Colorimetric and Fluorescent Sensing of Copper Ions in Water through o-Phenylenediamine-Derived Carbon Dots
Abstract
:1. Introduction
2. Materials and Experimental Procedure:
2.1. Materials
2.2. Synthesis of Carbon Dots
2.3. Agarose Hydrogel Film Preparation
2.4. Carbon Dots incorporated Agarose (Aga-NSCDs) Hydrogel Film Preparation
2.5. Instrumentation for Characterization and Sensitivity Measurements
2.6. Determination of Cu(II) Ions in Liquid and Solid State
3. Results
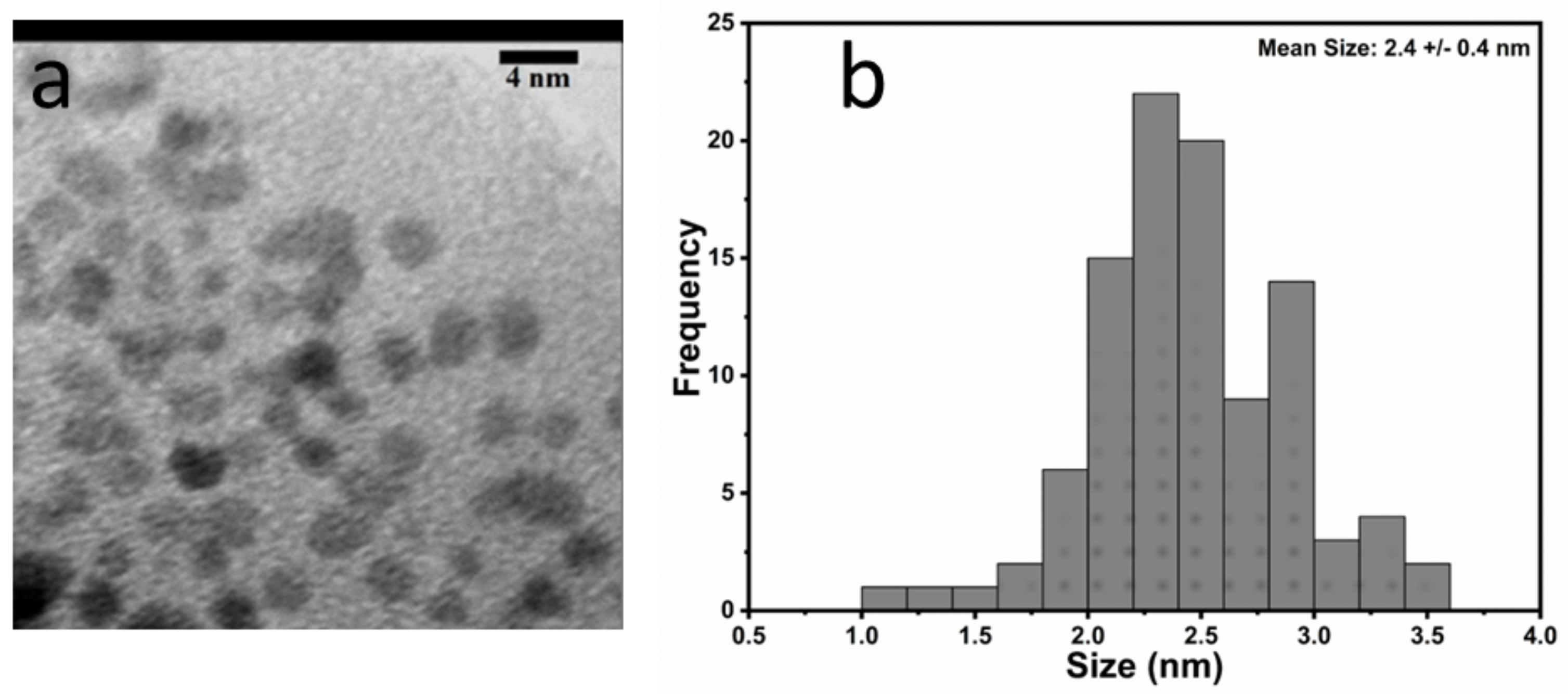
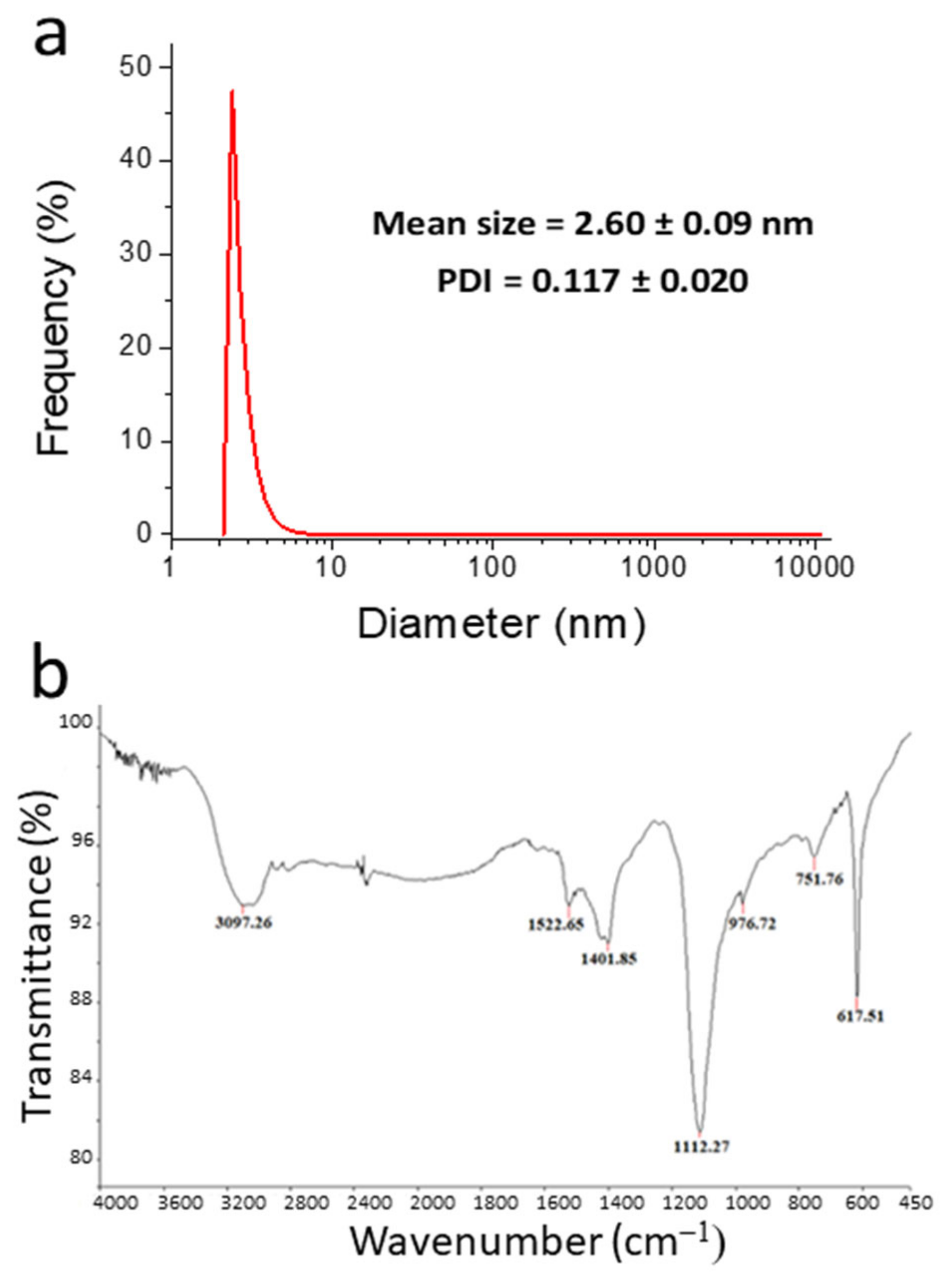
3.1. Morphological and Structural Characterization
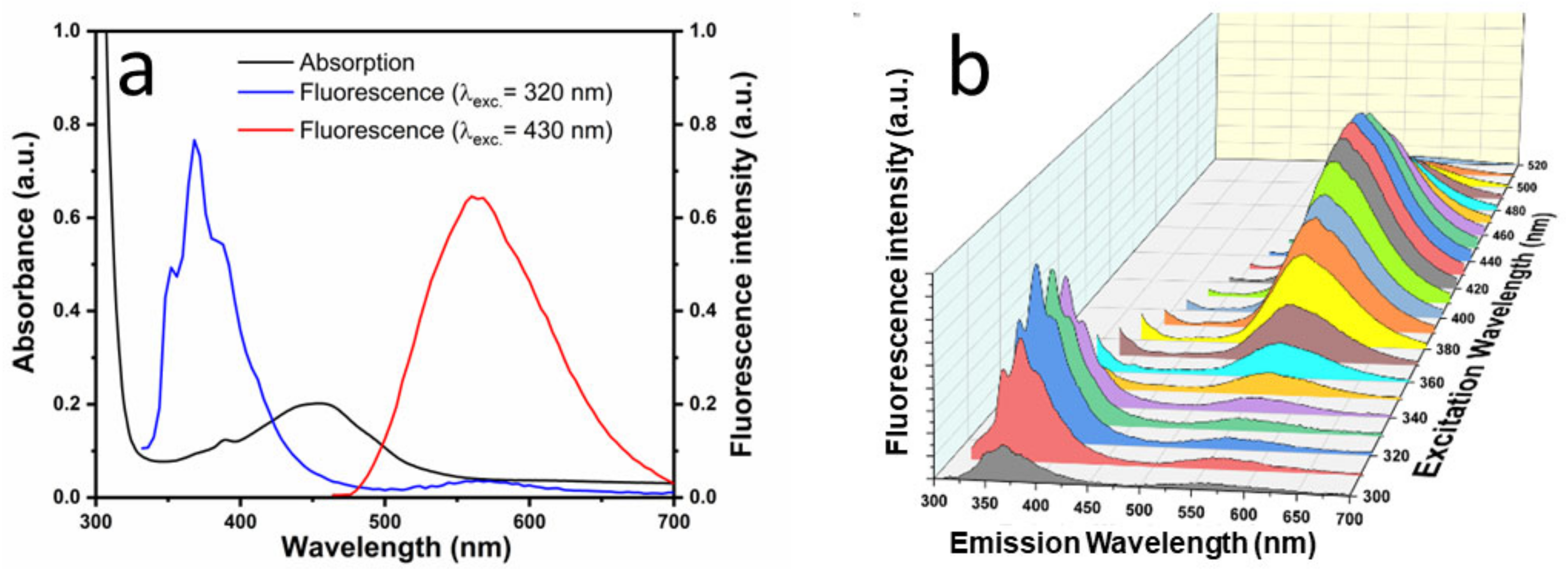
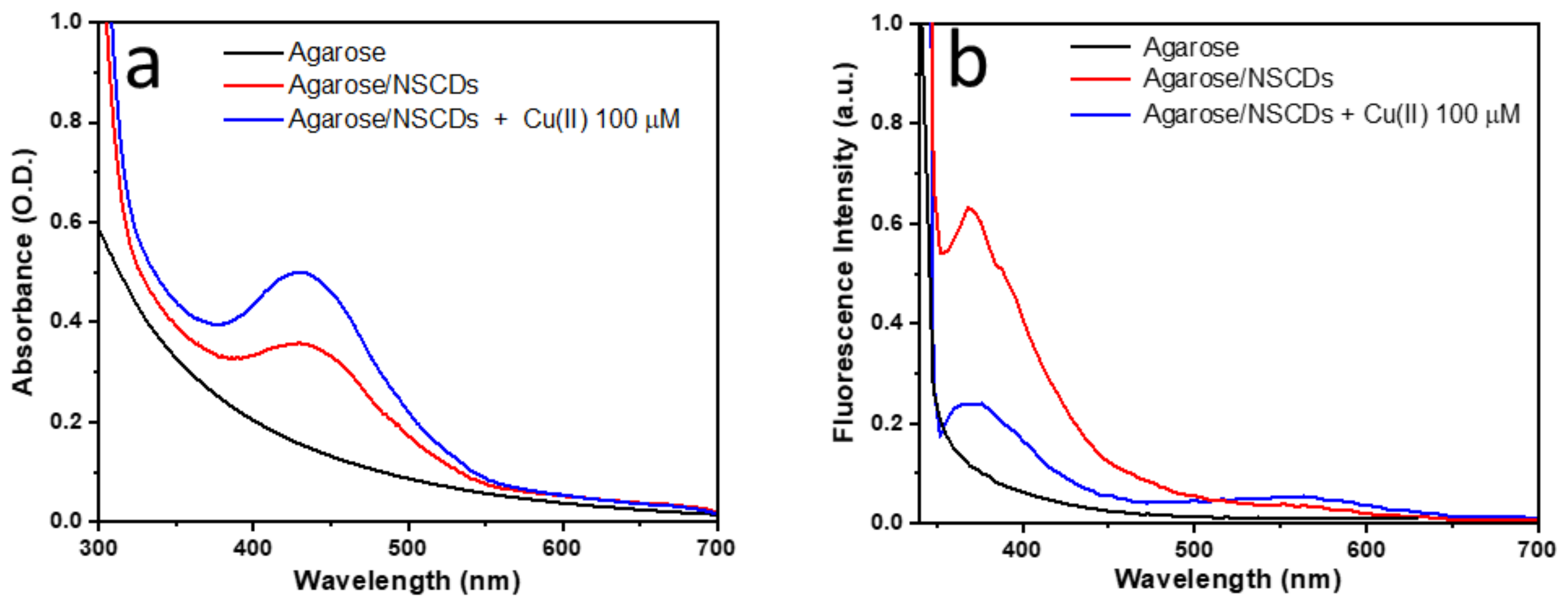
3.2. Spectroscopic Characterization
3.3. Results–Colorimetric and Fluorescence Sensing Properties
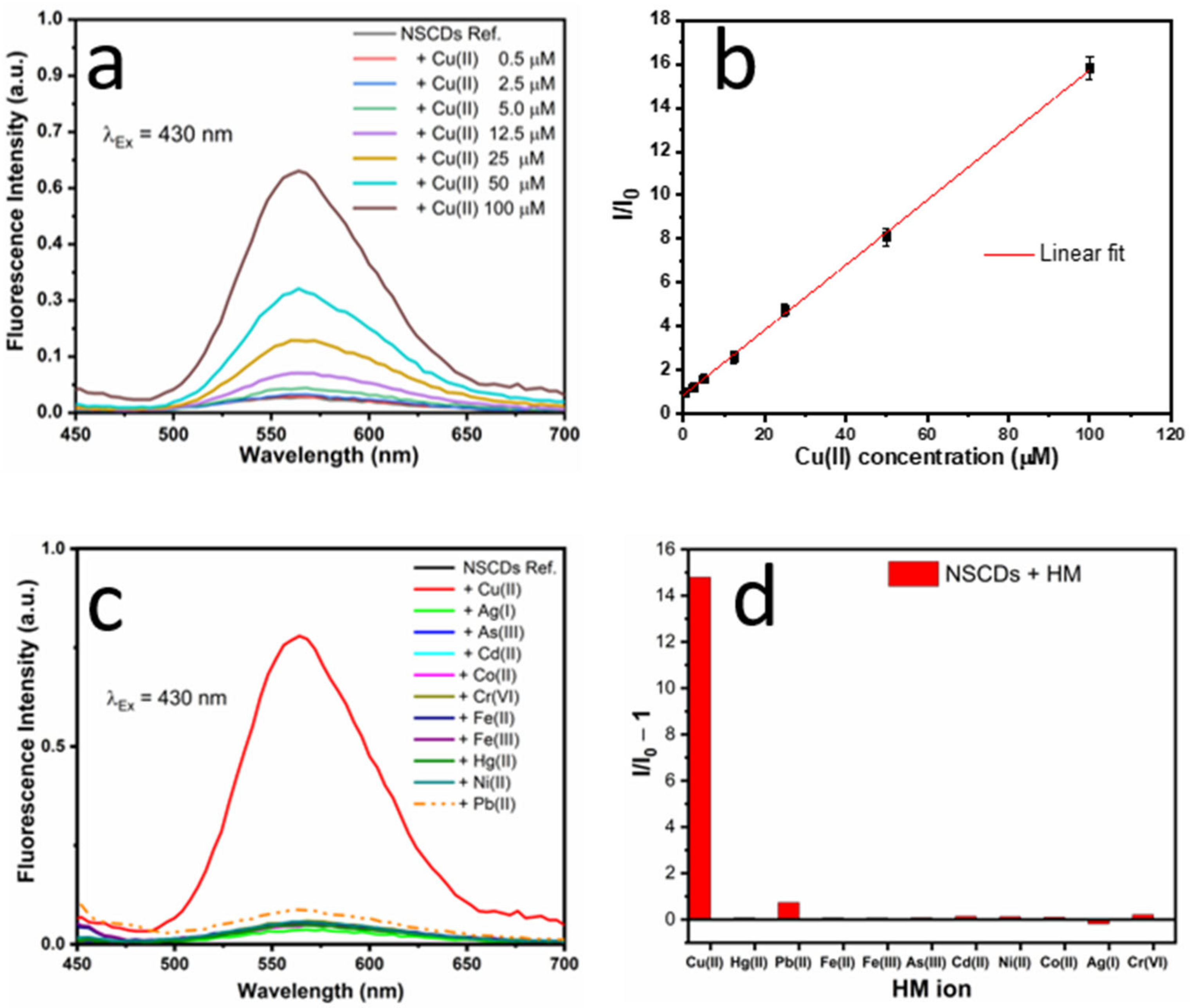
3.3.1. Absorption-based Sensing
3.3.2. Fluorescence-based Sensing
3.4. Comparison with Other Sensing Systems and Techniques
3.5. Stability and Reproducibility of NSCD Synthesis
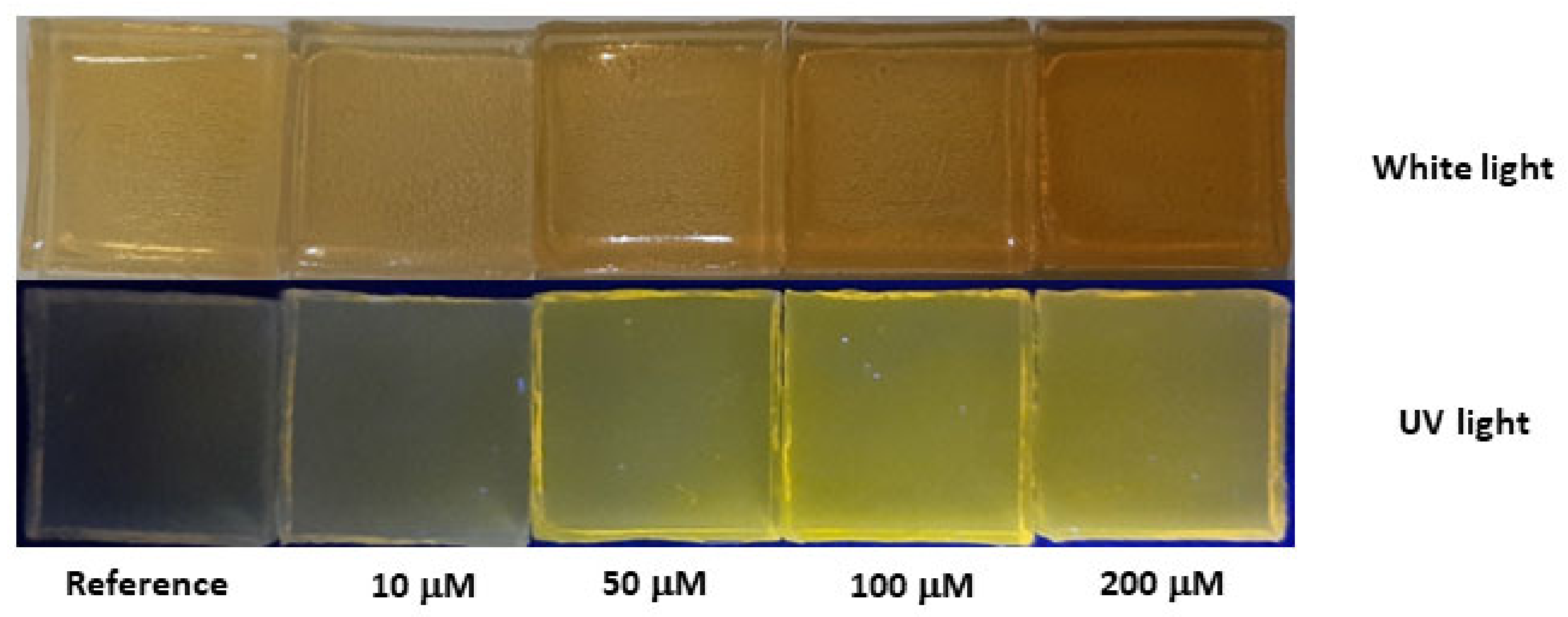
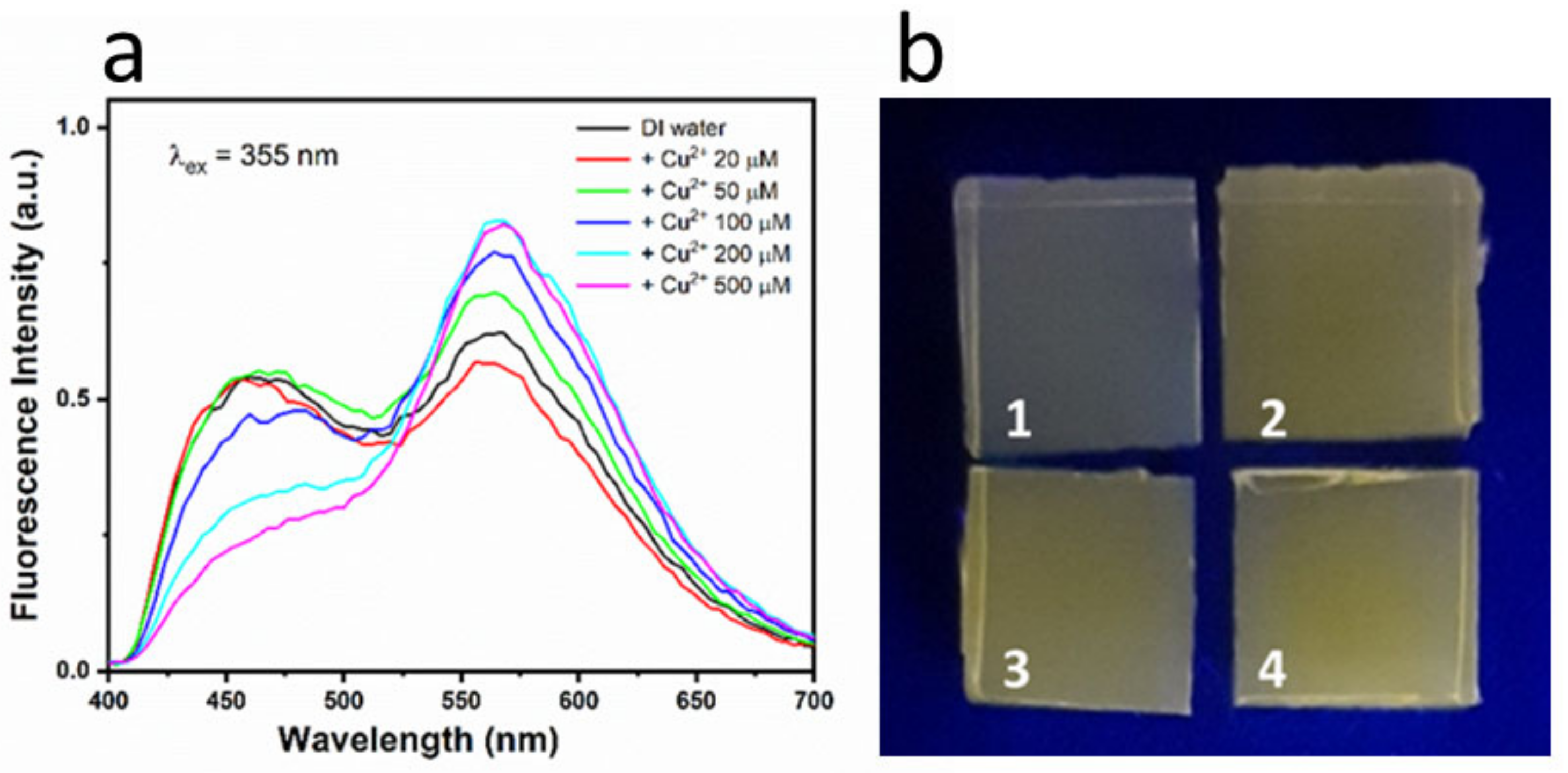
3.6. Results—Colorimetric and Fluorescent Sensing with Solid-State Agarose Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Mehta, J.; Bhardwaj, S.K.; Bhardwaj, N.; Paul, A.; Kumar, P.; Kim, K.H.; Deep, A. Progress in the biosensing techniques for trace-level heavy metals. Biotechnol. Adv. 2016, 34, 47–60. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Z.; Jiang, Z.; Zhang, J.; Wang, F.; Huang, X. Distribution and bioaccumulation of heavy metals in marine organisms in east and west Guangdong coastal regions, South China. Mar. Pollut. Bull. 2015, 101, 930–937. [Google Scholar] [CrossRef]
- Ofomaja, A.; Naidoo, E.B.; Modise, S. Biosorption of copper(II) and lead(II) onto potassium hydroxide treated pine cone powder. J. Environ. Manag. 2010, 91, 1674–1685. [Google Scholar] [CrossRef]
- Justino, C.I.; Freitas, A.C.; Duarte, A.C.; Santos, T.A. Sensors and biosensors for monitoring marine contaminants. Trends Environ. Anal. Chem. 2015, 6–7, 21–30. [Google Scholar] [CrossRef]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67, 952S–959S. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Huang, X.; Kou, E.; Cai, W.; Zhang, H.; Zhang, X.; Liu, Y.; Li, W.; Zheng, Y.; Lei, B. Carbon dot based sensing platform for real-time imaging Cu2+ distribution in plants and environment. Biosens. Bioelectron. 2023, 219, 114848. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.D.M.G.; Barcarolli, I.F.; de Menezes, E.J.; Giacomian, M.M.; Wood, C.M.; Bianchini, A. Acute toxicity, accumulation and tissue distribution of copper in the blue crab Callinectes sapidus acclimated to different salinities: In vivo and in vitro studies. Aquat. Toxicol. 2011, 101, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Deng, J.; Yi, Y.; Li, H.; Zhang, Y.; Yao, S. Label-free silicon quantum dots as fluorescent probe for selective and sensitive detection of copper ions. Talanta 2014, 125, 372–377. [Google Scholar] [CrossRef]
- Gedda, G.; Lee, C.-Y.; Lin, Y.-C.; Wu, H.-F. Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sens. Actuators B Chem. 2016, 224, 396–403. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Xu, C.; Ren, J.; Qu, X. Visual and quantitative detection of copper ions using magnetic silica nanoparticles clicked on multiwalled carbon nanotubes. Chem. Commun. 2010, 46, 6572–6574. [Google Scholar] [CrossRef]
- Sanni, S.O.; Moundzounga, T.H.G.; Oseghe, E.O.; Haneklaus, N.H.; Viljoen, E.L.; Brink, H.G. One-Step Green Synthesis of Water-Soluble Fluorescent Carbon Dots and Its Application in the Detection of Cu2+. Nanomaterials 2022, 12, 958. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-C.; Wu, J.; Yang, G.-P.; Wu, Y.-L.; Wang, Y.-Y. A microporous anionic metal–organic framework for a highly selective and sensitive electrochemical sensor of Cu2+ ions. Chem. Commun. 2016, 52, 8475–8478. [Google Scholar] [CrossRef] [PubMed]
- Cotton, D.; Jenkins, D. The determination of very low concentrations of copper, iron and lead in hydrocarbon fuels by atomic fluorescence spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1970, 25, 283–288. [Google Scholar] [CrossRef]
- Ashoka, S.; Peake, B.M.; Bremner, G.; Hageman, K.; Reid, M. Comparison of digestion methods for ICP-MS determination of trace elements in fish tissues. Anal. Chim. Acta 2009, 653, 191–199. [Google Scholar] [CrossRef]
- Acar, O. Determination of cadmium, copper and lead in soils, sediments and sea water samples by ETAAS using a Sc+ Pd+ NH4NO3 chemical modifier. Talanta 2005, 65, 672–677. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Shi, H.; Wang, H.; Liu, J. Rapid on-site/in-situ detection of heavy metal ions in environmental water using a structure-switching DNA optical biosensor. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Wang, Z.; Shao, H.; Jiang, X. Stable fluorescent gold nanoparticles for detection of Cu2+ with good sensitivity and selectivity. Analyst 2011, 137, 301–304. [Google Scholar] [CrossRef]
- Yu, C.; Wen, Y.; Zhang, J. Synthesis of a Cu2+-Selective Probe Derived from Rhodamine and Its Application in Cell Imaging. Sensors 2014, 14, 21375–21384. [Google Scholar] [CrossRef] [Green Version]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2003, 4, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Demchenko, A.P. Photobleaching of organic fluorophores: Quantitative characterization, mechanisms, protection. Methods Appl. Fluoresc. 2020, 8, 022001. [Google Scholar] [CrossRef]
- PPourreza, N.; Ghomi, M. Green synthesized carbon quantum dots from Prosopis juliflora leaves as a dual off-on fluorescence probe for sensing mercury (II) and chemet drug. Mater. Sci. Eng. C 2019, 98, 887–896. [Google Scholar] [CrossRef]
- Ye, Q.; Ren, S.; Huang, H.; Duan, G.; Liu, K.; Liu, J.-B. Fluorescent and Colorimetric Sensors Based on the Oxidation of o-Phenylenediamine. ACS Omega 2020, 5, 20698–20706. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, B.; Zhao, X.; Li, Z.-J.; Yang, X. Fluorescent and Colorimetric Dual-Readout Assay for Inorganic Pyrophosphatase with Cu2+-Triggered Oxidation of o-Phenylenediamine. Anal. Chem. 2016, 88, 1355–1361. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Zhang, L.; Wang, H.; Nie, J.; Qin, Z.; Li, J.; Xiao, W. Analyte-triggered autocatalytic amplification combined with gold nanoparticle probes for colorimetric detection of heavy-metal ions. Chem. Commun. 2017, 53, 7477–7480. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, E. A Nanoparticle Autocatalytic Sensor for Ag+ and Cu2+ Ions in Aqueous Solution with High Sensitivity and Selectivity and Its Application in Test Paper. Anal. Chem. 2011, 83, 5005–5011. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Wang, W.; Lv, Q.; Wang, Z.; Liu, Y.; Zhang, Q. Bicomponent colorimetric probe based on carbon quantum dots and o-phenylenediamine for sensitive and selective Cu2+ sensing. Anal. Sci. 2021, 21P267. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, S.G.; Han, L.; Luo, H.Q.; Li, N.B. A ratiometric fluorescent and colorimetric dual-signal sensing platform based on N-doped carbon dots for selective and sensitive detection of copper(II) and pyrophosphate ion. Sens. Actuators B Chem. 2018, 283, 215–221. [Google Scholar] [CrossRef]
- Zhao, X.-E.; Lei, C.; Gao, Y.; Gao, H.; Zhu, S.; Yang, X.; You, J.; Wang, H. A ratiometric fluorescent nanosensor for the detection of silver ions using graphene quantum dots. Sens. Actuators B Chem. 2017, 253, 239–246. [Google Scholar] [CrossRef]
- Vedamalai, M.; Periasamy, A.P.; Wang, C.-W.; Tseng, Y.-T.; Ho, L.-C.; Shih, C.-C.; Chang, H.-T. Carbon nanodots prepared from o-phenylenediamine for sensing of Cu2+ ions in cells. Nanoscale 2014, 6, 13119–13125. [Google Scholar] [CrossRef]
- Guo, J.J.; Zhou, M.J.; Yang, C.X. Fluorescent hydrogel waveguide for on-site detection of heavy metal ions. Sci. Rep. 2017, 7, e7902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Y.; Sun, L.; Qi, Q.; Zhao, X. Chitosan and κ-carrageenan-derived nitrogen and sulfur co-doped carbon dots “on-off-on” fluorescent probe for sequential detection of Fe3+ and ascorbic acid. Int. J. Biol. Macromol. 2021, 191, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Sahu, S.; Sinha, N.; Bhutia, S.K. Synthesis of a carbon-dot-based photoluminescent probe for selective and ultrasensitive detection of Hg2+ in water and living cells. Analyst 2014, 140, 1221–1228. [Google Scholar] [CrossRef]
- Wee, S.S.; Ng, Y.H.; Ng, S.M. Synthesis of fluorescent carbon dots via simple acid hydrolysis of bovine serum albumin and its potential as sensitive sensing probe for lead (II) ions. Talanta 2013, 116, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, S.; Bisauriya, R.; Carbone, M.; Roselli, L.; Cecchetti, D.; Bauer, E.M.; Sennato, S.; Prosposito, P.; Pizzoferrato, R. Colorimetric Detection of Chromium(VI) Ions in Water Using Unfolded-Fullerene Carbon Nanoparticles. Sensors 2021, 21, 6353. [Google Scholar] [CrossRef]
- Kaciulis, S.; Mezzi, A.; Soltani, P.; Pizzoferrato, R.; Ciotta, E.; Prosposito, P. Graphene quantum dots obtained by unfolding fullerene. Thin Solid Films 2019, 673, 19–25. [Google Scholar] [CrossRef]
- Limosani, F.; Bauer, E.M.; Cecchetti, D.; Biagioni, S.; Orlando, V.; Pizzoferrato, R.; Prosposito, P.; Carbone, M. Top-Down N-Doped Carbon Quantum Dots for Multiple Purposes: Heavy Metal Detection and Intracellular Fluorescence. Nanomaterials 2021, 11, 2249. [Google Scholar] [CrossRef]
- Rajendran, S.; Ben Zichri, S.; Vipinachandran, V.U.; Jelinek, R.; Bhunia, S.K. Triphenylphosphonium-Derived Bright Green Fluorescent Carbon Dots for Mitochondrial Targeting and Rapid Selective Detection of Tetracycline. ChemNanoMat 2021, 7, 545–552. [Google Scholar] [CrossRef]
- Martín-Pacheco, A.; Del Rió Castillo, A.E.; Martín, C.; Herrero, M.A.; Merino, S.; Garcia Fierro, J.L.; Díez-Barra, E.; Vázquez, E. Graphene quantum dot–aerogel: From nanoscopic to macroscopic fluorescent materials. sensing polyaromatic compounds in water. ACS Appl. Mater. Interfaces 2018, 10, 18192–18201. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.; Kennedy, S.; Steed, J.; Valcárcel, M. Fluorescent carbon quantum dot hydrogels for direct determination of silver ions. Talanta 2016, 151, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogoi, N.; Barooah, M.; Majumdar, G.; Chowdhury, D. Carbon Dots Rooted Agarose Hydrogel Hybrid Platform for Optical Detection and Separation of Heavy Metal Ions. ACS Appl. Mater. Interfaces 2015, 7, 3058–3067. [Google Scholar] [CrossRef]
- Yu, S.; Li, W.; Fujii, Y.; Omura, T.; Minami, H. Fluorescent Spherical Sponge Cellulose Sensors for Highly Selective and Semiquantitative Visual Analysis: Detection of Hg2+ and Cu2+ ions. ACS Sustain. Chem. Eng. 2019, 7, 19157–19166. [Google Scholar] [CrossRef]
- Bisauriya, R.; Antonaroli, S.; Ardini, M.; Angelucci, F.; Ricci, A.; Pizzoferrato, R. Tuning the Sensing Properties of N and S Co-Doped Carbon Dots for Colorimetric Detection of Copper and Cobalt in Water. Sensors 2022, 22, 2487. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Pang, Y.; Li, L.; Zhu, C.; Ma, C.; Gu, J.; Wu, Y.; Chen, G. One-Step Synthesis of the Nitrogen and Sulfur Codoped Carbon Dots for Detection of Lead and Copper Ions in Aqueous Solution. J. Sens. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Konar, S.; Samanta, D.; Mandal, S.; Das, S.; Mahto, M.K.; Shaw, M.; Mandal, M.; Pathak, A. Selective and sensitive detection of cinnamaldehyde by nitrogen and sulphur co-doped carbon dots: A detailed systematic study. RSC Adv. 2018, 8, 42361–42373. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Khandelwal, M.; Gupta, S.K.; Kumar, V.; Rani, R. Fourier transform infrared spectroscopy: Data interpretation and applications in structure elucidation and analysis of small molecules and nanostructures. In Data Processing Handbook for Complex Biological Data Sources; Academic Press: Cambridge, MA, USA, 2019; pp. 77–96. [Google Scholar]
- Qu, D.; Zheng, M.; Du, P.; Zhou, Y.; Zhang, L.; Li, D.; Tan, H.; Zhao, Z.; Xie, Z.; Sun, Z. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272–12277. [Google Scholar] [CrossRef]
- Moon, B.J.; Kim, S.J.; Lee, A.; Oh, Y.; Lee, S.-K.; Lee, S.H.; Kim, T.-W.; Hong, B.H.; Bae, S. Structure-controllable growth of nitrogenated graphene quantum dots via solvent catalysis for selective C-N bond activation. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Lee, N.E.; Jeong, J.-M.; Lim, H.S.; Lee, S.Y.; Cho, S.O. Ultraviolet/blue light emitting high-quality graphene quantum dots and their biocompatibility. Carbon 2020, 170, 213–219. [Google Scholar] [CrossRef]
- Yoon, H.; Chang, Y.H.; Song, S.H.; Lee, E.-S.; Jin, S.H.; Park, C.; Lee, J.; Kim, B.H.; Kang, H.J.; Kim, Y.-H.; et al. Intrinsic Photoluminescence Emission from Subdomained Graphene Quantum Dots. Adv. Mater. 2016, 28, 5255–5261. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology; Oxford: West Sussex, UK, 1997. [Google Scholar]
- Kalaiyarasan, G.; Joseph, J. Efficient dual-mode colorimetric/fluorometric sensor for the detection of copper ions and vitamin C based on pH-sensitive amino-terminated nitrogen-doped carbon quantum dots: Effect of reactive oxygen species and antioxidants. Anal. Bioanal. Chem. 2019, 411, 2619–2633. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Zhuang, Q.; Huang, S.; Hu, M.; Wang, Y.; Ni, Y. Adenine-stabilized carbon dots for highly sensitive and selective sensing of copper(II) ions and cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118531. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Long, W.; Liu, T.; Guan, Y.; Lei, G.; Suo, Y.; Jia, M.; He, J.; Chen, H.; She, Y.; et al. A sensitive fluorescence sensor based on a glutathione modified quantum dot for visual detection of copper ions in real samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 294, 122517. [Google Scholar] [CrossRef]
- Tabakci, B.; Ozcelik, E.; Erdemir, S.; Tabakci, M. A highly sensitive colorimetric and fluorogenic detection of copper(II) ion based on new picolylamine-armed calix [4]arene. Measurement 2023, 210, 112556. [Google Scholar] [CrossRef]
- Liu, M.; Wang, K.; Wang, H.; Lu, J.; Xu, S.; Zhao, L.; Wang, X.; Du, J. Simple and sensitive colorimetric sensors for the selective detection of Cu(II). RSC Adv. 2021, 11, 11732–11738. [Google Scholar] [CrossRef]
- Cheah, P.W.; Heng, M.P.; Saad, H.M.; Sim, K.S.; Tan, K.W. Specific detection of Cu2+ by a pH-independent colorimetric rhodamine based chemosensor. Opt. Mater. 2021, 114, 110990. [Google Scholar] [CrossRef]
- Zulfajri, M.; Dhandabani, G.K.; Chen, H.F.; Wang, J.J.; Huang, G.G. A Simple Visible Recognition Method for Copper Ions Using Dibenzo [b, j][1, 10] Phenanthroline Scaffold as a Colorimetric Sensor. Chemosensors 2021, 9, 7. [Google Scholar] [CrossRef]
- Huang, S.W.; Lin, Y.F.; Li, Y.X.; Hu, C.C.; Chiu, T.C. Synthesis of fluorescent carbon dots as selective and sensitive probes for cupric ions and cell imaging. Molecules 2019, 24, 1785. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Chen, C.; Wang, J.; Luo, F.; Guo, L.; Qiu, B.; Lin, Z. A Bright Nitrogen-doped-Carbon-Dots based Fluorescent Biosensor for Selective Detection of Copper Ions. J. Anal. Test. 2021, 5, 84–92. [Google Scholar] [CrossRef]


| Materials | Method | Linear Range (µM) | Limit of Detection (µM) | Reference |
|---|---|---|---|---|
| Receptor L | Colorimetric | 0–50 | 2.82 | [57] |
| Rhodamine | Colorimetric | 0–30 | 0.48 | [58] |
| Dibenzo[b,j][1,10]Phenanthroline | Colorimetric | 10–100 | 0.14 | [59] |
| picolylamine-armed calix [4]arene (4-PACX) | Colorimetric Fluorescence | 1–11 1–50 | 5.1 0.96 | [56] |
| GSH-CdTe QDs | Fluorescence | 0.02–1.1 | 0.0101 | [55] |
| CDs | Colorimetric Fluorescence | 0.01–10 0.1–2 | 0.004 0.09 | [53] |
| CDs | Fluorescence | 0.5–7 | 0.15 | [60] |
| N-CDs | Fluorescence | 0.05–25 | 0.023 | [61] |
| Adenine-stabilized CDs | Fluorescence | 0.001–0.75 | 0.0003 | [54] |
| CDs | Fluorescence | 0.002–0.080 | 0.0018 | [30] |
| NSCDs | Colorimetric Fluorescence | 1–100 0.5–100 | 0.1 1 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzoferrato, R.; Bisauriya, R.; Antonaroli, S.; Cabibbo, M.; Moro, A.J. Colorimetric and Fluorescent Sensing of Copper Ions in Water through o-Phenylenediamine-Derived Carbon Dots. Sensors 2023, 23, 3029. https://doi.org/10.3390/s23063029
Pizzoferrato R, Bisauriya R, Antonaroli S, Cabibbo M, Moro AJ. Colorimetric and Fluorescent Sensing of Copper Ions in Water through o-Phenylenediamine-Derived Carbon Dots. Sensors. 2023; 23(6):3029. https://doi.org/10.3390/s23063029
Chicago/Turabian StylePizzoferrato, Roberto, Ramanand Bisauriya, Simonetta Antonaroli, Marcello Cabibbo, and Artur J. Moro. 2023. "Colorimetric and Fluorescent Sensing of Copper Ions in Water through o-Phenylenediamine-Derived Carbon Dots" Sensors 23, no. 6: 3029. https://doi.org/10.3390/s23063029





