Tungsten Oxide Coated Liquid Metal Electrodes via Galvanic Replacement as Heavy Metal Ion Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Galvanic Replacement
2.2.2. Galinstan and WO-Galinstan Sensor Fabrication
2.2.3. Differential Pulse Stripping Voltammetry (DPSV) for Sensor Measurements
2.2.4. Characterization
3. Results and Discussion
3.1. Galinstan Electrode Fabrication and Galvanic Replacement
3.2. Application as a HMI Pb2+ Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Galvanic Replacement on Galinstan Droplets



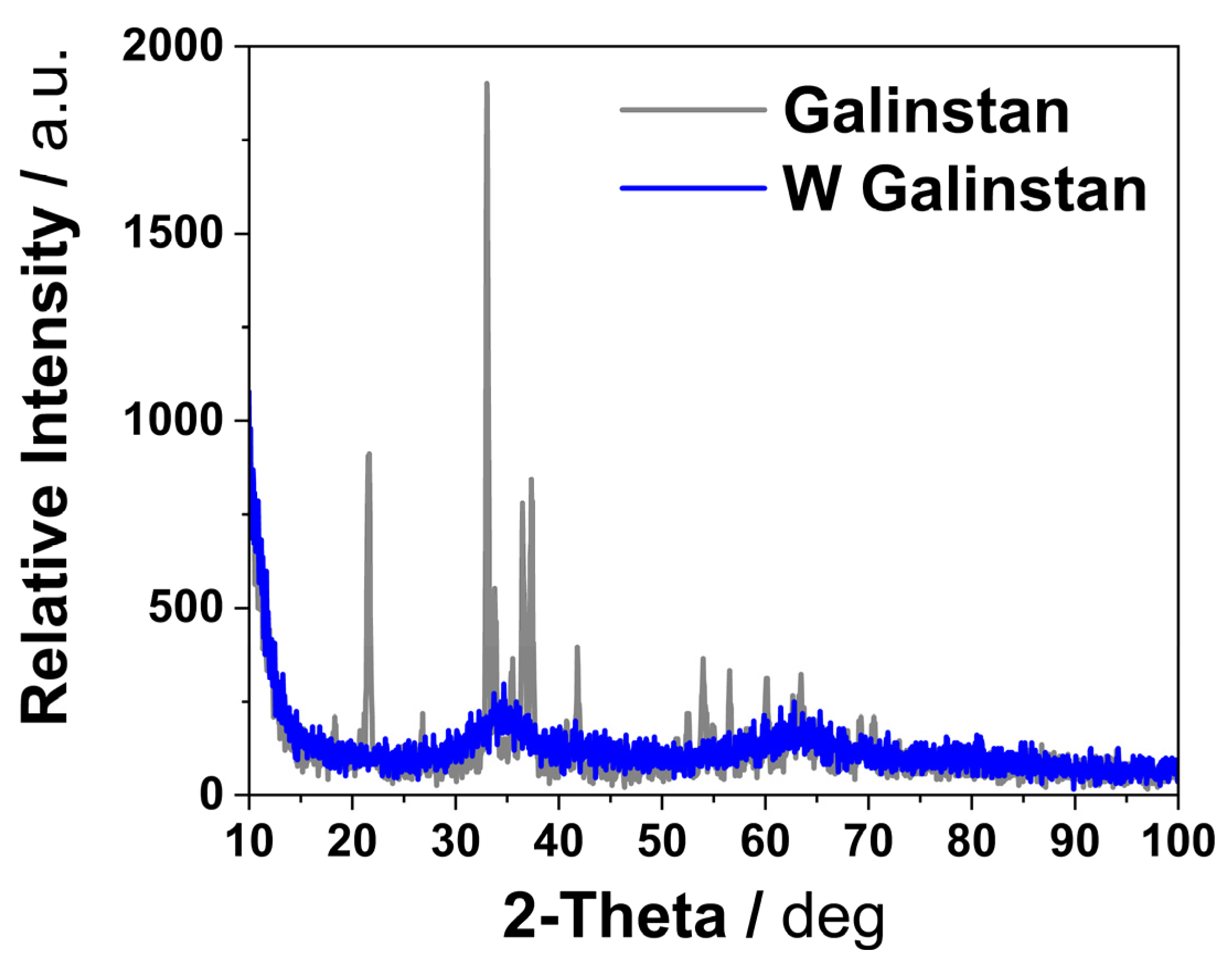
Appendix A.2. Sample Preparation for XRD Measurements
References
- Dickey, M.D.; Chiechi, R.C.; Larsen, R.J.; Weiss, E.A.; Weitz, D.A.; Whitesides, G.M. Eutectic Gallium-Indium (EGaIn): A Liquid Metal Alloy for the Formation of Stable Structures in Microchannels at Room Temperature. Adv. Funct. Mater. 2008, 18, 1097–1104. [Google Scholar] [CrossRef]
- Dickey, M.D. Emerging Applications of Liquid Metals Featuring Surface Oxides. ACS Appl. Mater. Interfaces 2014, 6, 18369–18379. [Google Scholar] [CrossRef] [PubMed]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-zadeh, K. Liquid Metals: Fundamentals and Applications in Chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wu, Z. Microfluidic Electronics. Lab. Chip. 2012, 12, 2782. [Google Scholar] [CrossRef]
- Khoshmanesh, K.; Tang, S.-Y.; Zhu, J.Y.; Schaefer, S.; Mitchell, A.; Kalantar-zadeh, K.; Dickey, M.D. Liquid Metal Enabled Microfluidics. Lab. Chip. 2017, 17, 974–993. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-Y.; Khoshmanesh, K.; Sivan, V.; Petersen, P.; O’Mullane, A.P.; Abbott, D.; Mitchell, A.; Kalantar-zadeh, K. Liquid Metal Enabled Pump. Proc. Natl. Acad. Sci. USA 2014, 111, 3304–3309. [Google Scholar] [CrossRef]
- Li, G.; Du, J.; Zhang, A.; Lee, D. Artificial Heart Based on Electrically Controlled Non-Toxic Liquid Metal Pump. Adv. Eng. Mater. 2019, 21, 1900381. [Google Scholar] [CrossRef]
- Lv, P.; Yang, X.; Bisoyi, H.K.; Zeng, H.; Zhang, X.; Chen, Y.; Xue, P.; Shi, S.; Priimagi, A.; Wang, L.; et al. Stimulus-Driven Liquid Metal and Liquid Crystal Network Actuators for Programmable Soft Robotics. Mater. Horiz. 2021, 8, 2475–2484. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Sivan, V.; Petersen, P.; Zhang, W.; Morrison, P.D.; Kalantar-zadeh, K.; Mitchell, A.; Khoshmanesh, K. Liquid Metal Actuator for Inducing Chaotic Advection. Adv. Funct. Mater. 2014, 24, 5851–5858. [Google Scholar] [CrossRef]
- Hutter, T.; Bauer, W.-A.C.; Elliott, S.R.; Huck, W.T.S. Formation of Spherical and Non-Spherical Eutectic Gallium-Indium Liquid-Metal Microdroplets in Microfluidic Channels at Room Temperature. Adv. Funct. Mater. 2012, 22, 2624–2631. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Joshipura, I.D.; Lin, Y.; Kalantar-Zadeh, K.; Mitchell, A.; Khoshmanesh, K.; Dickey, M.D. Liquid-Metal Microdroplets Formed Dynamically with Electrical Control of Size and Rate. Adv. Mater. 2016, 28, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Gol, B.; Tovar-Lopez, F.J.; Kurdzinski, M.E.; Tang, S.-Y.; Petersen, P.; Mitchell, A.; Khoshmanesh, K. Continuous Transfer of Liquid Metal Droplets across a Fluid–Fluid Interface within an Integrated Microfluidic Chip. Lab. Chip. 2015, 15, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; O’Brien, C.; Hamza, A.; Sharma, S.; Rein, C.; Sanjaya, M.; Helmer, D.; Kotz-Helmer, F.; Pezeshkpour, P.; Rapp, B.E. An On-Chip. Liquid Metal Plug Generator. Adv. Mater. 2022, 34, 2201469. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, N.; Cook, A.; Tabor, C.E. Designing Liquid Metal Interfaces to Enable Next Generation Flexible and Reconfigurable Electronics. Adv. Mater. Interfaces 2017, 4, 1700141. [Google Scholar] [CrossRef]
- Cumby, B.L.; Hayes, G.J.; Dickey, M.D.; Justice, R.S.; Tabor, C.E.; Heikenfeld, J.C. Reconfigurable Liquid Metal Circuits by Laplace Pressure Shaping. Appl. Phys. Lett. 2012, 101, 174102. [Google Scholar] [CrossRef]
- Boley, J.W.; White, E.L. Direct Writing of Gallium-Indium Alloy for Stretchable Electronics. Adv. Funct. Mater. 2014, 24, 3501–3507. [Google Scholar] [CrossRef]
- Dickey, M.D. Stretchable and Soft Electronics Using Liquid Metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef]
- Wang, M.; Trlica, C.; Khan, M.R.; Dickey, M.D.; Adams, J.J. A Reconfigurable Liquid Metal Antenna Driven by Electrochemically Controlled Capillarity. J. Appl. Phys. 2015, 117, 194901. [Google Scholar] [CrossRef]
- Rashed Khan, M.; Hayes, G.J.; So, J.-H.; Lazzi, G.; Dickey, M.D. A Frequency Shifting Liquid Metal Antenna with Pressure Responsiveness. Appl. Phys. Lett. 2011, 99, 013501. [Google Scholar] [CrossRef]
- Lin, Y.; Cooper, C.; Wang, M.; Adams, J.J.; Genzer, J.; Dickey, M.D. Handwritten, Soft Circuit Boards and Antennas Using Liquid Metal Nanoparticles. Small 2015, 11, 6397–6403. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, C.; Sun, X.; Liu, J. Liquid-Metal-Enhanced Wire Mesh as a Stiffness Variable Material for Making Soft Robotics. Adv. Eng. Mater. 2019, 21, 1900530. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Lu, Y.; Mei, D. 3D Printing of Liquid Metal Based Tactile Sensor for Simultaneously Sensing of Temperature and Forces. Int. J. Smart Nano Mater. 2021, 12, 269–285. [Google Scholar] [CrossRef]
- Surmann, P.; Zeyat, H. Voltammetric Analysis Using a Self-Renewable Non-Mercury Electrode. Anal. Bioanal. Chem. 2005, 383, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Surmann, P.; Channaa, H. Anodic Stripping Voltammetry with Galinstan as Working Electrode. Electroanalysis 2015, 27, 1726–1732. [Google Scholar] [CrossRef]
- Zheng, J.; Rahim, M.A.; Tang, J.; Allioux, F.; Kalantar-Zadeh, K. Post-Transition Metal Electrodes for Sensing Heavy Metal Ions by Stripping Voltammetry. Adv. Mater. Technol. 2022, 7, 2100760. [Google Scholar] [CrossRef]
- Baharfar, M.; Kalantar-Zadeh, K. Emerging Role of Liquid Metals in Sensing. ACS Sens. 2022, 7, 386–408. [Google Scholar] [CrossRef]
- Sivan, V.; Tang, S.-Y.; O’Mullane, A.P.; Petersen, P.; Eshtiaghi, N.; Kalantar-zadeh, K.; Mitchell, A. Liquid Metal Marbles. Adv. Funct. Mater. 2013, 23, 144–152. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, J.Z.; Tang, S.-Y.; Sivan, V.; Yao, D.D.; Latham, K.; Khoshmanesh, K.; Mitchell, A.; O’Mullane, A.P.; Kalantar-zadeh, K. Liquid Metal/Metal Oxide Frameworks. Adv. Funct. Mater. 2014, 24, 3799–3807. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Chang, H.; Rao, W. Galvanic Replacement of Liquid Metal/Reduced Graphene Oxide Frameworks. Adv. Mater. Interfaces 2020, 7, 2000626. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, T.; Li, Y.; Zhu, L.; Handschuh-Wang, S.; Zhu, D.; Zhou, X.; Liu, Z.; Gan, T.; Zhou, X. Robust Fabrication of Nonstick, Noncorrosive, Conductive Graphene-Coated Liquid Metal Droplets for Droplet-Based, Floating Electrodes. Adv. Funct. Mater. 2018, 28, 1706277. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zhang, Y.; Wu, J.; Li, S.; Li, L. A Disposable Electrochemical Sensor for Lead Ion Detection Based on In Situ Polymerization of Conductive Polypyrrole Coating. J. Electron. Mater. 2023, 52, 1819–1828. [Google Scholar] [CrossRef]
- Rubino, A.; Queirós, R. Electrochemical Determination of Heavy Metal Ions Applying Screen-Printed Electrodes Based Sensors. A Review on Water and Environmental Samples Analysis. Talanta Open 2023, 7, 100203. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Ruditskiy, A.; Xia, Y. 25th Anniversary Article: Galvanic Replacement: A Simple and Versatile Route to Hollow Nanostructures with Tunable and Well-Controlled Properties. Adv. Mater. 2013, 25, 6313–6333. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.G.M.; Rodrigues, T.S.; Haigh, S.J.; Camargo, P.H.C. Galvanic Replacement Reaction: Recent Developments for Engineering Metal Nanostructures towards Catalytic Applications. Chem. Commun. 2017, 53, 7135–7148. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Venkateshalu, S.; Jeong, S.; Tomboc, G.M.; Jo, J.; Park, J.; Lee, K. Galvanic Replacement Reaction to Prepare Catalytic Materials. Bull. Korean Chem. Soc. 2023, 44, 4–22. [Google Scholar] [CrossRef]
- Hoshyargar, F.; Crawford, J. Galvanic Replacement of the Liquid Metal Galinstan. J. Am. Chem. Soc. 2017, 139, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- David, R.; Miki, N. Tunable Noble Metal Thin Films on Ga Alloys via Galvanic Replacement. Langmuir 2018, 34, 10550–10559. [Google Scholar] [CrossRef]
- David, R.; Miki, N. Synthesis of Sub-Micrometer Biphasic Au–AuGa2/Liquid Metal Frameworks. Nanoscale 2019, 11, 21419–21432. [Google Scholar] [CrossRef]
- Oloye, O.; Tang, C.; Du, A.; Will, G.; O’Mullane, A.P. Galvanic Replacement of Liquid Metal Galinstan with Pt for the Synthesis of Electrocatalytically Active Nanomaterials. Nanoscale 2019, 11, 9705–9715. [Google Scholar] [CrossRef]
- Castilla-Amorós, L.; Stoian, D.; Pankhurst, J.R.; Varandili, S.B.; Buonsanti, R. Exploring the Chemical Reactivity of Gallium Liquid Metal Nanoparticles in Galvanic Replacement. J. Am. Chem. Soc. 2020, 142, 19283–19290. [Google Scholar] [CrossRef]
- Falchevskaya, A.S.; Prilepskii, A.Y.; Tsvetikova, S.A.; Koshel, E.I.; Vinogradov, V.V. Facile Synthesis of a Library of Hollow Metallic Particles through the Galvanic Replacement of Liquid Gallium. Chem. Mater. 2021, 33, 1571–1580. [Google Scholar] [CrossRef]
- Ghasemian, M.B.; Wang, Y.; Allioux, F.-M.; Zavabeti, A.; Kalantar-Zadeh, K. Coating of Gallium-Based Liquid Metal Particles with Molybdenum Oxide and Oxysulfide for Electronic Band Structure Modulation. Nanoscale 2023, 15, 5891–5898. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Ghasemian, M.B.; Rahim, M.A.; Baharfar, M.; Yang, J.; Tang, J.; Kalantar-Zadeh, K.; Allioux, F.-M. Formation of Inorganic Liquid Gallium Particle–Manganese Oxide Composites. Nanoscale 2023, 15, 4291–4300. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.Y.; Cheeseman, S.; Frias-De-Diego, A.; Hong, H.; Yang, J.; Jung, W.; Yin, H.; Murdoch, B.J.; Scholle, F.; Crook, N.; et al. A Liquid Metal Mediated Metallic Coating for Antimicrobial and Antiviral Fabrics. Adv. Mater. 2021, 33, 2104298. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, W.; Gao, X.; Lu, J.; Ye, J.; Li, Y.; Fahad, A.; Zhang, G.; Zhao, L. Nanoheterostructure by Liquid Metal Sandwich-Based Interfacial Galvanic Replacement for Cancer Targeted Theranostics. Small 2023, 19, 2300751. [Google Scholar] [CrossRef]
- Ren, L.; Cheng, N.; Man, X.; Qi, D.; Liu, Y.; Xu, G.; Cui, D.; Liu, N.; Zhong, J.; Peleckis, G.; et al. General Programmable Growth of Hybrid Core–Shell Nanostructures with Liquid Metal Nanodroplets. Adv. Mater. 2021, 33, 2008024. [Google Scholar] [CrossRef]
- Park, C.W.; Moon, Y.G.; Seong, H.; Jung, S.W.; Oh, J.-Y.; Na, B.S.; Park, N.-M.; Lee, S.S.; Im, S.G.; Koo, J.B. Photolithography-Based Patterning of Liquid Metal Interconnects for Monolithically Integrated Stretchable Circuits. ACS Appl. Mater. Interfaces 2016, 8, 15459–15465. [Google Scholar] [CrossRef]
- Barlag, R.; Nyasulu, F.; Starr, R.; Silverman, J.; Arthasery, P.; McMills, L. A Student-Made Silver–Silver Chloride Reference Electrode for the General Chemistry Laboratory: ∼10 Min Preparation. J. Chem. Educ. 2014, 91, 766–768. [Google Scholar] [CrossRef]




| O1s (at. %) | Ga2p1 (at. %) | Ga3d (at. %) | W4f (at. %) | |
|---|---|---|---|---|
| Galinstan | 64.62 | 33.63 | 1.75 | 0 |
| WO-Galinstan | 77.26 | 17.96 | 0 | 4.78 |
| Materials | Measurement Technique | Limit of Detection (mmol·L−1) | Comments | Reference |
|---|---|---|---|---|
| Galinstan (Hanging droplet) | Differential Pulse Voltammetry | 0.91 | Hanging droplets offer higher LM volume for HMI detection | [23] |
| Galinstan (Hanging droplet) | Differential Pulse Stripping Voltammetry | 0.008 | In addition to a higher volume, stripping voltammetry involves a pre-conditioning step wherein lead ions amalgamate, resulting in lower limit of detection values | [24] |
| Galinstan with WO3 coating (Hanging droplet) | Differential Pulse Stripping Voltammetry | 10 | WO3 micro-nanopowder coatings on Galinstan droplets | [27] |
| EGaIn-GO particles suspended on carbon paper | Differential Pulse Voltammetry | 10 | EGaIn-GO particles fabricated via sonication of EGaIn in GO suspension | [29] |
| Galinstan and WO-Galinstan planar electrodes via GR | Differential Pulse Stripping Voltammetry | 0.1 | Both Galinstan and WO-Galinstan (via GR) planar electrodes show excellent sensitivity to 0.1 mmol·L−1 Pb2+ | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhagwat, S.; Hambitzer, L.; Prediger, R.; Zhu, P.; Hamza, A.; Kilian, S.K.; Kluck, S.; Pezeshkpour, P.; Kotz-Helmer, F.; Rapp, B.E. Tungsten Oxide Coated Liquid Metal Electrodes via Galvanic Replacement as Heavy Metal Ion Sensors. Sensors 2024, 24, 416. https://doi.org/10.3390/s24020416
Bhagwat S, Hambitzer L, Prediger R, Zhu P, Hamza A, Kilian SK, Kluck S, Pezeshkpour P, Kotz-Helmer F, Rapp BE. Tungsten Oxide Coated Liquid Metal Electrodes via Galvanic Replacement as Heavy Metal Ion Sensors. Sensors. 2024; 24(2):416. https://doi.org/10.3390/s24020416
Chicago/Turabian StyleBhagwat, Sagar, Leonhard Hambitzer, Richard Prediger, Pang Zhu, Ahmed Hamza, Sophia K. Kilian, Sebastian Kluck, Pegah Pezeshkpour, Frederik Kotz-Helmer, and Bastian E. Rapp. 2024. "Tungsten Oxide Coated Liquid Metal Electrodes via Galvanic Replacement as Heavy Metal Ion Sensors" Sensors 24, no. 2: 416. https://doi.org/10.3390/s24020416





