The Use of a Droplet Collar Accessory Attached to a Portable near Infrared Instrument to Identify Methanol Contamination in Whisky
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Preparation
2.2. Near Infrared Spectra Collection
2.3. Data Analysis
3. Results and Discussion
3.1. Spectra Interpretation
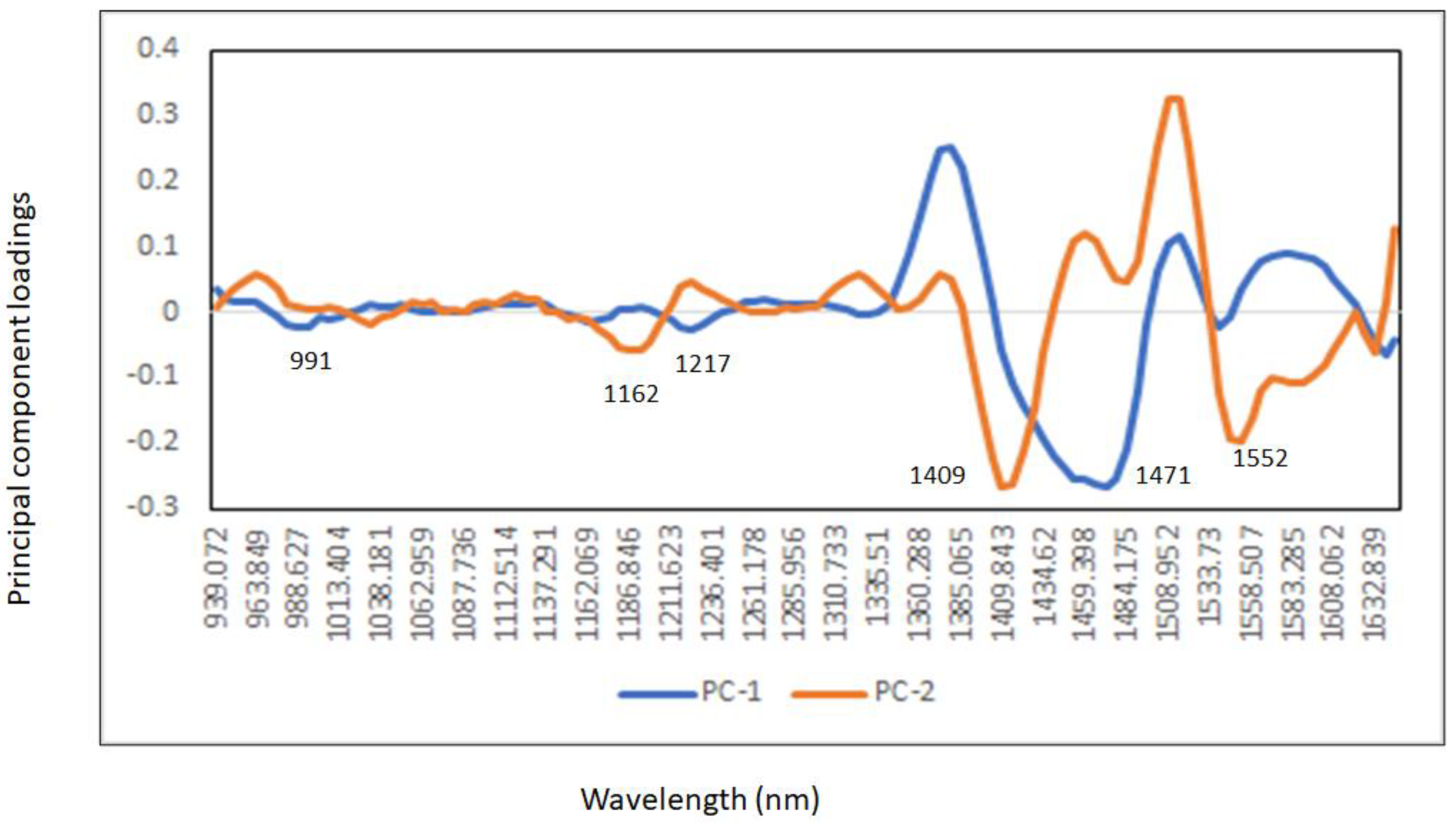
3.2. Principal Component Analysis
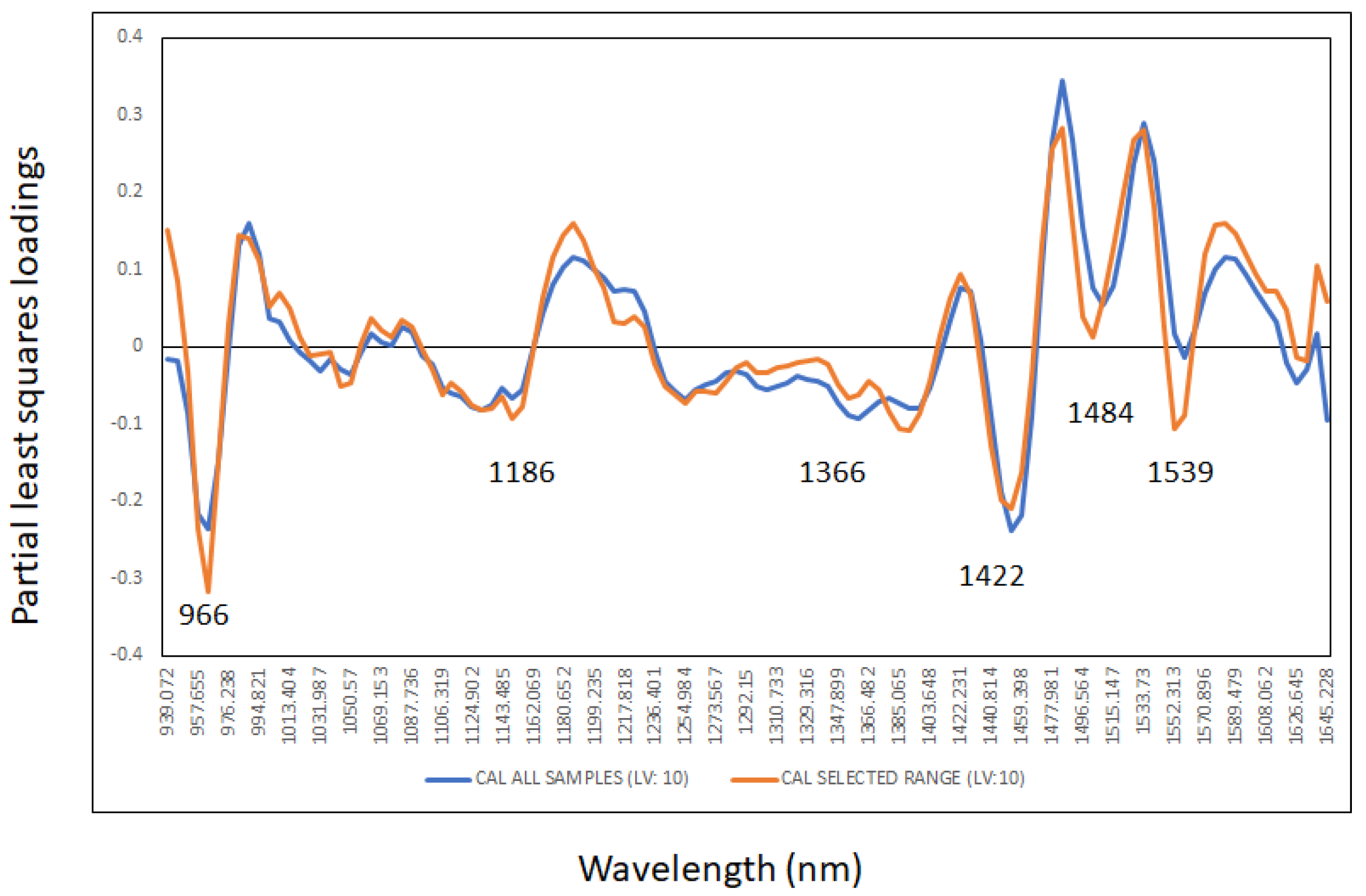
3.3. Cross Validation Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Power, A.C.; Néill, C.N.; Geoghegan, S.; Currivan, S.; Deasy, M.; Cozzolino, D. A Brief History of Whiskey Adulteration and the Role of Spectroscopy Combined with Chemometrics in the Detection of Modern Whiskey Fraud. Beverages 2020, 6, 49. [Google Scholar] [CrossRef]
- Ohimain, E.I. Methanol contamination in traditionally fermented alcoholic beverages: The microbial dimension. SpringerPlus 2016, 5, 1607. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.E.; Hodge, H.C.; Smith, R.P.; Gleason, M.N. Clinical Toxicology of Commercial Products; Williams & Wilkins: Baltimore, MD, USA, 1976; pp. 229–233. [Google Scholar]
- Vašková, H. Spectroscopic determination of methanol content in alcoholic drinks. Int. J. Biol. Biotechnol. 2014, 8, 27–34. [Google Scholar]
- Spink, J.; Moyer, D.C. Defining the public health threat of food fraud. J. Food Sci. 2011, 76, 157–163. [Google Scholar] [CrossRef]
- Spink, J.; Fortin, N.D.; Moyer, D.C.; Miao, H.; Wu, Y. Food fraud prevention: Policy, strategy, and decision-making—Implementation steps for a government agency or industry. Chimia 2016, 70, 320–328. [Google Scholar] [CrossRef]
- Spink, J.; Ortega, D.L.; Chen, C.; Wu, F. Food fraud prevention shifts the food risk focus to vulnerability. Trends Food Sci. Technol. 2017, 62, 215–220. [Google Scholar] [CrossRef]
- Dabbene, F.; Gay, P.; Tortia, C. Traceability issues in food supply chain management: A review. Biosyst. Eng. 2014, 120, 65–80. [Google Scholar] [CrossRef]
- Spink, J. The current state of food fraud prevention: Overview and requirements to address How to Start? and How Much is Enough? Curr. Opin. Food Sci. 2019, 27, 130–138. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Brusic, V.; Georgiou, C.A. Food authentication: State of the art and prospects. Curr. Opin. Food Sci. 2010, 10, 22–31. [Google Scholar] [CrossRef]
- van Ruth, S.M.; Huisman, W.; Luning, P.A. Food fraud vulnerability and its key factors. Trends Food Sci. Technol. 2017, 67, 70–75. [Google Scholar] [CrossRef]
- van Ruth, S.M.; Lunning, P.A.; Silvis, L.C.J.; Yang, Y.; Huisman, W. Differences in fraud vulnerability in a various food supply chains and their tiers. Food Control 2018, 84, 375–381. [Google Scholar] [CrossRef]
- Folinas, D.; Manikas, I.; Manos, B. Traceability data management for food chains. Br. Food J. 2006, 108, 622–633. [Google Scholar] [CrossRef]
- Olsen, O.; Borit, M. The components of a food traceability system. Trends Food Sci. Technol. 2018, 77, 143–149. [Google Scholar] [CrossRef]
- Robson, K.; Dean, M.; Haughey, S.; Elliott, C. A comprehensive review of food fraud terminologies and food fraud mitigation guides. Food Control 2021, 120, 107516. [Google Scholar] [CrossRef]
- Primrose, S.; Woolfe, M.; Rollinson, S. Food forensics: Methods for determining the authenticity of foodstuffs. Trends Food Sci. Technol. 2010, 21, 582–590. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, S.P.; Lahiri, S. Novel method for identification and quantification of methanol and ethanol in alcoholic beverages by gas chromatography–Fourier transform infrared spectroscopy and horizontal attenuated total reflectance Fourier transform infrared spectroscopy. J. AOAC Int. 2009, 92, 518–526. [Google Scholar] [CrossRef]
- Ellis, D.I.; Brewster, V.L.; Dunn, W.B.; Allwood, J.W.; Golovanov, A.P.; Goodacre, R. Fingerprinting food: Current technologies for the detection of food adulteration and contamination. Chem. Soc. Rev. 2012, 41, 5706–5727. [Google Scholar] [CrossRef]
- Ellis, D.I.; Muhamadali, H.; Haughey, S.A.; Elliott, C.T.; Goodacre, R. Point and shoot: Rapid quantitative detection methods for on-site food fraud analysis—Moving out the laboratory and into the food supply chain. Anal. Methods 2015, 7, 9401–9414. [Google Scholar] [CrossRef]
- Wang, M.L.; Wang, J.T.; Choong, Y.M. A rapid and accurate method for determination of methanol in alcoholic beverage by direct injection capillary gas chromatography. J. Food Compos. Anal. 2004, 17, 187–196. [Google Scholar] [CrossRef]
- Dambergs, R.G.; Kambouris, A.; Francis, I.L.; Gishen, M. Rapid analysis of methanol in grape-derived distillation products using near-infrared transmission spectroscopy. J. Agric. Food Chem. 2002, 50, 3079–3084. [Google Scholar] [CrossRef]
- Kun, T.; Ye, Y.-Y.; Du, P.-J. Identification and classification of near-infrared spectrum of adulterated wine based on support vector machine. Acta Photonica Sin. 2013, 42, 69–73. [Google Scholar] [CrossRef]
- Nagarajan, R.; Mehrotra, R.; Bajaj, M.M. Quantitative analysis of methanol, an adulterant in alcoholic beverages, using attenuated total reflectance spectroscopy. J. Autom. Methods Manag. Chem. 2006, 65, 416–419. [Google Scholar]
- Pérez-Ponce, A.; de la Guardia, M. Partial least-squares Fourier transform infrared spectrometric determination of methanol and ethanol by vapor-phase generation. Analyst 1998, 123, 1253–1258. [Google Scholar] [CrossRef]
- Wynne, L.; Clark, S.; Adams, M.J.; Barnett, N.W. Compositional dynamics of a commercial wine fermentation using two-dimensional FTIR correlation analysis. Vib. Spectrosc. 2007, 44, 394–400. [Google Scholar] [CrossRef]
- Yu, H.; Yuan, W.J.; Qi, D. Fast and nondestructive determination on excess methanol in liquor based on near-infrared spectroscopy. Food Sci. 2012, 33, 201–204. [Google Scholar] [CrossRef]
- Coldea, T.E.; Socaciu, C.; Fetea, F.; Ranga, F.; Pop, R.M.; Florea, M. Rapid quantitative analysis of ethanol and prediction of methanol content in traditional fruit brandies from Romania, using FTIR spectroscopy and chemometrics. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 143–149. [Google Scholar] [CrossRef]
- Pontes, M.J.; Santos, S.R.; Araujo, M.C.; Almeida, L.F.; Lima, R.A.; Gaiao, E.N.; Souto, U.T. Classification of distilled alcoholic beverages and verification of adulteration by near infrared spectrometry. Food Res. Int. 2006, 39, 182–189. [Google Scholar] [CrossRef]
- Saviztky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer Aided Design of Experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Williams, P.; Dardenne, P.; Flinn, P. Tutorial: Items to be included in a report on a near infrared spectroscopy project. J. Near Infrared Spectrosc. 2017, 25, 85–90. [Google Scholar] [CrossRef]
- Fearn, T. Assessing calibrations: SEP, RPD, RER and R2. NIR News 2002, 13, 12–13. [Google Scholar] [CrossRef]
- Cozzolino, D.; Liu, L.; Cynkar, W.U.; Dambergs, R.G.; Janik, L.; Colby, C.B.; Gishen, M. Effect of temperature variation on the visible and near infrared spectra of wine and the consequences on the partial least square calibrations developed to measure chemical composition. Anal. Chim. Acta 2007, 588, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Wülfert, F.; Kok, W.T.; Smilde, A.K. Influence of Temperature on Vibrational Spectra and Consequences for the Predictive Ability of Multivariate Models. Anal. Chem. 1998, 70, 1761–17671. [Google Scholar] [CrossRef] [PubMed]
- Workman, J., Jr.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy, 2nd ed.; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Li, D.; Li, L.; Quan, S.; Dong, Q.; Liu, R.; Sun, Z.; Zang, H. A feasibility study on quantitative analysis of low concentration methanol by FT-NIR spectroscopy and aquaphotomics. J. Mol. Struct. 2019, 1182, 197–203. [Google Scholar] [CrossRef]
- Kojić, D.; Tsenkova, R.; Tomobe, K.; Yasuoka, K.; Yasui, M. Water confined in the local field of ions. ChemPhysChem 2014, 15, 4077–4086. [Google Scholar] [CrossRef] [PubMed]
- Menevseoglu, A.; Aykas, D.P.; Hatta-Sakoda, B.; Toledo-Herrera, V.H.; Rodriguez-Saona, L.E. Non-Invasive Monitoring of Ethanol and Methanol Levels in Grape-Derived Pisco Distillate by Vibrational Spectroscopy. Sensors 2021, 21, 6278. [Google Scholar] [CrossRef]
- Ellis, D.I.; Muhamadali, H.; Xu, Y.; Eccles, R.; Goodall, I.; Goodacre, R. Rapid through-container detection of fake spirits and methanol quantification with handheld Raman spectroscopy. Analyst 2019, 144, 324–330. [Google Scholar] [CrossRef]
- Czarnecki, M.; Maeda, H.; Ozaki, Y.; Susuki, M.; Iwashi, M. A near infrared study of hydrogen bonds in alcohol—Comparison of chemometrics ans spectroscopic analysis. Chemom. Intell. Lab. Syst. 1999, 45, 121–130. [Google Scholar]
- Prado, R.; Gastl, M.; Becker, T. Aroma and color development during the production of specialty malts: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4816–4840. [Google Scholar] [CrossRef]
- Anjos, O.; Caldeira, I.; Fernandes, T.A.; Pedro, S.I.; Vitória, C.; Oliveira-Alves, S.; Catarino, S.; Canas, S. PLS-R Calibration Models for Wine Spirit Volatile Phenols Prediction by Near-Infrared Spectroscopy. Sensors 2022, 22, 286. [Google Scholar] [CrossRef] [PubMed]
- Joshi, I.; Truong, V.K.; Chapman, J.; Cozzolino, D. The use of two-dimensional spectroscopy to interpret the effect of temperature on the near infrared spectra of whisky. J. Near Infrared Spectrosc. 2020, 28, 148–152. [Google Scholar] [CrossRef]
- dos Santos, C.A.T.; Lopo, M.; Páscoa, R.N.M.J.; Lopes, J.A. A review on the applications of portable near-infrared spectrometers in the agro-food industry. Appl. Spectrosc. 2013, 67, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Crocombe, R.A. Portable spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef] [PubMed]
- Kademi, H.I.; Ulusoy, B.H.; Hecer, C. Applications of miniaturized and portable near infrared spectroscopy (NIRS) for inspection and control of meat and meat products. Food Rev. Int. 2018, 35, 201–220. [Google Scholar] [CrossRef]



| Brand | ABV% | Colour | Hue | Ageing | Country |
|---|---|---|---|---|---|
| Finnlaigh | 40 | Golden | Dark | n/a | Ireland |
| Canadian Club | 37 | Brown | Light | n/a | Canada |
| Jim Beam Black | 40 | Brown | Dark | American white oak | USA |
| Crown Royal | 40 | Brown | Light | New oak | Canada |
| Johnny Walker Black Label | 40 | Brown | Dark | n/a | Scotland |
| Wild Turkey | 43 | Brown | Dark | n/a | USA |
| Jim Beam | 37 | Brown | Dark | American white oak | USA |
| Chivas Regal | 40 | Brown | Dark | n/a | Scotland |
| Jameson | 40 | Golden | Dark | Oak | Irish |
| Johnny Walker Red Label | 40 | Brown | Dark | n/a | Scotland |
| Kilbeggan | 40 | Golden | Dark | n/a | Ireland |
| Southern Comfort | 35 | Brown | Light | n/a | USA |
| N | R2CV | SECV | Slope | Bias | SEP | RPD | LV | |
|---|---|---|---|---|---|---|---|---|
| CAL (all samples) | 150 | 0.95 | 0.35 | 0.96 | 0.009 | 10 | ||
| CAL (removed 0.5 and 1%) | 78 | 0.97 | 0.28 | 0.98 | 0.005 | 10 | ||
| VAL (CAL all samples) | 66 | 0.94 | 0.94 | 0.07 | 0.36 | 4.4 | ||
| VAL (CAL selected samples) | 66 | 0.93 | 0.94 | 0.07 | 0.42 | 4.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolobaric, A.; Orrell-Trigg, R.; Orloff, S.; Fraser, V.; Chapman, J.; Cozzolino, D. The Use of a Droplet Collar Accessory Attached to a Portable near Infrared Instrument to Identify Methanol Contamination in Whisky. Sensors 2023, 23, 8969. https://doi.org/10.3390/s23218969
Kolobaric A, Orrell-Trigg R, Orloff S, Fraser V, Chapman J, Cozzolino D. The Use of a Droplet Collar Accessory Attached to a Portable near Infrared Instrument to Identify Methanol Contamination in Whisky. Sensors. 2023; 23(21):8969. https://doi.org/10.3390/s23218969
Chicago/Turabian StyleKolobaric, Adam, Rebecca Orrell-Trigg, Seth Orloff, Vanessa Fraser, James Chapman, and Daniel Cozzolino. 2023. "The Use of a Droplet Collar Accessory Attached to a Portable near Infrared Instrument to Identify Methanol Contamination in Whisky" Sensors 23, no. 21: 8969. https://doi.org/10.3390/s23218969





