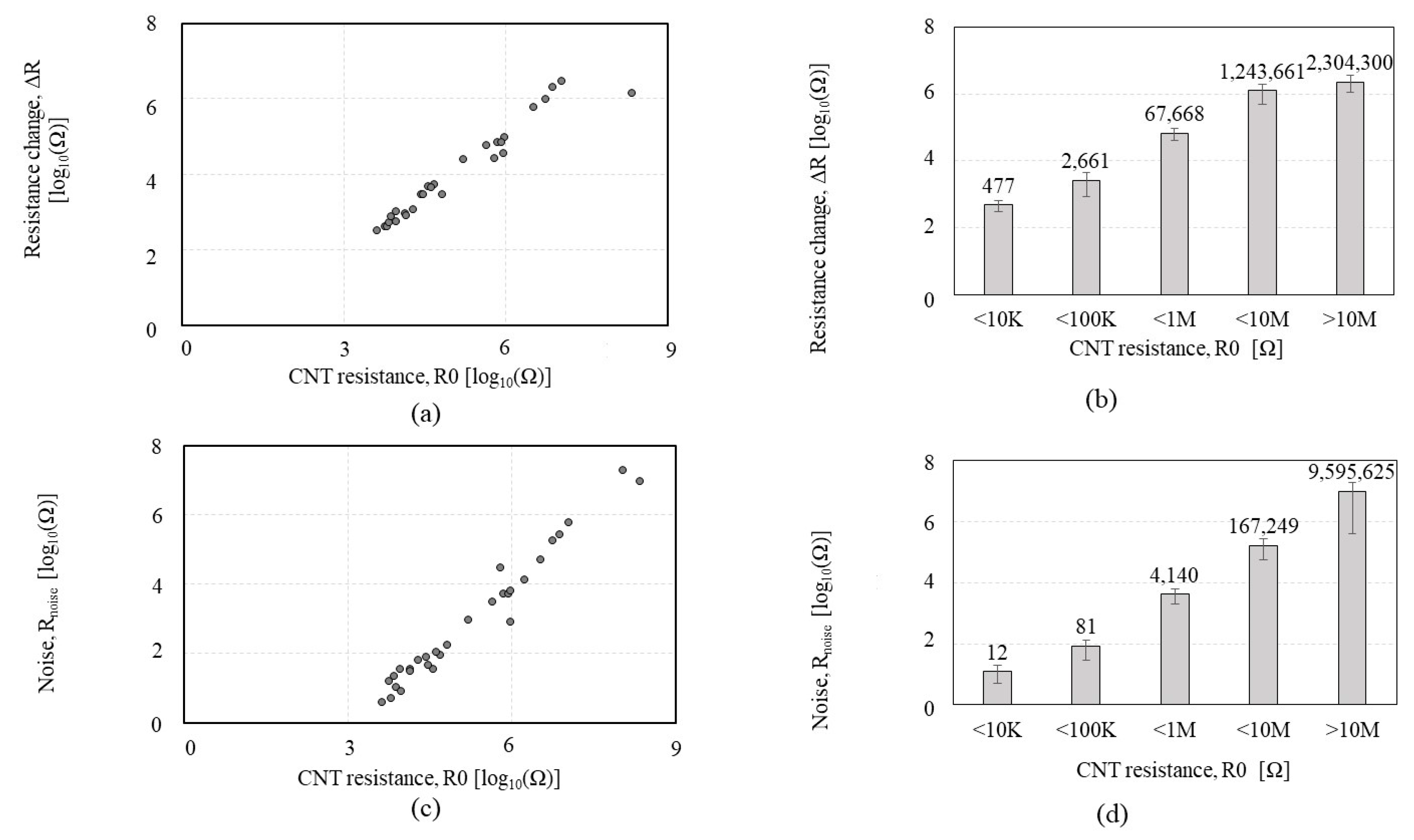



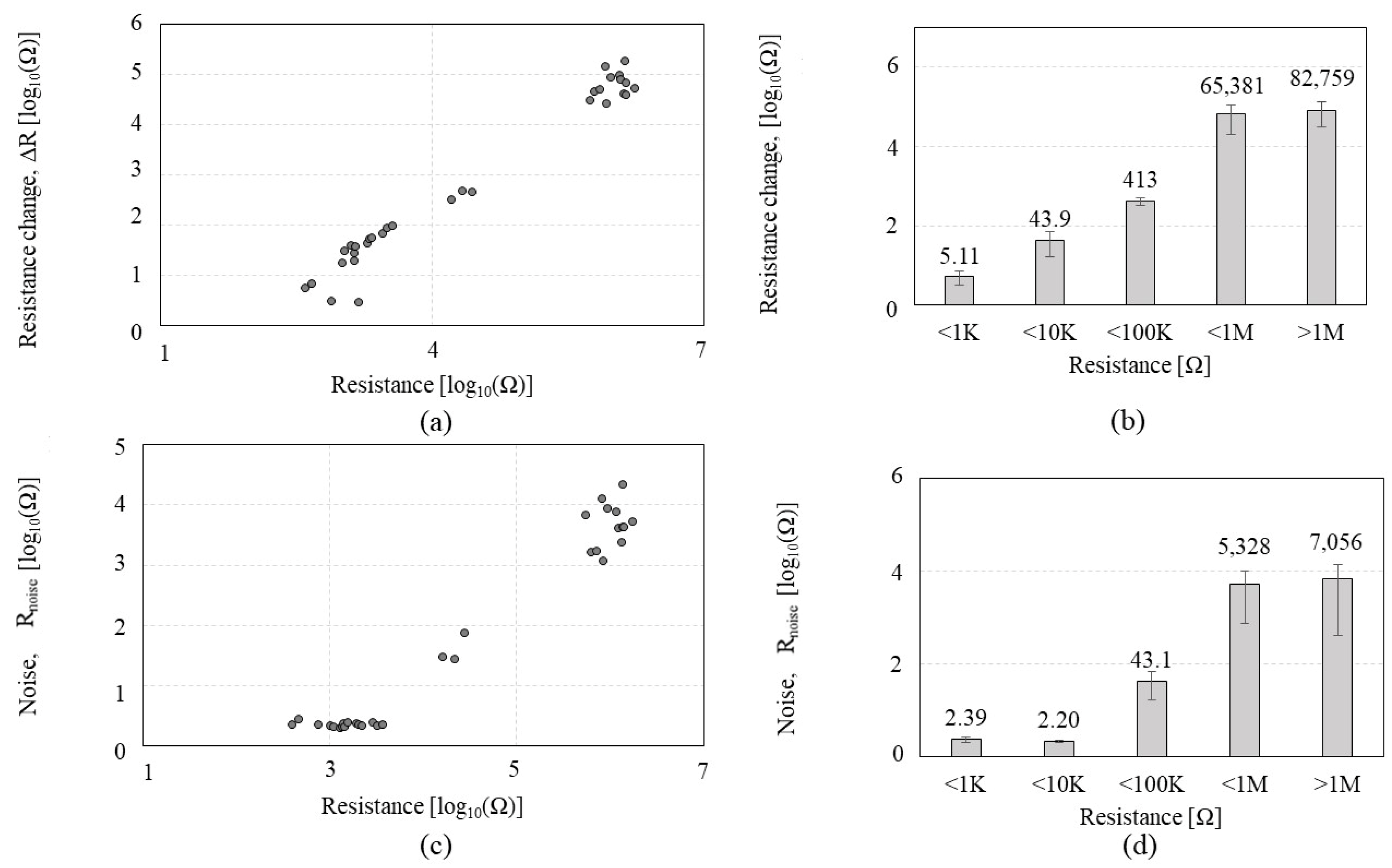



| CNT Chemiresistor-1 (CCR1) | CNT Chemiresistor-2 (CCR2) | Bare CNT Chemiresistor | ||
|---|---|---|---|---|
| Functionalized peptide sequence | FYYYLLQ | GSVQKLSATPWV | - | |
| DEP Conditions (to tune CNT resistance) |
| 0 Ω, 100 Ω, 1.2 KΩ | 0 Ω | 0 Ω |
| 1.0 mg/mL | 1.0 mg/mL, 0.5 mg/mL, 0.1 mg/mL | 1.0 mg/mL | |
| 60 s | 0–60 s | 0–60 s | |
| 6 Vp-p, 10 MHz | |||
| DEP results |
| 37 (out of 51) | 45 (out of 56) | 21 (out of 24) |
| 3.43 KΩ–233 MΩ | 0.48 KΩ–82.5 MΩ | 0.26–372 KΩ | |
| CNT Resistance Range (Ω) | <500 Ω | <1 KΩ | <10 KΩ | <100 KΩ | <1 MΩ | >1 MΩ |
|---|---|---|---|---|---|---|
| SNR Standard deviation (Ω) | 3.40 | 16.89 | 38.41 | 59.75 | 82.05 | 17.63 |
| SNR CV (%) | 29.50 | 74.91 | 55.76 | 63.83 | 166.36 | 53.99 |
| Characterized Sensing Performance | CNT Types | Tested Devices # | Target to Detect | Results (Findings) | |
|---|---|---|---|---|---|
| Ref. [29] |
|
| 3 | Streptavidin | Lower density offers higher sensitivity |
| Ref. [30] |
|
| 8 | NO2 | Density proportionally relates to senstivity |
| Present work |
|
| 103 | IPA Acetone | Moderately high density is desirable for the optimized SNR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, D.; Huang, T.; Kim, S.S. Peptide-Functionalized Carbon Nanotube Chemiresistors: The Effect of Nanotube Density on Gas Sensing. Sensors 2023, 23, 8469. https://doi.org/10.3390/s23208469
Sim D, Huang T, Kim SS. Peptide-Functionalized Carbon Nanotube Chemiresistors: The Effect of Nanotube Density on Gas Sensing. Sensors. 2023; 23(20):8469. https://doi.org/10.3390/s23208469
Chicago/Turabian StyleSim, Daniel, Tiffany Huang, and Steve S. Kim. 2023. "Peptide-Functionalized Carbon Nanotube Chemiresistors: The Effect of Nanotube Density on Gas Sensing" Sensors 23, no. 20: 8469. https://doi.org/10.3390/s23208469






