Recent Trends in Chemical Sensors for Detecting Toxic Materials
Abstract
1. Introduction
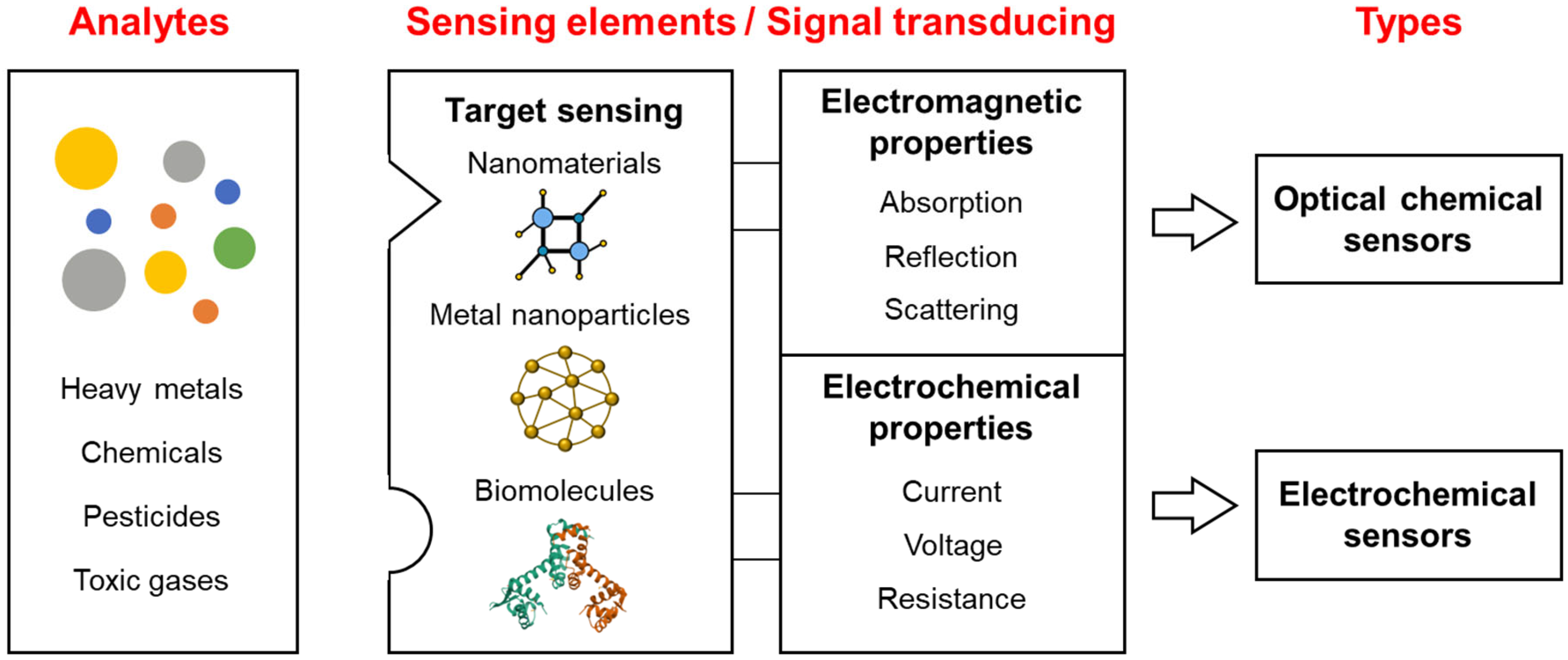
2. Overview of Chemical Sensors for Detecting Toxic Materials
3. Optical Chemical Sensors
3.1. Fiber Optic Chemical Sensors
3.2. Microfluidic System-Based Optical Chemical Sensors
3.3. Nanoparticle-Based Optical Chemical Sensors
3.4. Nanomaterial-Based Optical Chemical Sensors
4. Electrochemical Sensors
4.1. CNM-Based Electrochemical Sensors
4.2. NP-Based Electrochemical Sensors
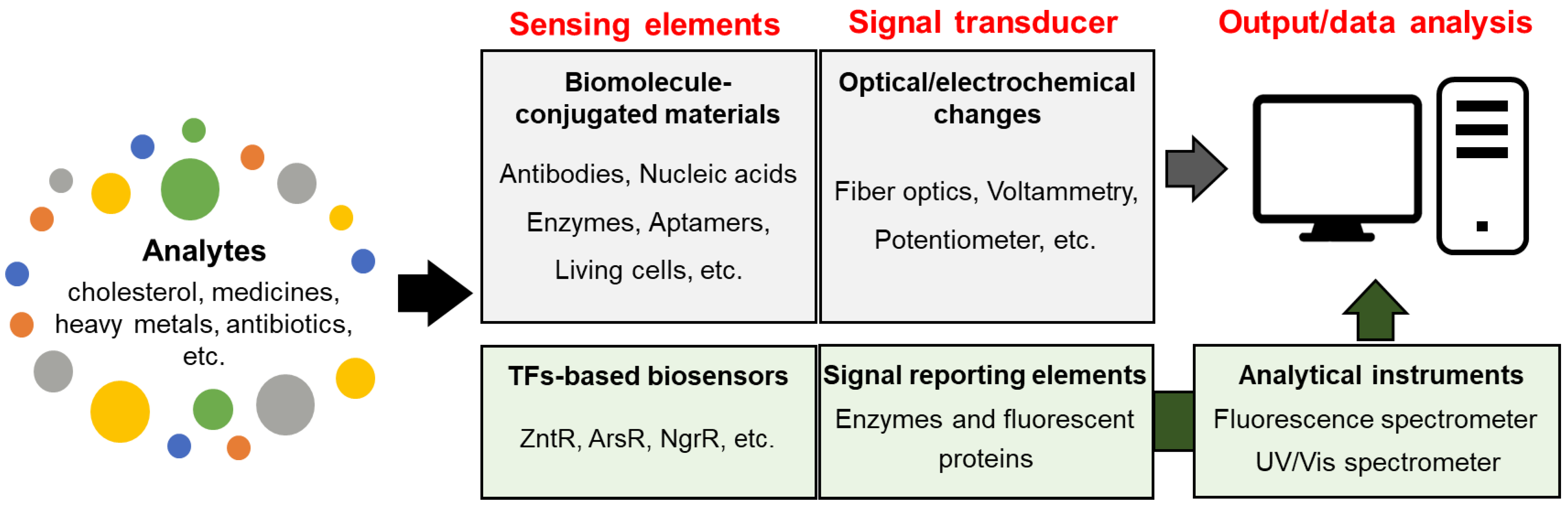
5. Biosensors
5.1. Optical Biosensors and Electrochemical Biosensors
5.2. Enzyme-Based Biosensors
5.3. Biomolecule-Based Biosensors
5.4. TF-Based Biosensors
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. Environmental pollution: Causes, effects, and the remedies. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. [Google Scholar]
- Ajibade, F.O.; Adelodun, B.; Lasisi, K.H.; Fadare, O.O.; Ajibade, T.F.; Nwogwu, N.A.; Sulaymon, I.D.; Ugya, A.Y.; Wang, H.C.; Wang, A. Environmental pollution and their socioeconomic impacts. In Microbe Mediated Remediation of Environmental Contaminants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 321–354. [Google Scholar]
- Young, S.; Balluz, L.; Malilay, J. Natural and technologic hazardous material releases during and after natural disasters: A review. Sci. Total Environ. 2004, 322, 3–20. [Google Scholar] [CrossRef]
- Corn, M. Handbook of Hazardous Materials; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Ha, S.; Seidle, T.; Lim, K.-M. Act on the Registration and Evaluation of Chemicals (K-REACH) and replacement, reduction or refinement best practices. Environ. Health Toxicol. 2016, 31, e2016026. [Google Scholar] [CrossRef]
- Rouessac, F.; Rouessac, A. Chemical Analysis: Modern Instrumentation Methods and Techniques; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Chen, H.; Zheng, J.; Zhang, X.; Luo, M.; Wang, Z.; Qiao, X. Surface desorption atmospheric pressure chemical ionization mass spectrometry for direct ambient sample analysis without toxic chemical contamination. J. Mass Spectrom. 2007, 42, 1045–1056. [Google Scholar] [CrossRef]
- Leary, P.E.; Kammrath, B.W.; Lattman, K.J.; Beals, G.L. Deploying portable gas chromatography–mass spectrometry (GC-MS) to military users for the identification of toxic chemical agents in theater. Appl. Spectrosc. 2019, 73, 841–858. [Google Scholar] [CrossRef]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Cammann, K.; Lemke, U.; Rohen, A.; Sander, J.; Wilken, H.; Winter, B. Chemical sensors and biosensors—Principles and applications. Angew. Chem. Int. Ed. Engl. 1991, 30, 516–539. [Google Scholar] [CrossRef]
- Janata, J. Principles of Chemical Sensors; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical sensors. Anal. Chem. 2010, 82, 4723–4741. [Google Scholar] [CrossRef] [PubMed]
- Karker, N.; Dharmalingam, G.; Carpenter, M.A. Thermal energy harvesting plasmonic based chemical sensors. ACS Nano 2014, 8, 10953–10962. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors. Anal. Chem. 2006, 78, 3859–3874. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-J.; Choi, Y.-K. Chemical sensors based on nanostructured materials. Sens. Actuators B Chem. 2007, 122, 659–671. [Google Scholar] [CrossRef]
- Jimenez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Star, A. Carbon nanotube gas and vapor sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Wolfbeis, O.S. Optical biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Meyyappan, M. Carbon nanotube-based chemical sensors. Small 2016, 12, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Yavari, F.; Koratkar, N. Graphene-based chemical sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Faddah, L.M. Nanoparticles as biochemical sensors. Nanotechnol. Sci. Appl. 2010, 3, 65–76. [Google Scholar] [CrossRef]
- Ispas, C.R.; Crivat, G.; Andreescu, S. Recent developments in enzyme-based biosensors for biomedical analysis. Anal. Lett. 2012, 45, 168–186. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Balaji, T.; El-Safty, S.A.; Matsunaga, H.; Hanaoka, T.; Mizukami, F. Optical sensors based on nanostructured cage materials for the detection of toxic metal ions. Angew. Chem. 2006, 118, 7360–7366. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Baghayeri, M.; Karimi, F.; Fu, L.; Rouhi, J.; Niculina, D.E.; Gündüz, E.S.; Dragoi, E. Recent developments in carbon nanomaterials-based electrochemical sensors for methyl parathion detection. J. Food Meas. Charact. 2023, 17, 5371–5389. [Google Scholar] [CrossRef]
- Dong, J.; Wang, X.; Qiao, F.; Liu, P.; Ai, S. Highly sensitive electrochemical stripping analysis of methyl parathion at MWCNTs–CeO2–Au nanocomposite modified electrode. Sens. Actuators B Chem. 2013, 186, 774–780. [Google Scholar] [CrossRef]
- Qazi, H.H.; Mohammad, A.B.b.; Akram, M. Recent progress in optical chemical sensors. Sensors 2012, 12, 16522–16556. [Google Scholar] [CrossRef]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical chemical sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. Materials for fluorescence-based optical chemical sensors. J. Mater. Chem. 2005, 15, 2657–2669. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Lisse, C.; Medawala, W.; Brady, P.N.; Bwambok, D.K.; Anum, D.; Alonge, T.; Taylor, M.E.; Baker, G.A.; Mehari, T.F. Fluorescent chemical sensors: Applications in analytical, environmental, forensic, pharmaceutical, biological, and biomedical sample measurement, and clinical diagnosis. Appl. Spectrosc. Rev. 2023, 59, 1–89. [Google Scholar] [CrossRef]
- Singh, A.K.; Mittal, S.; Das, M.; Saharia, A.; Tiwari, M. Optical biosensors: A decade in review. Alex. Eng. J. 2023, 67, 673–691. [Google Scholar] [CrossRef]
- Lee, B.; Roh, S.; Park, J. Current status of micro-and nano-structured optical fiber sensors. Opt. Fiber Technol. 2009, 15, 209–221. [Google Scholar] [CrossRef]
- Lin, J. Recent development and applications of optical and fiber-optic pH sensors. TrAC Trends Anal. Chem. 2000, 19, 541–552. [Google Scholar] [CrossRef]
- Kim, J.A.; Hwang, T.; Dugasani, S.R.; Amin, R.; Kulkarni, A.; Park, S.H.; Kim, T. Graphene based fiber optic surface plasmon resonance for bio-chemical sensor applications. Sens. Actuators B Chem. 2013, 187, 426–433. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R. An optical fiber based surface plasmon resonance technique for sensing of lead ions: A toxic water pollutant. Opt. Fiber Technol. 2018, 46, 152–156. [Google Scholar] [CrossRef]
- Ozcariz, A.; Ruiz-Zamarreno, C.; Arregui, F.J. A comprehensive review: Materials for the fabrication of optical fiber refractometers based on lossy mode resonance. Sensors 2020, 20, 1972. [Google Scholar] [CrossRef]
- Ju, S.; Nguyen, V.L.; Watekar, P.R.; Kim, B.H.; Jeong, C.; Boo, S.; Kim, C.J.; Han, W.-T. Fabrication and optical characteristics of a novel optical fiber doped with the Au nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 3555–3558. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.M.; Nevill, J.T.; Pettigrew, K.I.; Votaw, G.; Kung, P.-J.; Crenshaw, H.C. A low-cost, manufacturable method for fabricating capillary and optical fiber interconnects for microfluidic devices. Lab A Chip 2008, 8, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Usha, S.P.; Gupta, B.D. A lossy mode resonance-based fiber optic hydrogen gas sensor for room temperature using coatings of ITO thin film and nanoparticles. Meas. Sci. Technol. 2016, 27, 045103. [Google Scholar] [CrossRef]
- Usha, S.P.; Gupta, B.D. Performance analysis of zinc oxide-implemented lossy mode resonance-based optical fiber refractive index sensor utilizing thin film/nanostructure. Appl. Opt. 2017, 56, 5716–5725. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Teng, P.; Kong, D.; Gao, S.; Liu, Z.; Yang, J.; Gao, D.; Luo, M.; Wen, X. Determination of the antibiotic minocycline by integrated optofluidic microstructured polymer optical fiber chemiluminescence. Instrum. Sci. Technol. 2021, 49, 571–584. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.-j.; Wei, J.-f.; Xu, J.-r.; Wang, Y.-h.; Zheng, G.-x. Paper-based three-dimensional microfluidic device for monitoring of heavy metals with a camera cell phone. Anal. Bioanal. Chem. 2014, 406, 2799–2807. [Google Scholar] [CrossRef]
- Jayawardane, B.M.; Wei, S.; McKelvie, I.D.; Kolev, S.D. Microfluidic paper-based analytical device for the determination of nitrite and nitrate. Anal. Chem. 2014, 86, 7274–7279. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, K.-B.; Shin, K.-S.; Park, H.-D.; Kim, M.-C.; Kim, J.-R.; Park, S.-J.; Song, Y.-H. Design, fabrication and characterization of an integrated micro ammonia analysis system (IMAAS) with microreactor and in-plane type optical detector based on the Berthelot reaction. Sens. Actuators B Chem. 2006, 117, 516–522. [Google Scholar] [CrossRef]
- He, S.; Li, D.; Zhu, C.; Song, S.; Wang, L.; Long, Y.; Fan, C. Design of a gold nanoprobe for rapid and portable mercury detection with the naked eye. Chem. Commun. 2008, 40, 4885–4887. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, C.-J.; Tao, T.; Duan, M.; Fang, S.-W.; Zheng, M. A miniaturized fiber-optic colorimetric sensor for nitrite determination by coupling with a microfluidic capillary waveguide. Anal. Bioanal. Chem. 2016, 408, 3413–3423. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, N.; Takarada, T.; Maeda, M. Rapid naked-eye detection of mercury ions based on non-crosslinking aggregation of double-stranded DNA-carrying gold nanoparticles. Chem. Commun. 2011, 47, 2077–2079. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Jin, J.; Chen, Y.; Shao, N.; Kang, H.; Xiao, Z.; Tang, Z.; Wu, Y.; Zhu, Z.; Tan, W. Carbon nanotube-quenched fluorescent oligonucleotides: Probes that fluoresce upon hybridization. J. Am. Chem. Soc. 2008, 130, 8351–8358. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Yan, Y.; Hou, Y.; Li, G.; Hao, C. Novel carbon quantum dots for fluorescent detection of phenol and insights into the mechanism. New J. Chem. 2018, 42, 11485–11492. [Google Scholar] [CrossRef]
- Babar, D.G.; Garje, S.S. Nitrogen and phosphorus co-doped carbon dots for selective detection of nitro explosives. ACS Omega 2020, 5, 2710–2717. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jia, H.; Xu, D.; Wang, J. Novel method in emerging environmental contaminants detection: Fiber optic sensors based on microfluidic chips. Sci. Total Environ. 2023, 857, 159563. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, G.; Lee, L.M. Optical imaging techniques in microfluidics and their applications. Lab A Chip 2012, 12, 3566–3575. [Google Scholar] [CrossRef]
- Orti, V.; Audran, M.; Gibert, P.; Bougard, G.; Bressolle, F. High-performance liquid chromatographic assay for minocycline in human plasma and parotid saliva. J. Chromatogr. B Biomed. Sci. Appl. 2000, 738, 357–365. [Google Scholar] [CrossRef]
- Jaywant, S.A.; Arif, K.M. A comprehensive review of microfluidic water quality monitoring sensors. Sensors 2019, 19, 4781. [Google Scholar] [CrossRef]
- Yin, M.-j.; Gu, B.; An, Q.-F.; Yang, C.; Guan, Y.L.; Yong, K.-T. Recent development of fiber-optic chemical sensors and biosensors: Mechanisms, materials, micro/nano-fabrications and applications. Coord. Chem. Rev. 2018, 376, 348–392. [Google Scholar] [CrossRef]
- Kuswandi, B.; Huskens, J.; Verboom, W. Optical sensing systems for microfluidic devices: A review. Anal. Chim. Acta 2007, 601, 141–155. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef] [PubMed]
- Chemchem, M.; Chemchem, A.; Aydıner, B.; Seferoğlu, Z. Recent advances in colorimetric and fluorometric sensing of neurotransmitters by organic scaffolds. Eur. J. Med. Chem. 2022, 244, 114820. [Google Scholar] [CrossRef] [PubMed]
- Montes-García, V.; Squillaci, M.A.; Diez-Castellnou, M.; Ong, Q.K.; Stellacci, F.; Samori, P. Chemical sensing with Au and Ag nanoparticles. Chem. Soc. Rev. 2021, 50, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, C. Detection of chemical pollutants in water using gold nanoparticles as sensors: A review. Rev. Anal. Chem. 2013, 32, 1–14. [Google Scholar] [CrossRef]
- Cho, H.H.; Jung, D.H.; Heo, J.H.; Lee, C.Y.; Jeong, S.Y.; Lee, J.H. Gold Nanoparticles as Exquisite Colorimetric Transducers for Water Pollutant Detection. ACS Appl. Mater. Interfaces 2023, 15, 19785–19806. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Sharma, R.K.; Wangoo, N. Highly sensitive colorimetric detection of ethyl parathion using gold nanoprobes. Sens. Actuators B Chem. 2015, 210, 425–430. [Google Scholar] [CrossRef]
- Li, D.; Wang, S.; Wang, L.; Zhang, H.; Hu, J. A simple colorimetric probe based on anti-aggregation of AuNPs for rapid and sensitive detection of malathion in environmental samples. Anal. Bioanal. Chem. 2019, 411, 2645–2652. [Google Scholar] [CrossRef]
- Khattab, T.A.; Abdelrahman, M.S. Advances in gold nanoparticles for optical detection of nerve agents. In Sensing of Deadly Toxic Chemical Warfare Agents, Nerve Agent Simulants, and Their Toxicological Aspects; Elsevier: Amsterdam, The Netherlands, 2023; pp. 111–131. [Google Scholar]
- Sahu, B.; Kurrey, R.; Deb, M.K.; Khalkho, B.R.; Manikpuri, S. Recognition of malathion pesticides in agricultural samples by using α-CD functionalized gold nanoparticles as a colorimetric sensor. Talanta 2023, 259, 124526. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lou, X.-Y.; Liang, F.; Yang, Y.-W. Surface-functionalized gold and silver nanoparticles for colorimetric and fluorescent sensing of metal ions and biomolecules. Coord. Chem. Rev. 2022, 459, 214461. [Google Scholar] [CrossRef]
- Ding, X.; Kong, L.; Wang, J.; Fang, F.; Li, D.; Liu, J. Highly sensitive SERS detection of Hg2+ ions in aqueous media using gold nanoparticles/graphene heterojunctions. ACS Appl. Mater. Interfaces 2013, 5, 7072–7078. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. TrAC Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Bhasin, K.; Baskoutas, S. Chemical sensing applications of ZnO nanomaterials. Materials 2018, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H. Chemical preparation of graphene-based nanomaterials and their applications in chemical and biological sensors. Small 2011, 7, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, T.; Qamar, A.Z.; Slaughter, G. Application of nanomaterials for chemical and biological sensors: A review. IEEE Sens. J. 2020, 21, 12407–12425. [Google Scholar] [CrossRef]
- Baptista, F.R.; Belhout, S.A.; Giordani, S.; Quinn, S.J. Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef]
- Speranza, G. Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Amirfazli, M.; Massah, F.; Bagheri, Z. Application of functionalized carbon dots in detection, diagnostic, disease treatment, and desalination: A review. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 025017. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.L.; Li, C.M.; Huang, C.Z. Fluorescent carbon dots functionalization. Adv. Colloid Interface Sci. 2019, 270, 165–190. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2018, 119, 599–663. [Google Scholar] [CrossRef] [PubMed]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.A.A.; Yuce, M.R. Recent progress in nanomaterial enabled chemical sensors for wearable environmental monitoring applications. Adv. Funct. Mater. 2020, 30, 2005703. [Google Scholar] [CrossRef]
- Bakker, E.; Telting-Diaz, M. Electrochemical sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, G.; Patil, D.G.; Wang, J. Electrochemical sensors for environmental monitoring: Design, development and applications. J. Environ. Monit. 2004, 6, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Stradiotto, N.R.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef]
- Wu, G.; Zheng, H.; Xing, Y.; Wang, C.; Yuan, X.; Zhu, X. A sensitive electrochemical sensor for environmental toxicity monitoring based on tungsten disulfide nanosheets/hydroxylated carbon nanotubes nanocomposite. Chemosphere 2022, 286, 131602. [Google Scholar] [CrossRef]
- Wanjari, V.P.; Duttagupta, S.P.; Singh, S.P. Dual Linear Range Laser-Induced Graphene-Based Sensor for 4-Nitrophenol Detection in Water. ACS Appl. Nano Mater. 2023, 6, 11351–11360. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Y.; Guo, Y.; Ren, H.; Gao, C. Nitrogen dioxide sensing based on multiple-morphology cuprous oxide mixed structures anchored on reduced graphene oxide nanosheets at room temperature. Nanotechnology 2019, 30, 455502. [Google Scholar] [CrossRef]
- Yoon, H.J.; Yang, J.H.; Zhou, Z.; Yang, S.S.; Cheng, M.M.-C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B Chem. 2011, 157, 310–313. [Google Scholar] [CrossRef]
- Shaikshavali, P.; Reddy, T.M.; Palakollu, V.; Karpoormath, R.; Rao, Y.S.; Venkataprasad, G.; Gopal, T.V.; Gopal, P. Multi walled carbon nanotubes supported CuO-Au hybrid nanocomposite for the effective application towards the electrochemical determination of acetaminophen and 4-aminophenol. Synth. Met. 2019, 252, 29–39. [Google Scholar] [CrossRef]
- Castañeda, M.e.T.; Alegret, S.; Merkoci, A. Electrochemical sensing of DNA using gold nanoparticles. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2007, 19, 743–753. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Li, P.; Zhang, W.; Lian, K.; Hu, J.; Chen, Y. Preparation and characterization of AuNPs/CNTs-ErGO electrochemical sensors for highly sensitive detection of hydrazine. Talanta 2016, 158, 283–291. [Google Scholar] [CrossRef] [PubMed]
- de Lima, C.A.; Santana, E.R.; Piovesan, J.V.; Spinelli, A. Silver nanoparticle-modified electrode for the determination of nitro compound-containing pesticides. Anal. Bioanal. Chem. 2016, 408, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Kumar, R.; Umar, A.; Kim, S.; Bumajdad, A.; Ansari, Z.; Baskoutas, S. Cauliflower-shaped ZnO nanomaterials for electrochemical sensing and photocatalytic applications. Electrochim. Acta 2016, 222, 463–472. [Google Scholar] [CrossRef]
- Frontera, P.; Malara, A.; Stelitano, S.; Leonardi, S.G.; Bonavita, A.; Fazio, E.; Antonucci, P.; Neri, G.; Neri, F.; Santangelo, S. Characterisation and H2O2 sensing properties of TiO2-CNTs/Pt electro-catalysts. Mater. Chem. Phys. 2016, 170, 129–137. [Google Scholar] [CrossRef]
- Meskher, H.; Ragdi, T.; Thakur, A.K.; Ha, S.; Khelfaoui, I.; Sathyamurthy, R.; Sharshir, S.W.; Pandey, A.; Saidur, R.; Singh, P. A review on CNTs-based electrochemical sensors and biosensors: Unique properties and potential applications. Crit. Rev. Anal. Chem. 2023, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zheng, Y.; Li, X.; Liu, X.; Lin, C.-T.; Karimi-Maleh, H. Strategies and Applications of Graphene and Its Derivatives-Based Electrochemical Sensors in Cancer Diagnosis. Molecules 2023, 28, 6719. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Goud, K.Y.; Kailasa, S.K.; Kumar, V.; Tsang, Y.F.; Gobi, K.V.; Kim, K.-H. Progress on nanostructured electrochemical sensors and their recognition elements for detection of mycotoxins: A review. Biosens. Bioelectron. 2018, 121, 205–222. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Yang, Y.; Yuan, Q. Aptamer-functionalized carbon nanomaterials electrochemical sensors for detecting cancer relevant biomolecules. Carbon 2018, 129, 380–395. [Google Scholar] [CrossRef]
- Deji, R.; Rahul; Choudhary, B.; Sharma, R.K. Role of Graphene-Based Materials in Gas Sensing Applications: From Synthesis to Device Fabrication. In Handbook of Porous Carbon Materials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 493–518. [Google Scholar]
- Krishna Perumal, P.; Chen, C.-w.; Giri, B.S.; Singhania, R.R.; Patel, A.K.; Dong, C.-D. Graphene-based functional electrochemical sensors for the detection of chlorpyrifos in water and food samples: A review. J. Food Sci. Technol. 2023, 1–11. [Google Scholar] [CrossRef]
- Das, P.K.; Mohanty, C.; Purohit, G.K.; Mishra, S.; Palo, S. Nanoparticle assisted environmental remediation: Applications, toxicological implications and recommendations for a sustainable environment. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100679. [Google Scholar]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Murphy, M.; Ting, K.; Zhang, X.; Soo, C.; Zheng, Z. Current development of silver nanoparticle preparation, investigation, and application in the field of medicine. J. Nanomater. 2015, 2015, 696918. [Google Scholar] [CrossRef]
- Gajbhiye, S.; Sakharwade, S. Silver nanoparticles in cosmetics. J. Cosmet. Dermatol. Sci. Appl. 2016, 6, 48–53. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Sakamoto, M.; Fujistuka, M.; Majima, T. Light as a construction tool of metal nanoparticles: Synthesis and mechanism. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 33–56. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Zahran, M.; Khalifa, Z.; Zahran, M.A.-H.; Azzem, M.A. Recent advances in silver nanoparticle-based electrochemical sensors for determining organic pollutants in water: A review. Mater. Adv. 2021, 2, 7350–7365. [Google Scholar] [CrossRef]
- Yari, A.; Saidikhah, M. Trithiane silver-nanoparticles-decorated polyaniline nanofibers as sensing element for electrochemical determination of Adenine and Guanine in DNA. J. Electroanal. Chem. 2016, 783, 288–294. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A review on metal-and metal oxide-based nanozymes: Properties, mechanisms, and applications. Nano-Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-Warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Zhao, R.; Du, J.; Li, J. nanostructural ZnO-based electrochemical sensor for environmental application. J. Electrochem. Soc. 2022, 169, 020573. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Nejad, F.G.; Safaei, M. Recent advances in ZnO nanostructure-based electrochemical sensors and biosensors. J. Mater. Chem. B 2020, 8, 5826–5844. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef]
- Bhanjana, G.; Dilbaghi, N.; Kumar, R.; Kumar, S. Zinc oxide quantum dots as efficient electron mediator for ultrasensitive and selective electrochemical sensing of mercury. Electrochim. Acta 2015, 178, 361–367. [Google Scholar] [CrossRef]
- Hu, P.; Chen, L.; Kang, X.; Chen, S. Surface functionalization of metal nanoparticles by conjugated metal–ligand interfacial bonds: Impacts on intraparticle charge transfer. Acc. Chem. Res. 2016, 49, 2251–2260. [Google Scholar] [CrossRef]
- Tran, P.H.; Duan, W.; Tran, T.T. Fucoidan-based nanostructures: A focus on its combination with chitosan and the surface functionalization of metallic nanoparticles for drug delivery. Int. J. Pharm. 2020, 575, 118956. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef]
- Stetter, J.R.; Penrose, W.R.; Yao, S. Sensors, chemical sensors, electrochemical sensors, and ECS. J. Electrochem. Soc. 2003, 150, S11. [Google Scholar] [CrossRef]
- Banica, F.-G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Holford, T.R.; Davis, F.; Higson, S.P. Recent trends in antibody based sensors. Biosens. Bioelectron. 2012, 34, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, J.; Deng, H.; Zhang, L. Progress of mimetic enzymes and their applications in chemical sensors. Crit. Rev. Anal. Chem. 2016, 46, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Willner, B. Biomolecule-based nanomaterials and nanostructures. Nano Lett. 2010, 10, 3805–3815. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef] [PubMed]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent advances in optical biosensors for sensing applications: A review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Recent advancements in optical biosensors for cancer detection. Biosens. Bioelectron. 2022, 197, 113805. [Google Scholar] [CrossRef]
- Reddy, Y.V.M.; Shin, J.H.; Palakollu, V.N.; Sravani, B.; Choi, C.-H.; Park, K.; Kim, S.-K.; Madhavi, G.; Park, J.P.; Shetti, N.P. Strategies, advances, and challenges associated with the use of graphene-based nanocomposites for electrochemical biosensors. Adv. Colloid Interface Sci. 2022, 304, 102664. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Zare, Y.; Rhee, K.Y. Electrochemical biosensors based on polymer nanocomposites for detecting breast cancer: Recent progress and future prospects. Adv. Colloid Interface Sci. 2022, 309, 102795. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhu, A.; Tian, Y. In vivo electrochemical biosensors: Recent advances in molecular design, electrode materials, and electrochemical devices. Anal. Chem. 2023, 95, 388–406. [Google Scholar] [CrossRef]
- Loewenthal, D.; Kamber, D.; Bisker, G. Monitoring the activity and inhibition of cholinesterase enzymes using single-walled carbon nanotube fluorescent sensors. Anal. Chem. 2022, 94, 14223–14231. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Fritzsche, M.; Pieper, S.B.; Wood, T.K.; Lear, K.L.; Dandy, D.S.; Reardon, K.F. Fiber optic monooxygenase biosensor for toluene concentration measurement in aqueous samples. Biosens. Bioelectron. 2011, 26, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Atailia, S.; Baraket, A.; Rabai, S.; Benounis, M.; Jaffrezic, N.; Araar, H.; Naït-Bouda, A.; Boumaza, A.; Errachid, A.; Houhamdi, M. Electrochemical urea biosensor based on Proteus mirabilis urease immobilized over polyaniline PANi-Glassy carbon electrode. Electroanalysis 2023, 35, e202200502. [Google Scholar] [CrossRef]
- Liu, S.; Kang, M.; Yan, F.; Peng, D.; Yang, Y.; He, L.; Wang, M.; Fang, S.; Zhang, Z. Electrochemical DNA biosensor based on microspheres of cuprous oxide and nano-chitosan for Hg (II) detection. Electrochim. Acta 2015, 160, 64–73. [Google Scholar] [CrossRef]
- Mehto, N.K.; Sharma, P.; Kumar, S.; Khanuja, M.; Rawal, R.; Narang, J. Towards papertronics based electrode decorated with zinc oxide nanoparticles for the detection of the yellow fever virus consensus sequence. Process Biochem. 2022, 123, 36–43. [Google Scholar] [CrossRef]
- Wang, H.; Chi, Z.; Cong, Y.; Wang, Z.; Jiang, F.; Geng, J.; Zhang, P.; Ju, P.; Dong, Q.; Liu, C. Development of a fluorescence assay for highly sensitive detection of Pseudomonas aeruginosa based on an aptamer-carbon dots/graphene oxide system. RSC Adv. 2018, 8, 32454–32460. [Google Scholar] [CrossRef]
- Hu, J.; Fu, K.; Bohn, P.W. Whole-cell Pseudomonas aeruginosa localized surface plasmon resonance aptasensor. Anal. Chem. 2018, 90, 2326–2332. [Google Scholar] [CrossRef]
- Stocker, J.; Balluch, D.; Gsell, M.; Harms, H.; Feliciano, J.; Daunert, S.; Malik, K.A.; Van der Meer, J.R. Development of a set of simple bacterial biosensors for quantitative and rapid measurements of arsenite and arsenate in potable water. Environ. Sci. Technol. 2003, 37, 4743–4750. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, S.; Chae, Y.; Jeong, S.-W.; An, Y.-J. Evaluation of bioavailable arsenic and remediation performance using a whole-cell bioreporter. Sci. Total Environ. 2016, 547, 125–131. [Google Scholar] [CrossRef]
- Riether, K.; Dollard, M.-A.; Billard, P. Assessment of heavy metal bioavailability using Escherichia coli zntAp::lux and copAp::lux-based biosensors. Appl. Microbiol. Biotechnol. 2001, 57, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Kim, S.; Chae, Y.; Kang, Y.; Lee, Y.; Jeong, S.-W.; An, Y.-J. Use of tunable whole-cell bioreporters to assess bioavailable cadmium and remediation performance in soils. PLoS ONE 2016, 11, e0154506. [Google Scholar] [CrossRef] [PubMed]
- Tecon, R.; Beggah, S.; Czechowska, K.; Sentchilo, V.; Chronopoulou, P.-M.; McGenity, T.J.; van der Meer, J.R. Development of a multistrain bacterial bioreporter platform for the monitoring of hydrocarbon contaminants in marine environments. Environ. Sci. Technol. 2010, 44, 1049–1055. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, Y.; Kim, Y.; Park, C.; Choi, H.; Jang, G.; Yoon, Y. Development of novel Escherichia coli cell-based biosensors to monitor Mn (II) in environmental systems. Front. Microbiol. 2022, 13, 1051926. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.K.; Jung, J.K.; Verosloff, M.S.; Clauer, P.R.; Lee, J.W.; Capdevila, D.A.; Pastén, P.A.; Giedroc, D.P.; Collins, J.J.; Lucks, J.B. Rapid, low-cost detection of water contaminants using regulated in vitro transcription. BioRxiv 2019, 619296. [Google Scholar]
- Zhang, P.; Feng, H.; Yang, J.; Jiang, H.; Zhou, H.; Lu, Y. Detection of inorganic ions and organic molecules with cell-free biosensing systems. J. Biotechnol. 2019, 300, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Nigam, V.K.; Shukla, P. Enzyme based biosensors for detection of environmental pollutants-a review. J. Microbiol. Biotechnol. 2015, 25, 1773–1781. [Google Scholar] [CrossRef]
- Coronado-Apodaca, K.G.; González-Meza, G.M.; Aguayo-Acosta, A.; Araújo, R.G.; Gonzalez-Gonzalez, R.B.; Oyervides-Muñoz, M.A.; Martínez-Ruiz, M.; Melchor-Martínez, E.M.; Barceló, D.; Parra-Saldívar, R.; et al. Immobilized Enzyme-based Novel Biosensing System for Recognition of Toxic Elements in the Aqueous Environment. Top. Catal. 2023, 66, 606–624. [Google Scholar] [CrossRef]
- Pundir, C.S.; Chauhan, N. Acetylcholinesterase inhibition-based biosensors for pesticide determination: A review. Anal. Biochem. 2012, 429, 19–31. [Google Scholar] [CrossRef]
- Andreescu, S.; Marty, J.-L. Twenty years research in cholinesterase biosensors: From basic research to practical applications. Biomol. Eng. 2006, 23, 1–15. [Google Scholar] [CrossRef]
- Economou, A.; Karapetis, S.K.; Nikoleli, G.P.; Nikolelis, D.P.; Bratakou, S.; Varzakas, T.H. Enzyme-Based Sensors. Adv. Food Diagn. 2017, 231–250. [Google Scholar] [CrossRef]
- Wilson, G.S.; Hu, Y. Enzyme-based biosensors for in vivo measurements. Chem. Rev. 2000, 100, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yu, X.; Zheng, Y.; Yu, J. DNA based chemical sensor for the detection of nitrogen dioxide enabled by organic field-effect transistor. Sens. Actuators B Chem. 2016, 222, 1003–1011. [Google Scholar] [CrossRef]
- Schoukroun-Barnes, L.R.; Macazo, F.C.; Gutierrez, B.; Lottermoser, J.; Liu, J.; White, R.J. Reagentless, structure-switching, electrochemical aptamer-based sensors. Annu. Rev. Anal. Chem. 2016, 9, 163–181. [Google Scholar] [CrossRef]
- Xu, K.; Purahmad, M.; Brenneman, K.; Meshik, X.; Farid, S.; Poduri, S.; Pratap, P.; Abell, J.; Zhao, Y.; Nichols, B. Design and applications of nanomaterial-based and biomolecule-based nanodevices and nanosensors. Des. Appl. Nanomater. Sens. 2014, 16, 61–97. [Google Scholar]
- Dunn, M.R.; Jimenez, R.M.; Chaput, J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017, 1, 0076. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, M.; Zhao, F.; Han, S. Application of nanomaterial modified aptamer-based electrochemical sensor in detection of heavy metal ions. Foods 2022, 11, 1404. [Google Scholar] [CrossRef]
- Sargazi, S.; Simge, E.; Mobashar, A.; Gelen, S.S.; Rahdar, A.; Ebrahimi, N.; Hosseinikhah, S.M.; Bilal, M.; Kyzas, G.Z. Aptamer-conjugated carbon-based nanomaterials for cancer and bacteria theranostics: A review. Chem. Biol. Interact. 2022, 361, 109964. [Google Scholar] [CrossRef]
- Lou, B.; Liu, Y.; Shi, M.; Chen, J.; Li, K.; Tan, Y.; Chen, L.; Wu, Y.; Wang, T.; Liu, X. Aptamer-based biosensors for virus protein detection. TrAC Trends Anal. Chem. 2022, 157, 116738. [Google Scholar] [CrossRef]
- Kadam, U.S.; Hong, J.C. Recent advances in aptameric biosensors designed to detect toxic contaminants from food, water, human fluids, and the environment. Trends Environ. Anal. Chem. 2022, 36, e00184. [Google Scholar] [CrossRef]
- Rahman, M.M.; Li, X.-B.; Lopa, N.S.; Ahn, S.J.; Lee, J.-J. Electrochemical DNA hybridization sensors based on conducting polymers. Sensors 2015, 15, 3801–3829. [Google Scholar] [CrossRef]
- Pham, C.; Stogios, P.J.; Savchenko, A.; Mahadevan, R. Advances in engineering and optimization of transcription factor-based biosensors for plug-and-play small molecule detection. Curr. Opin. Biotechnol. 2022, 76, 102753. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, H.; Zhang, B.; Liu, S.; Liu, C.-g.; Li, F.; Song, H. Engineering whole-cell microbial biosensors: Design principles and applications in monitoring and treatment of heavy metals and organic pollutants. Biotechnol. Adv. 2022, 60, 108019. [Google Scholar] [CrossRef]
- Mahr, R.; Frunzke, J. Transcription factor-based biosensors in biotechnology: Current state and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Mannan, A.A.; Liu, D.; Zhang, F.; Oyarzún, D.A. Fundamental design principles for transcription-factor-based metabolite biosensors. ACS Synth. Biol. 2017, 6, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Wells, M.C.; Van der Meer, J.R. Whole-cell living biosensors—Are they ready for environmental application? Appl. Microbiol. Biotechnol. 2006, 70, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Elcin, E.; Öktem, H. Whole-cell fluorescent bacterial bioreporter for arsenic detection in water. Int. J. Environ. Sci. Technol. 2019, 16, 5489–5500. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, W.; Lu, Y. Advances in cell-free biosensors: Principle, mechanism, and applications. Biotechnol. J. 2020, 15, 2000187. [Google Scholar] [CrossRef]
- Jung, J.K.; Alam, K.K.; Verosloff, M.S.; Capdevila, D.A.; Desmau, M.; Clauer, P.R.; Lee, J.W.; Nguyen, P.Q.; Pastén, P.A.; Matiasek, S.J. Cell-free biosensors for rapid detection of water contaminants. Nat. Biotechnol. 2020, 38, 1451–1459. [Google Scholar] [CrossRef]
- Tricoli, A.; Neri, G. Miniaturized bio-and chemical-sensors for point-of-care monitoring of chronic kidney diseases. Sensors 2018, 18, 942. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Davis, J.J. Point of care sensors for infectious pathogens. Anal. Chem. 2020, 93, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.J.; Burmølle, M.; Hansen, L.H. Making bio-sense of toxicity: New developments in whole-cell biosensors. Curr. Opin. Biotechnol. 2006, 17, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Li, G.; Xing, Y.; Zhang, D.; Jia, J.; Cui, Z.; Luan, X.; Tang, H. A whole-cell bioreporter assay for quantitative genotoxicity evaluation of environmental samples. Chemosphere 2017, 184, 384–392. [Google Scholar] [CrossRef] [PubMed]
| Type of Transducer | Sensing Element | Target | LOD/Detection Ranges | Optical Response | Ref. |
|---|---|---|---|---|---|
| LMR-based refractometer | Indium tin oxide NPs Zinc oxide nanorods | Hydrogen gas Sulfide gas | - - | LMR | [40] [41] |
| SPR based-optic fiber | Graphene film Chitosan-optic fiber | Streptavidin Pb(II) | - 1–7 ppb | Reflective index | [35] [36] |
| In-fiber optofluidic device | mPOF | Minocycline | 100 ppb | Chemilumin. | [42] |
| Microfluidic device | Chemicals | Cu(II), Ni(II), Cr(VI) | 0.29 ppm, 0.33 ppm, 0.35 ppm | Colorimetric | [43] |
| Zinc microparticles | Nitrate | 19 µM | Colorimetric | [44] | |
| Berthelot reaction | Ammonia | - | Absorbance | [45] | |
| AuNPs | Hg(II) | - | Colorimetric | [46] | |
| Microfluidic capillary waveguide | Griess reagents | Nitrite | 7 ppb | Colorimetric | [47] |
| Naked eyes/UV-Vis spec. | Nanostructured cages | Sb(III), Hg(II), Pb(II) | 33.7 nM, 6.34 nM, 2.38 nM | Absorbance | [25] |
| Naked eyes | DNA hybridized AuNPs | Mercury ions (Hg2+) | 0.5 mM | Colorimetric | [48] |
| Fluorescence spectroscopy | SWCNTs | DNA sequences | 4.0 nM | Fluorescence | [49] |
| Chemiluminescence analyzer Fluorescence spectroscopy | CDs wsNP-CDs | Phenol Trinitrophenol | 0.76 mM 23 µM | Fluorescence Fluorescence | [50] [51] |
| Type of Transducer | Sensing Element | Target | LOD | Electrochemical Response | Ref. |
|---|---|---|---|---|---|
| SPCE | WS2/MWCNTs-OH | 2,4,6-trichlorophenol | - | Cyclic voltammetry | [83] |
| bisphenol AF PSNP | - - | ||||
| Electrochemical analyzer | LIG | 4-nitrophenol | 95 nM | Cyclic voltammetry | [84] |
| Metal electrode | Cu2O-rGO | NO2 | 50 ppb | Resistance | [85] |
| Graphene flake | CO2 | - | Resistance | [86] | |
| GCE | MWCNTs/CuO-Au | 4-aminophenol Acetaminophen | 0.105 µM 0.016 µM | Differential pulse voltammetry | [87] |
| AuNPs/DNA | DNA | 0.78 fmol | Cyclic voltammetry | [88] | |
| AuNPs/CNTs-ErGO | Hydrazine | 0.065 µM | [89] | ||
| AgNPs | Pendimethalin Ethyl parathion | 36 nmol/L 40 nmol/L | Square-wave adsorptive Stripping voltammetry | [90] | |
| Metal electrode | cauliflower-shaped ZnO | Picric acid Nitrophenol | 0.078 mM - | Current voltage technique | [91] |
| TiO2-CNTs/Pt | H2O2 | 0.016 µM | Cyclic voltammetry | [92] |
| Type of Transducer | Sensing Element | Target | LOD | Response | Ref. |
|---|---|---|---|---|---|
| Fluorescence spec. | ChE-SWCNT | Pesticides Heavy metals | - - | NIR fluorescence | [130] |
| Optic fiber | Toluene monooxygenase | Toluene | 3 µM | Absorbance | [131] |
| GCE | Urease-polyaniline | Urea | 0.1 mM | Cyclic Voltammetry | [132] |
| Au electrode | DNA-Cu2O@NCs | Hg(II) | 0.15 nM | [133] | |
| Potentiostat | DNA-ZnO NPs | Yellow fever virus | 0.01 µM | Cyclic voltammetry | [134] |
| Fluorescence spec. SPR | Aptamer-CDs/GO Aptamer-Biotin | Pseudomonas aeruginosa | 9 CFU/mL 10 CFU/mL | Fluorescence Reflective index | [135] [136] |
| TFs-based biosensors | ArsR | As(III), As(V) | 10 µg/L | Fluorescence | [137,138] |
| CueR | Cu(II) | 10 nM | [139] | ||
| ZntR | Pb(II), Hg(II), Cd(II) | - | [139,140] | ||
| TbuT | BTEX | 0.24 ± 0.22 µM | [141] | ||
| MntR | Mn(II) | 0.01 µM | [142] | ||
| MobR TetR mphR | 3-hydroxybenzoate Tetracycline erythromycin | 2 mM 1.25 µM 50 µM | [143] | ||
| BenR | benzoate | 1 nM | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Jeon, Y.; Na, M.; Hwang, S.-J.; Yoon, Y. Recent Trends in Chemical Sensors for Detecting Toxic Materials. Sensors 2024, 24, 431. https://doi.org/10.3390/s24020431
Kim Y, Jeon Y, Na M, Hwang S-J, Yoon Y. Recent Trends in Chemical Sensors for Detecting Toxic Materials. Sensors. 2024; 24(2):431. https://doi.org/10.3390/s24020431
Chicago/Turabian StyleKim, Yeonhong, Yangwon Jeon, Minyoung Na, Soon-Jin Hwang, and Youngdae Yoon. 2024. "Recent Trends in Chemical Sensors for Detecting Toxic Materials" Sensors 24, no. 2: 431. https://doi.org/10.3390/s24020431
APA StyleKim, Y., Jeon, Y., Na, M., Hwang, S.-J., & Yoon, Y. (2024). Recent Trends in Chemical Sensors for Detecting Toxic Materials. Sensors, 24(2), 431. https://doi.org/10.3390/s24020431






