Ethanol-Gas-Sensing Performances of Built-in ZrO2/Co3O4 Hybrid Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Synthesis of ZrO2/Co3O4 Junctions
2.3. Sample Characterization
2.4. Sensor Fabrication and Gas-Sensing Measurements
3. Results
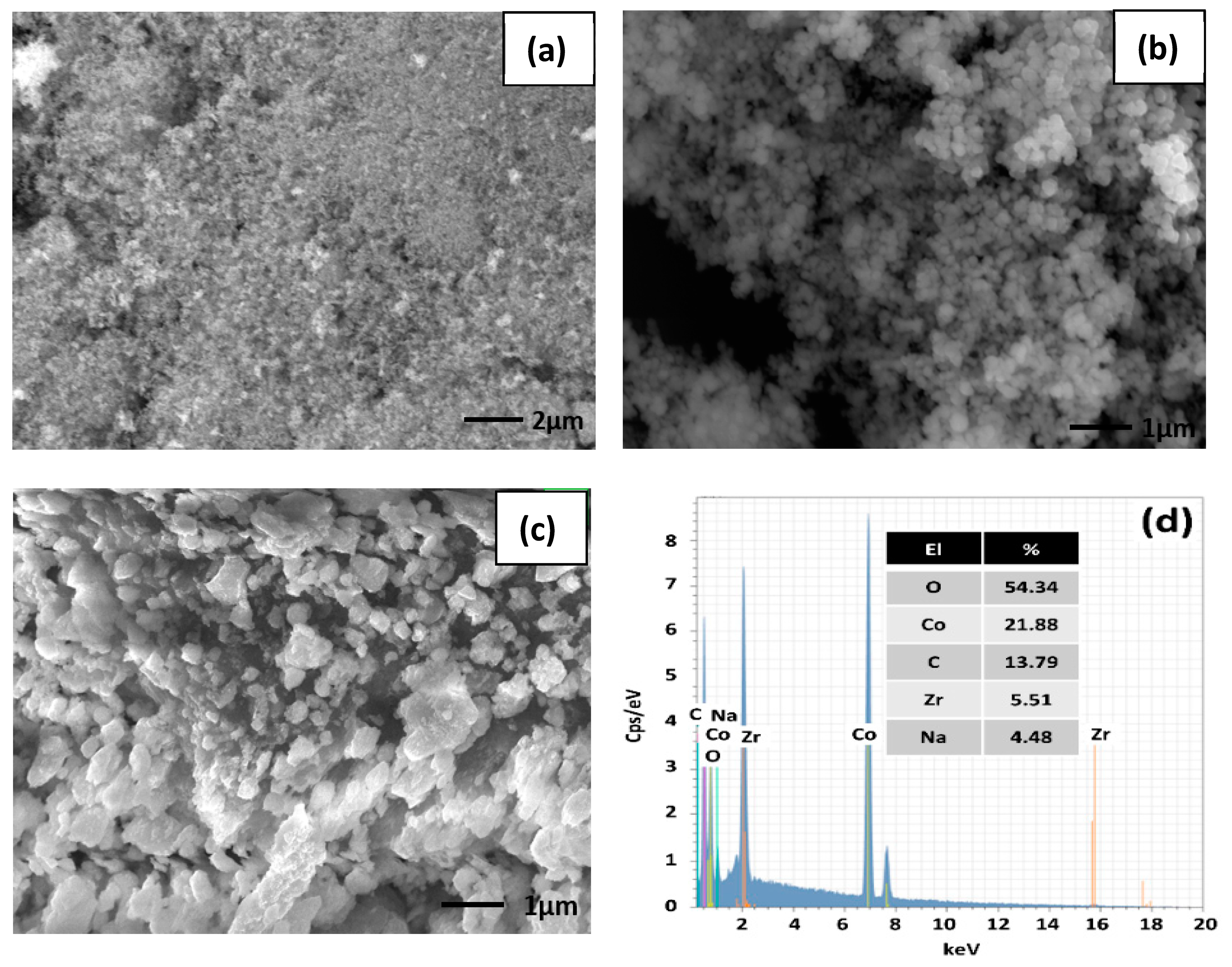
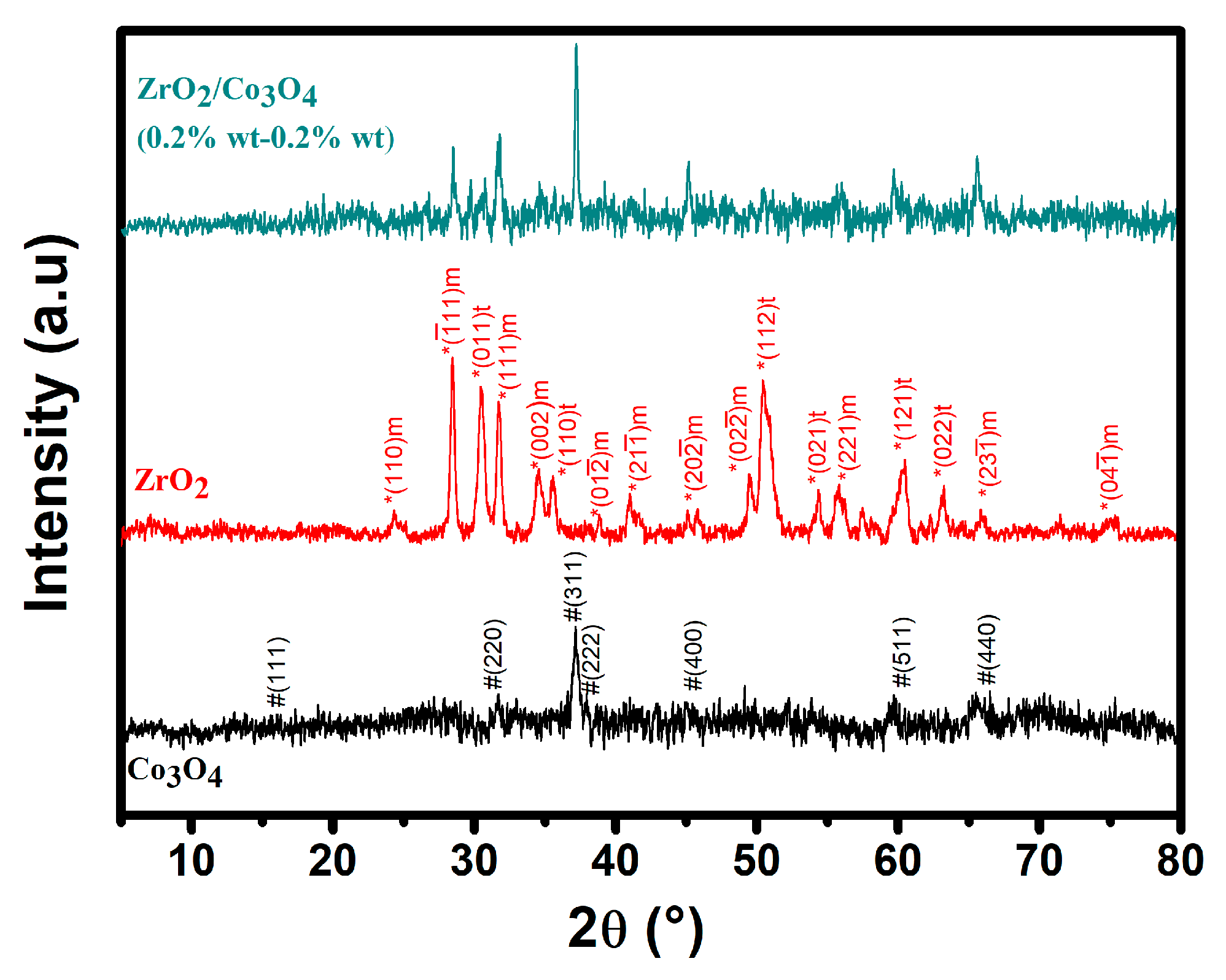
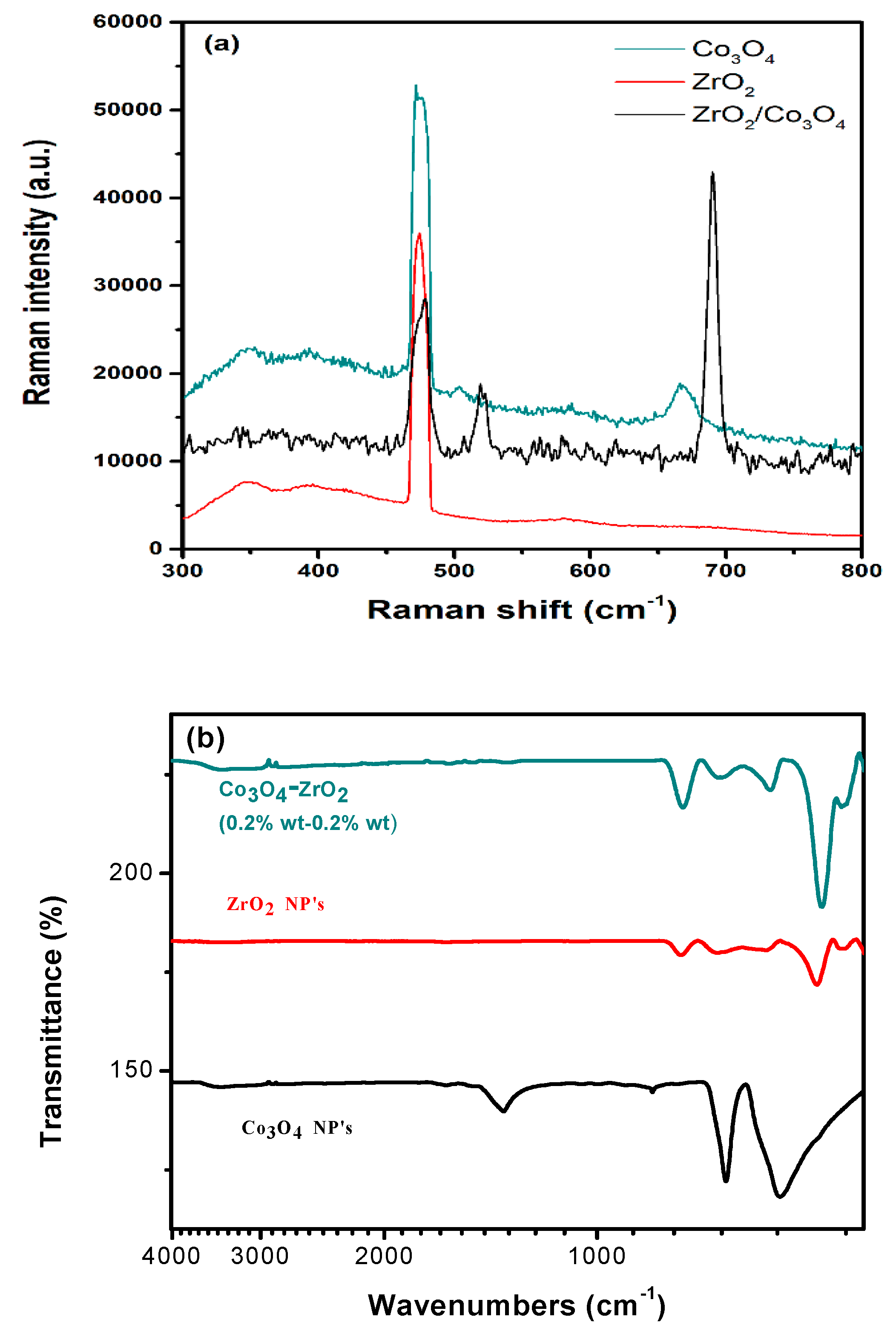
3.1. Structure and Morphology Studies
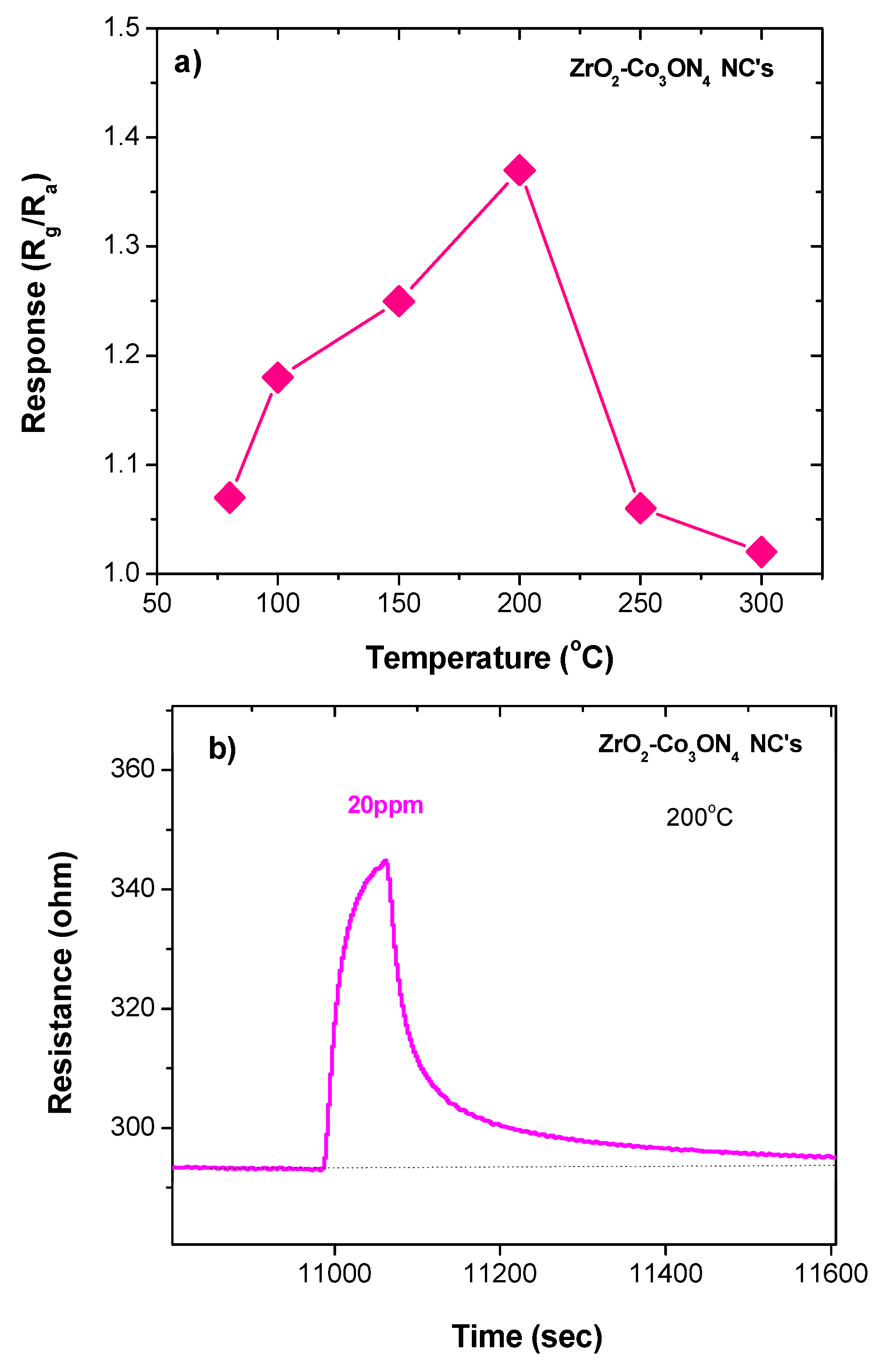
3.2. Electrical and Gas-Sensing Measurements
3.3. Gas-Sensing Mechanism
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef]
- Kim, I.D.; Rothschild, A.; Tuller, H.L. Advances and new directions in gas-sensing devices. Acta Mater. 2013, 61, 974–1000. [Google Scholar] [CrossRef]
- Khan, M.; Crispi, S.; Hussain, M.; Sarfraz, Z.; Neri, G. Gas sensing performance of Fe2O3-Co3O4 nano heterojunctions for ethanol detection. J. Mater. Sci. Mater. Electron. 2023, 34, 1982. [Google Scholar] [CrossRef]
- Huo, L.; Yang, X.; Liu, Z.; Tian, X.; Qi, T.; Wang, X.; Yu, K.; Sun, J.; Fan, M. Modulation of potential barrier heights in Co3O4/SnO2 heterojunctions for highly H2-selective sensors. Sens. Actuators B Chem. 2017, 244, 694–700. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Espro, C.; Iannazzo, D.; Moulaee, K.; Neri, G. A novel yttria-doped ZrO2 based conductometric sensor for hydrogen leak monitoring. Int. J. Hydrogen Energy 2022, 47, 9819–9828. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Ww Fazio, E.; Spadaro, S.; Corsaro, C.; Neri, G.; Leonardi, S.G.; Neri, F.; Lavanya, N.; Sekar, C.; Donato, N.; Neri, G. Metal-Oxide Based Nanomaterials: Synthesis, Characterization and Their Applications in Electrical and Electrochemical Sensors. Sensors 2021, 21, 2494. [Google Scholar] [CrossRef]
- Chen, K.W.; Tsai, J.H.; Chen, C.H. NiO functionalized Co3O4 hetero-nanocomposites with a novel apple-like architecture for CO gas sensing applications. Mater. Lett. 2019, 255, 126508. [Google Scholar] [CrossRef]
- Liu, S.; Teng, L.; Zhao, Y.; Liu, Z.; Zhang, J.; Ikram, M.; Rehman, A.U.; Li, L.; Shi, K. Facile route to synthesize porous hierarchical Co3O4/CuO nanosheets with high porosity and excellent NOx sensing properties at room temperature. Appl. Surf. Sci. 2018, 450, 91–101. [Google Scholar] [CrossRef]
- Zegebreal, L.T.; Tegegne, N.A.; Hone, F.G. Recent progress in hybrid conducting polymers and metal oxide nanocomposite for room-temperature gas sensor applications: A Review. Sens. Actuators A Phys. 2023, 359, 114472. [Google Scholar] [CrossRef]
- Manikandan, A.; Selvam, N.; Kennedy, L.J.; Kumar, R.T.; Vijaya, J.J. Structural and optical properties of novel ZrO2 nanostructures by microwave and solution combustion method. J. Nanosci. Nanotechnol. 2013, 13, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, A.; Espro, C.; Iannazzo, D.; Bonavita, A.; Neri, G. Yttria-zirconia electrochemical sensor for the detection of tyrosine. Mater. Today Commun. 2023, 35, 106036. [Google Scholar] [CrossRef]
- Feng, G.; Che, Y.; Wang, S.; Wang, S.; Hu, J.; Xiao, J.; Song, C.; Jiang, L. Sensitivity enhancement of In2O3/ZrO2 composite based acetone gas sensor: A promising collaborative approach of ZrO2 as the heterojunction and dopant for in-situ grown octahedron-like particles. Sens. Actuators B Chem. 2022, 367, 132087. [Google Scholar] [CrossRef]
- Yan, Y.; Ma, Z.; Sun, J.; Bu, M.; Huo, Y.; Wang, Z.; Li, Y.; Hu, N. Surface microstructure-controlled ZrO2 for highly sensitive room-temperature NO2 sensors. Nano Mater. Sci. 2021, 3, 268–275. [Google Scholar] [CrossRef]
- Dutta, P.K.; Akbar, S.A. High-Temperature Ceramic Electrochemical Sensors. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K.-i., Savinell, R.F., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Zeng, J.; Xu, Y.; Yu, J.; Zhang, X.; Zhang, X.; Jin, H.; Jin, Q.; Shen, W.; Zou, J.; Deng, S.; et al. Compact Yttria-Stabilized Zirconia Based Total NOx Sensor with a Dual Functional Co3O4/NiO Sensing Electrode. ACS Sens. 2019, 4, 2150–2155. [Google Scholar] [CrossRef]
- Pandit, N.A.; Ahmad, T. ZrO2/CeO2-Heterostructured Nanocomposites for Enhanced Carbon Monoxide Gas Sensing. ACS Appl. Nano Mater. 2023, 6, 7299–7309. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Wang, H.; Qiao, G.; Liu, W.; Xiong, Y.; Tian, J.; Wu, N.; Wang, X. Comparative and mechanistic analysis of the ethanol gas sensing properties of ZnO@In2O3 and In2O3@ZnO spherical heterostructures. J. Alloys Compd. 2023, 930, 167468. [Google Scholar] [CrossRef]
- Fu, Q.; Lu, K.; Li, N.; Dong, Z. Advances in the development of MOS-based sensors for detection of ethanol: A review. Mater. Res. Bull. 2023, 168, 112457. [Google Scholar] [CrossRef]
- Jamnani, S.R.; Moghaddam, H.M.; Leonardi, S.G.; Neri, G.; Ferlazzo, A. VOCs sensing properties of samarium oxide nanorods. Ceram. Int. 2023. [Google Scholar] [CrossRef]
- Paul, R.; Das, B.; Ghosh, R. Novel approaches towards design of metal oxide based hetero-structures for room temperature gas sensor and its sensing mechanism: Recent progress. J. Alloys Compd. 2023, 941, 168943. [Google Scholar] [CrossRef]
- Khan, M.; Abid, K.; Ferlazzo, A.; Bressi, V.; Espro, C.; Hussain, M.; Foti, A.; Gucciardi, P.G.; Neri, G. A Sensitive and Selective Non-Enzymatic Dopamine Sensor Based on Nanostructured Co3O4–Fe2O3 Heterojunctions. Chemosensors 2023, 11, 379. [Google Scholar] [CrossRef]
- Prabaharan, D.D.M.; Sadaiyandi, K.; Mahendran, M.; Sagadevan, S. Precipitation method and characterization of cobalt oxide nanoparticles. Appl. Phys. A 2017, 123, 1–6. [Google Scholar] [CrossRef]
- Manoharan, D.; Loganathan, A.; Kurapati, V.; Nesamony, V.J. Unique sharp photoluminescence of size-controlled sonochemically synthesized zirconia nanoparticles. Ultrason. Sonochemistry 2015, 23, 174–184. [Google Scholar] [CrossRef]
- Rhodes, M.D.; Bell, A.T. The effects of zirconia morphology on methanol synthesis from CO and H2 over Cu/ZrO2 catalysts: Part I. Steady-state studies. J. Catal. 2005, 233, 198–209. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199. [Google Scholar] [CrossRef]
- Chai, G.; Zhang, W.; Liotta, L.F.; Li, M.; Guo, Y.; Giroir-Fendler, A. Total oxidation of propane over Co3O4-based catalysts: Elucidating the influence of Zr dopant. Appl. Catal. B Environ. 2021, 298, 120606. [Google Scholar] [CrossRef]
- Farhadi, S.; Safabakhsh, J.; Zaringhadam, P. Synthesis, characterization, and investigation of optical and magnetic properties of cobalt oxide (Co3O4) nanoparticles. J. Nanostructure Chem. 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Awad, R. Study of the Structural and Physical Properties of Co3O4 Nanoparticles Synthesized by Co-Precipitation Method. J Supercond Nov Magn. 2020, 33, 1395–1404. [Google Scholar] [CrossRef]
- Packiaraj, R.; Devendran, P.; Venkatesh, K.S.; Asath bahadur, S.; Manikandan, A.; Nallamuthu, N. Electrochemical investigations of magnetic Co3O4 nanoparticles as an active electrode for supercapacitor applications. J. Supercond. Nov. Magn. 2018, 32, 2427. [Google Scholar] [CrossRef]
- Guo, G.Y.; Chen, Y.L.; Ying, W.J. Thermal, spectroscopic and X-ray diffractional analyses of zirconium hydroxides precipitated at low pH values. Mater. Chem. Phys. 2004, 84, 308–314. [Google Scholar] [CrossRef]
- Horti, N.C.; Kamatagi, M.D.; Nataraj, S.K.; Wari, M.N.; Inamdar, S.R. Structural and optical properties of zirconium oxide (ZrO2) nanoparticles: Effect of calcination temperature. Nano Express 2020, 1, 010022. [Google Scholar] [CrossRef]
- Rashad, M.M.; Baioumy, H.M. Effect of thermal treatment on the crystal structure and morphology of zirconia nanopowders produced by three different routes. J. Mater. Process. Technol. 2008, 195, 178–185. [Google Scholar] [CrossRef]
- Hemalatha, E.; Gopalakrishnan, N. Synthesis of ZrO2 nanostructure for gas sensing application. Bull. Mater. Sci. 2020, 43, 12. [Google Scholar]
- Keiteb, A.S.; Saion, E.; Zakaria, A.; Soltani, N. Structural and Optical Properties of Zirconia Nanoparticles by Thermal Treatment Synthesis. J. Nanomater. 2016, 2016, 1913609. [Google Scholar] [CrossRef]
- Madvar, H.R.; Kordrostami, Z.; Mirzaei, A. Sensitivity enhancement of resistive ethanol gas sensor by optimized sputtered-assisted CuO decoration of ZnO nanorods. Sensors 2022, 23, 365. [Google Scholar] [CrossRef]
- Vaezi, M.R.; Khoby Shendy, S.; Ebadzadeh, T. Synthesis of TiO2/SnO2 core shell nanocomposite by chemical route and its gas sensing properties. Indian J. Phys. 2012, 86, 9–13. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Sun, G.J.; Lee, C. Synthesis, structure, and ethanol gas sensing properties of In2O3 nanorods decorated with Bi2O3 nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 8138–8146. [Google Scholar] [CrossRef]
- Pakhare, K.S.; Sargar, B.M.; Mane, R.K.; Wategaonkar, S.B. Facile Synthesis and Characterization of CdO-ZnO Nanocomposite for Gas Sensor. Macromol. Symp. 2020, 393, 2000084. [Google Scholar] [CrossRef]
- Singh, S.; Raj, S.; Sharma, S. Ethanol sensing using MoS2/TiO2 composite prepared via hydrothermal method. Mater. Today Proc. 2021, 46, 6083–6086. [Google Scholar] [CrossRef]
- Phuoc, P.H.; Viet, N.N.; Chien, N.V.; Van Hoang, N.; Hung, C.M.; Hoa, N.D.; Van Duy, N.; Hong, H.S.; Van Hieu, N. Comparative study of CuO/Co3O4 external and CuO-Co3O4 internal heterojunctions: Do these factors always enhance gas-sensing performance? Sens. Actuators B 2023, 384, 133620. [Google Scholar] [CrossRef]
- Khan, M.; Ferlazzo, A.; Crispi, S.; Hussain, M.; Neri, G. Easy preparation of cobalt oxide/copper oxide composites for gas sensing application. Phys. Scr. 2023, 98, 125927. [Google Scholar] [CrossRef]




| Sensor | Temp. (°C) | Ethanol ppm | Response Time (s) | Recovery Time (s) | Response (Rg/Ra) | Ref. |
|---|---|---|---|---|---|---|
| CuO-ZnO | 350 | 100 | 3.2 | 2.8 | 7.5 | [38] |
| TiO2/SnO3 | 200 | 10,000 | 60 | 50 | 12.27 | [39] |
| Bi2O3-In2O3 | 200 | 200 | 24 | 180 | 171 | [40] |
| CdO/ZnO | 275 | 24 | NA | NA | 29.11 | [41] |
| MoS2/TiO2 | 350 | 100 | 52 | 155 | NA | [42] |
| Co3O4/Fe2O3 | 250 | 100 | 95 | 305 | 1.7 | [4] |
| ZrO2/Co3O4 | 200 | 20 | 56 | 363 | 1.07 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; Ferlazzo, A.; Hussain, M.; Fazio, E.; Corsaro, C.; Mezzasalma, A.M.; Neri, G. Ethanol-Gas-Sensing Performances of Built-in ZrO2/Co3O4 Hybrid Nanostructures. Sensors 2023, 23, 9578. https://doi.org/10.3390/s23239578
Khan M, Ferlazzo A, Hussain M, Fazio E, Corsaro C, Mezzasalma AM, Neri G. Ethanol-Gas-Sensing Performances of Built-in ZrO2/Co3O4 Hybrid Nanostructures. Sensors. 2023; 23(23):9578. https://doi.org/10.3390/s23239578
Chicago/Turabian StyleKhan, Madiha, Angelo Ferlazzo, Mozaffar Hussain, Enza Fazio, Carmelo Corsaro, Angela Maria Mezzasalma, and Giovanni Neri. 2023. "Ethanol-Gas-Sensing Performances of Built-in ZrO2/Co3O4 Hybrid Nanostructures" Sensors 23, no. 23: 9578. https://doi.org/10.3390/s23239578






