Disclosing Fast Detection Opportunities with Nanostructured Chemiresistor Gas Sensors Based on Metal Oxides, Carbon, and Transition Metal Dichalcogenides
Abstract
:1. Introduction
1.1. General Context
1.2. Aim of the Work and Outline
2. Chemiresistors: Dynamical Response and Time Scales in Adsorption and Desorption Processes
2.1. Chemiresistors
2.2. Adsorption-Desorption Processes and Models
3. Selected Categories of Ultrafast Chemiresistors
3.1. Metal Oxide Chemiresistors
3.2. Chemiresistors Based on Nanostructured Carbon
3.3. Chemiresistors Based on Transition Metal Dichalcogenides (TMD)
| Ref. | Year | Active Layer | Gas | Conc. (ppm) | T (°) | Response | t/t (s) |
|---|---|---|---|---|---|---|---|
| [116] | 2020 | NSs + NPs | 50 | 22 | 91.26 | 23/1.6 s | |
| [117] | 2022 | MXene + | 100 | RT | 0.82 | 3/2.4 s | |
| [118] | 2022 | MXene + NSs + NFs | 50 | RT | 55.16 | 1.6/n.a. | |
| [119] | 2022 | NFs | 3 | RT | 0.03 | 9/3 s | |
| [120] | 2019 | MoS2 NSs + ML- | 50 | RT | 26.12 | 1.6/27.7 s | |
| [121] | 2019 | nanoplates + ML- | 100 | RT | 19.4 | 1.06/22.9 s | |
| [122] | 2019 | FL- NSs | 100 | RT | 4.4 | 42/2 s | |
| [123] | 2021 | / composites | 5 | RT | 6 | 28/3 s | |
| [124] | 2019 | /graphene 2D heterostructures | 10 | 200 | 0.69 | 0.7/0.9 s | |
| [125] | 2021 | NFs + NTs | 100 | RT | 34.67 | 2.2/10.5 s | |
| [126] | 2022 | UV-activated / heterostructures | 0.5 | RT | 0.51 | 9/8 s | |
| [127] | 2019 | WS2/ZnS heterostructures | 5 | RT | 32.5 | 4/1000 s | |
| [128] | 2019 | NSs on mesoporous cubic | 100 | RT | 10.13 | 1/n.a. s | |
| [129] | 2019 | UNCD + NRs + | 100 | RT | 0.50 | 8/12 s | |
| [113] | 2020 | + Pt NPs | 100 | 150 | 10 | 4/19 s | |
| [130] | 2019 | NSs | 100 | RT | 0.49 | 10/9 s | |
| [131] | 2021 | nanoflowers + NPs | ethanol | 50 | RT | 7.78 | 7/5 s |
| [132] | 2020 | / | ethanol | 40 | RT | 9.2 | 9.7/6.6 s |
| [133] | 2023 | NSs + Zno | ethanol | 500 | RT | 37.8 | 8.4/14.7 s |
| [134] | 2019 | ZnO + core/shell heterojunctions | acetone | 0.5 | 350 | 1.50 | 9/17 s |
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Ref. | LOD (ppm) | Range of Det. (Min–Max) (ppm) | Target Gas | Gases Used to Test Selectivity |
|---|---|---|---|---|
| [52] | 0.6–3 | acetone, ethanol, toluene | ||
| [53] | ethanol, methanol, acetone, toluene | |||
| [54] | 0.026 | 0.1–10 | n-hexane, methanol, benzene, , CO, ethanol | |
| [55] | , , , | |||
| [56] | 100–1000 | , , , CO, | ||
| [57] | 0.05 | 0.5–100 | , , , hexanal | |
| [58] | 1–20 | , , , ethanol, , | ||
| [59] | 0.001 | 0.001–250 | ethanol, acetone, xylene, methylbenzene, formaldehyde, | |
| [60] | 0.01 | 0.01–100 | NO | , , , CO, |
| [61] | 0.1 | 0.1–400 | , CO, , , dichloromethane, ethanol | |
| [62] | 1–5 | 1.0–100 | acetone, toluene, propanol, ethanol, hydrogen | |
| [63] | 0.001 | 0.3–200 | acetaldehyde, methanol, ethanol, acetone, N-amyl alcohol, methane, ethylene, and CO | |
| [64] | 10 | 10.0–100 | ammonia, ethanol, methane | |
| [65] | 4.8 | 20–5000 | CO, , | |
| [66] | 0.012 | 100–1000 | ammonia, methanol, ethanol | |
| [67] | 2 | 2.0–200 | acetone | Formaldehyde, methanol, ethanol, ammonia, hydrogen, toluene, CO |
| [68] | 0.5–8, 200–1000 | acetone | ethanol, formaldehyde, ammonia | |
| [69] | 0.001 | 0.17–500 | acetone | Ammonia, ethanol, formaldehyde, isopropanol |
| [70] | 0.05 | acetone | methanol, ethanol, ammonia, formaldehyde, toluene, n-hexane, methylbenzene | |
| [71] | 0.5–5000 | CO | Methane, ammonia, hydrogen, | |
| [72] | 10.0–500 | CO | , , toluene, formaldehyde and methanol | |
| [73] | methane | |||
| [74] | 10.0–100 | toluene | methanol, acetone, glycol, formaldehyde, ethanol, , , , CO | |
| [75] | 0.32 | 10.0–50 | toluene | Ethanol, , acetone, methanol |
| [76] | 5 | 5.0–500 | toluene | ethanol, formaldehyde, acetone, benzene trimethylamine, ammonia |
| [77] | 2.0–100 | triethylamine | Benzene, methylbenzene, ammonia, methanal, trimethylamine, triethylamine | |
| [78] | 0.008 | 1.0–100 | triethylamine | Ammonia, ethanol, acetone, methanol, toluene |
| [79] | 1 | 1.0–200 | formaldehyde | methylbenzene, methanol, ethanol acetone |
| [80] | 0.002 | 1.0–50 | formaldehyde | Xylene, n-butyl alcohol, carbinol, toluene, 2-methoxy ethanol, methanol, ethanol, acetone, ammonia |
| [81] | 5.0–300 | benzene | acetone, propanol, ethanol, ammonia, triethylamine, benzene. | |
| [82] | 0.001 | 0.5–100 | ethanol | Acetone, toluene, formaldehyde, 2-butanone, ammonia, , |
| [83] | 40 | 5–70 | 2-methoxy ethanol | Xylene, n-butyl alcohol, carbinol, toluene, 2-methoxy ethanol, methanol, ethanol, acetone, formaldehyde, ammonia |
| Ref. | LOD (ppm) | Range of Det. (Min–Max) (ppm) | Target Gas | Gases Used to Test Selectivity |
|---|---|---|---|---|
| [100] | 2–15 | Ammonia, hydrogen, acetone, LPG | ||
| [101] | 0.069 | 0.5–16 | ||
| [102] | 0.05 | 50–500 | , , , , | |
| [103] | 0.1 | 0–300 | , | |
| [104] | 5.0–800 | Acetone | ammonia, ethanol, methanal, toluene | |
| [105] | 0.024 | , , , , 75% RH, 100% RH | ||
| [106] | 25–500 | ethanol | methanol, acetone, toluene, isopropyl alcohol, ammonia | |
| [107] | 10 | 10.0–100 | CO | , , |
| [108] | 50–500 | Toluene | diethylamine, acetone, DMF, ammonia, ethanol, methanol, isopropanol, formalin, , | |
| [109] | LPG |
| Ref. | LOD (ppm) | Range of Det. (Min–Max) (ppm) | Target Gas | Gases Used to Test Selectivity |
|---|---|---|---|---|
| [116] | 1.0–200 | ethanol, , , , , | ||
| [117] | 0.2 | 0.2–100 | Ethanol, acetone, ethylene, toluene, ammonia, , , | |
| [118] | ||||
| [119] | 0.190 | 3.0–150 | , 2NT, , , , | |
| [120] | 0.01 | 0.01–50 | , CO, ,, , | |
| [121] | 0.1 | 0.1–100 | , CO, | |
| [122] | 5.0–200 | |||
| [123] | 5.0–50 | , ethanol, formaldehyde, acetone, methanol | ||
| [124] | 0.2 | 0.2–10 | ||
| [125] | 0.01 | 0.01–100 | , , , CO | |
| [126] | 0.5–20 | , , , , CO, | ||
| [127] | 0.01 | 0.01–5 | Ethanol, methanol, toluene, acetone, ammonia | |
| [128] | 0.1–100 | NO | , CO, | |
| [129] | 5.0–500 | , , CO, | ||
| [113] | 10.0–100 | , , CO | ||
| [130] | 10 | 10.0–500 | , | |
| [131] | 1.0–50 | ethanol | , , , , | |
| [132] | 1.0–40 | ethanol | Methanol, acetone, hexane, benzene, toluene | |
| [133] | 0.3 | 10.0–500 | ethanol | Formaldehyde, benzene, acetone |
| [134] | 0.005 | 0.01–0.5 | acetone | , , , , |
References
- Wilson, J.S. Sensor Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Dhall, S.; Mehta, B.; Tyagi, A.; Sood, K. A review on environmental gas sensors: Materials and technologies. Sens. Int. 2021, 2, 100116. [Google Scholar] [CrossRef]
- Hayat, H.; Griffiths, T.; Brennan, D.; Lewis, R.P.; Barclay, M.; Weirman, C.; Philip, B.; Searle, J.R. The state-of-the-art of sensors and environmental monitoring technologies in buildings. Sensors 2019, 19, 3648. [Google Scholar] [CrossRef]
- Sagar, M.S.I.; Allison, N.R.; Jalajamony, H.M.; Fernandez, R.E.; Sekhar, P.K. Review–Modern Data Analysis in Gas Sensors. J. Electrochem. Soc. 2022, 169, 127512. [Google Scholar] [CrossRef]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on smart gas sensing technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef] [PubMed]
- Milone, A.; Monteduro, A.G.; Rizzato, S.; Leo, A.; Di Natale, C.; Kim, S.S.; Maruccio, G. Advances in Materials and Technologies for Gas Sensing from Environmental and Food Monitoring to Breath Analysis. Adv. Sustain. Syst. 2023, 7, 2200083. [Google Scholar] [CrossRef]
- Caron, A.; Redon, N.; Thevenet, F.; Hanoune, B.; Coddeville, P. Performances and limitations of electronic gas sensors to investigate an indoor air quality event. Build. Environ. 2016, 107, 19–28. [Google Scholar]
- Vichi, F.; Ianniello, A.; Frattoni, M.; Imperiali, A.; Esposito, G.; Tomasi Scianò, M.C.; Perilli, M.; Cecinato, A. Air quality assessment in the central mediterranean sea (Tyrrhenian sea): Anthropic impact and miscellaneous natural sources, including volcanic contribution, on the budget of volatile organic compounds (VOCs). Atmosphere 2021, 12, 1609. [Google Scholar] [CrossRef]
- Sofia, D.; Giuliano, A.; Gioiella, F. Air quality monitoring network for tracking pollutants: The case study of Salerno city center. Chem. Eng. Trans. 2018, 68, 67–72. [Google Scholar]
- Lay-Ekuakille, A.; Ikezawa, S.; Mugnaini, M.; Morello, I.; De Capua, C. Detection of specific macro and micropollutants in air monitoring: Review of methods and techniques. Measurement 2017, 98, 49–59. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Drix, D.; Schmuker, M. Resolving fast gas transients with metal oxide sensors. ACS Sens. 2021, 6, 688–692. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Ahmed, F.; Saber, O.; Kumar, S. Gases in food production and monitoring: Recent advances in target chemiresistive gas sensors. Chemosensors 2022, 10, 338. [Google Scholar] [CrossRef]
- Scarabottolo, N.; Fedel, M.; Cocola, L.; Poletto, L. In-line inspecting device for leak detection from gas-filled food packages. In Proceedings of the Sensing for Agriculture and Food Quality and Safety XII, Online, 27 April–9 May 2020; Volume 11421, p. 1142103. [Google Scholar]
- Andrighetto, C.; Cocola, L.; De Dea, P.; Fedel, M.; Lombardi, A.; Melison, F.; Poletto, L. Determination of CO2 and H2 content in the headspace of spore contaminated milk by Raman gas analysis. In Proceedings of the Sensing for Agriculture and Food Quality and Safety XIV, Orlando, FL, USA, 3 April–13 June 2022; Volume 12120, pp. 8–15. [Google Scholar]
- Clarivate Web of Science. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 23 May 2023).
- Moumen, A.; Kumarage, G.C.; Comini, E. P-type metal oxide semiconductor thin films: Synthesis and chemical sensor applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Goldoni, A.; Alijani, V.; Sangaletti, L.; D’Arsiè, L. Advanced promising routes of carbon/metal oxides hybrids in sensors: A review. Electrochim. Acta 2018, 266, 139–150. [Google Scholar] [CrossRef]
- Dariyal, P.; Sharma, S.; Chauhan, G.S.; Singh, B.P.; Dhakate, S.R. Recent trends in gas sensing via carbon nanomaterials: Outlook and challenges. Nanoscale Adv. 2021, 3, 6514–6544. [Google Scholar] [CrossRef]
- Parichenko, A.; Huang, S.; Pang, J.; Ibarlucea, B.; Cuniberti, G. Recent advances in technologies toward the development of 2D materials-based electronic noses. TrAC Trends Anal. Chem. 2023, 166, 117185. [Google Scholar] [CrossRef]
- Wang, Z.; Bu, M.; Hu, N.; Zhao, L. An overview on room-temperature chemiresistor gas sensors based on 2D materials: Research status and challenge. Compos. Part B Eng. 2023, 248, 110378. [Google Scholar] [CrossRef]
- Kim, Y.; Sohn, I.; Shin, D.; Yoo, J.; Lee, S.; Yoon, H.; Park, J.; Chung, S.m.; Kim, H. Recent Advances in Functionalization and Hybridization of Two-dimensional Transition Metal Dichalcogenide for Gas Sensor. Adv. Eng. Mater. 2024, 26, 2301063. [Google Scholar] [CrossRef]
- Yaqoob, U.; Younis, M.I. Chemical gas sensors: Recent developments, challenges, and the potential of machine learning—A review. Sensors 2021, 21, 2877. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ionics 2021, 360, 115544. [Google Scholar] [CrossRef]
- Tian, X.; Wang, S.; Li, H.; Li, M.; Chen, T.; Xiao, X.; Wang, Y. Recent advances in MoS2 based nanomaterials sensors for room temperature gas detection: A review. Sens. Diagn. 2023, 5, 1901062. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, C.; Lin, X.; Guo, Y. UV light activated NO2 gas sensing based on Au nanoparticles decorated few-layer MoS2 thin film at room temperature. Appl. Phys. Lett. 2018, 113, 082103. [Google Scholar] [CrossRef]
- Suematsu, K.; Harano, W.; Oyama, T.; Shin, Y.; Watanabe, K.; Shimanoe, K. Pulse-driven semiconductor gas sensors toward ppt level toluene detection. Anal. Chem. 2018, 90, 11219–11223. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Singh, S.G. Reduction of the Measurement Time of a Chemiresistive Gas Sensor Using Transient Analysis and the Cantor Pairing Function. ACS Meas. Sci. Au 2021, 2, 113–119. [Google Scholar] [CrossRef]
- Awang, Z. Gas sensors: A review. Sens. Transducers 2014, 168, 61–75. [Google Scholar]
- Elosua, C.; Matias, I.R.; Bariain, C.; Arregui, F.J. Volatile organic compound optical fiber sensors: A review. Sensors 2006, 6, 1440–1465. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro-and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.V.; Choi, I.Y.; Son, Y.S.; Kim, J.C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Banica, F.G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Lucas, D.; Ewing, G.E. Spontaneous desorption of vibrationally excited molecules physically adsorbed on surfaces. Chem. Phys. 1981, 58, 385–393. [Google Scholar] [CrossRef]
- de Boer, J.H. The Dynamical Character of Adsorption; Oxford University Press: Oxford, UK, 1953; Volume 76. [Google Scholar]
- Chambers, A. Modern Vacuum Physics; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Butt, H.J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Morse, P.M. Diatomic molecules according to the wave mechanics. II. Vibrational levels. Phys. Rev. 1929, 34, 57. [Google Scholar] [CrossRef]
- Giraud, F.; Geantet, C.; Guilhaume, N.; Loridant, S.; Gros, S.; Porcheron, L.; Kanniche, M.; Bianchi, D. Individual amounts of Lewis and Brønsted acid sites on metal oxides from NH3 adsorption equilibrium: Case of TiO2 based solids. Catal. Today 2021, 373, 69–79. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K.; Qi, P.; Dai, H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
- Bagsican, F.R.; Winchester, A.; Ghosh, S.; Zhang, X.; Ma, L.; Wang, M.; Murakami, H.; Talapatra, S.; Vajtai, R.; Ajayan, P.M.; et al. Adsorption energy of oxygen molecules on graphene and two-dimensional tungsten disulfide. Sci. Rep. 2017, 7, 1774. [Google Scholar] [PubMed]
- Somorjai, G.A.; Li, Y. Introduction to Surface Chemistry and Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Liang, S.Z.; Chen, G.; Harutyunyan, A.R.; Cole, M.W.; Sofo, J.O. Analysis and optimization of carbon nanotubes and graphene sensors based on adsorption-desorption kinetics. Appl. Phys. Lett. 2013, 103, 233108. [Google Scholar] [CrossRef]
- Ponzoni, A. A Statistical Analysis of Response and Recovery Times: The Case of Ethanol Chemiresistors Based on Pure SnO2. Sensors 2022, 22, 6346. [Google Scholar] [CrossRef]
- Rigoni, F.; Freddi, S.; Pagliara, S.; Drera, G.; Sangaletti, L.; Suisse, J.M.; Bouvet, M.; Malovichko, A.; Emelianov, A.; Bobrinetskiy, I. Humidity-enhanced sub-ppm sensitivity to ammonia of covalently functionalized single-wall carbon nanotube bundle layers. Nanotechnology 2017, 28, 255502. [Google Scholar] [CrossRef]
- Meixner, H.; Lampe, U. Metal oxide sensors. Sens. Actuators B Chem. 1996, 33, 198–202. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Singh, S.G. Highly sensitive and ultra-fast responsive ammonia gas sensor based on 2D ZnO nanoflakes. Mater. Sci. Energy Technol. 2020, 3, 91–96. [Google Scholar]
- Shaikh, S.F.; Ghule, B.G.; Shinde, P.V.; Raut, S.D.; Gore, S.K.; Ubaidullah, M.; Mane, R.S.; Al-Enizi, A.M. Continuous hydrothermal flow-inspired synthesis and ultra-fast ammonia and humidity room-temperature sensor activities of WO3 nanobricks. Mater. Res. Express 2020, 7, 015076. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Wang, D.; Wang, T.; Zhang, J.; Zhang, D. High performance ammonia gas sensor based on electrospinned Co3O4 nanofibers decorated with hydrothermally synthesized MoTe2 nanoparticles. J. Alloys Compd. 2022, 923, 166355. [Google Scholar] [CrossRef]
- Mathankumar, G.; Harish, S.; Mohan, M.K.; Bharathi, P.; Kannan, S.K.; Archana, J.; Navaneethan, M. Enhanced selectivity and ultra-fast detection of NO2 gas sensor via Ag modified WO3 nanostructures for gas sensing applications. Sens. Actuators B Chem. 2023, 381, 133374. [Google Scholar] [CrossRef]
- Chen, T.; Yan, W.; Wang, Y.; Li, J.; Hu, H.; Ho, D. SnS2/MXene derived TiO2 hybrid for ultra-fast room temperature NO2 gas sensing. J. Mater. Chem. C 2021, 9, 7407–7416. [Google Scholar] [CrossRef]
- Fan, C.; Shi, J.; Zhang, Y.; Quan, W.; Chen, X.; Yang, J.; Zeng, M.; Zhou, Z.; Su, Y.; Wei, H.; et al. Fast and recoverable NO2 detection achieved by assembling ZnO on Ti3C2Tx MXene nanosheets under UV illumination at room temperature. Nanoscale 2022, 14, 3441–3451. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, S.; Zhou, P.; Cui, B.; Liu, W.; Wei, D.; Shen, Y. Room-temperature NO2 sensing properties and mechanism of CuO nanorods with Au functionalization. Sens. Actuators B Chem. 2021, 328, 129070. [Google Scholar] [CrossRef]
- Zhao, H.; Ge, W.; Tian, Y.; Wang, P.; Li, X.; Liu, Z. Pr2Sn2O7/NiO heterojunction for ultra-fast and low operating temperature to NO2 gas sensing. Sens. Actuators A Phys. 2023, 349, 114100. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, X.; Ullah, M.; He, L.; Ikram, M.; Khan, M.; Ma, L.; Li, L.; Zhang, G.; Shi, K. Three-dimensional flower-like Ni9S8/NiAl2O4 nanocomposites composed of ultra-thin porous nanosheets: Fabricated, characterized and ultra-fast NOx gas sensors at room temperature. J. Alloys Compd. 2020, 825, 154151. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Z.; Li, H.; Sun, M.; Han, S.; Cai, C.; Shen, W.; Fu, Y. Ultrafast response/recovery and high selectivity of the H2S gas sensor based on α-Fe2O3 nano-ellipsoids from one-step hydrothermal synthesis. ACS Appl. Mater. Interfaces 2019, 11, 12761–12769. [Google Scholar] [CrossRef]
- Dun, M.; Tan, J.; Tan, W.; Tang, M.; Huang, X. CdS quantum dots supported by ultrathin porous nanosheets assembled into hollowed-out Co3O4 microspheres: A room-temperature H2S gas sensor with ultra-fast response and recovery. Sens. Actuators B Chem. 2019, 298, 126839. [Google Scholar] [CrossRef]
- Wang, X.; Lu, J.; Han, W.; Cheng, P.; Wang, Y.; Sun, J.; Ma, J.; Sun, P.; Zhang, H.; Sun, Y.; et al. Carbon modification endows WO3 with anti-humidity property and long-term stability for ultrafast H2S detection. Sens. Actuators B Chem. 2022, 350, 130884. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultra-fast response and highly selectivity hydrogen gas sensor based on Pd/SnO2 nanoparticles. Int. J. Hydrogen Energy 2022, 47, 3157–3169. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Tang, M.; Chen, Q.; Zhang, H.; Shao, X. Construction of ultra-fast hydrogen sensor for dissolved gas detection in oil-immersed transformers based on titanium dioxide quantum dots modified tin dioxide nanosheets. Sens. Actuators B Chem. 2023, 393, 134141. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Gao, W. PdAg alloy modified SnO2 nanoparticles for ultrafast detection of hydrogen. Sens. Actuators B Chem. 2023, 382, 133515. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, H.; Wang, Y.; Li, J.; Yang, Y.; Cheng, X.; Luo, Y.; An, B.; Pan, X.; Xie, E. Ex-situ XPS analysis of yolk-shell Sb2O3/WO3 for ultra-fast acetone resistive sensor. J. Hazard. Mater. 2021, 412, 125175. [Google Scholar] [CrossRef]
- Ge, W.; Jiao, S.; Chang, Z.; He, X.; Li, Y. Ultrafast response and high selectivity toward acetone vapor using hierarchical structured TiO2 nanosheets. ACS Appl. Mater. Interfaces 2020, 12, 13200–13207. [Google Scholar] [CrossRef]
- Chang, X.; Xu, S.; Liu, S.; Wang, N.; Sun, S.; Zhu, X.; Li, J.; Ola, O.; Zhu, Y. Highly sensitive acetone sensor based on WO3 nanosheets derived from WS2 nanoparticles with inorganic fullerene-like structures. Sens. Actuators B Chem. 2021, 343, 130135. [Google Scholar] [CrossRef]
- He, K.; Jin, Z.; Chu, X.; Bi, W.; Wang, W.; Wang, C.; Liu, S. Fast response–recovery time toward acetone by a sensor prepared with Pd doped WO3 nanosheets. RSC Adv. 2019, 9, 28439–28450. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, P.; Wang, B.; Wang, Y. Flame-annealed porous TiO2/CeO2 nanosheets for enhenced CO gas sensors. Appl. Surf. Sci. 2022, 593, 153418. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Wang, X.; Sun, G.; Cao, J.; Wang, Y. Surface modification of In2O3 porous nanospheres with Au single atoms for ultrafast and highly sensitive detection of CO. Appl. Surf. Sci. 2023, 613, 155987. [Google Scholar] [CrossRef]
- Sertel, B.C.; Sonmez, N.A.; Kaya, M.D.; Ozcelik, S. Development of MgO: TiO2 thin films for gas sensor applications. Ceram. Int. 2019, 45, 2917–2921. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Yang, M.; Guo, L.; Xie, N.; Kou, X.; Song, Y.; Wang, Q.; Sun, Y.; Lu, G. Dispersed WO3 nanoparticles with porous nanostructure for ultrafast toluene sensing. Sens. Actuators B Chem. 2019, 289, 195–206. [Google Scholar] [CrossRef]
- Hermawan, A.; Zhang, B.; Taufik, A.; Asakura, Y.; Hasegawa, T.; Zhu, J.; Shi, P.; Yin, S. CuO Nanoparticles/Ti3C2Tx MXene Hybrid Nanocomposites for Detection of Toluene Gas. ACS Appl. Nano Mater. 2020, 3, 4755–4766. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, S.; Zhou, T.; Tu, J.; Zhang, T. Facile preparation of hierarchical structure based on p-type Co3O4 as toluene detecting sensor. Appl. Surf. Sci. 2020, 503, 144167. [Google Scholar] [CrossRef]
- Zhai, C.; Zhao, Q.; Gu, K.; Xing, D.; Zhang, M. Ultra-fast response and recovery of triethylamine gas sensors using a MOF-based ZnO/ZnFe2O4 structures. J. Alloys Compd. 2019, 784, 660–667. [Google Scholar] [CrossRef]
- Cheng, L.; Li, Y.; Sun, G.; Cao, J.; Wang, Y. Modification of Bi2O3 on ZnO porous nanosheets-assembled architecture for ultrafast detection of TEA with high sensitivity. Sens. Actuators B Chem. 2023, 376, 132986. [Google Scholar] [CrossRef]
- Fu, X.; Yang, P.; Xiao, X.; Zhou, D.; Huang, R.; Zhang, X.; Cao, F.; Xiong, J.; Hu, Y.; Tu, Y.; et al. Ultra-fast and highly selective room-temperature formaldehyde gas sensing of Pt-decorated MoO3 nanobelts. J. Alloys Compd. 2019, 797, 666–675. [Google Scholar] [CrossRef]
- John, R.A.B.; Shruthi, J.; Ramana Reddy, M.; Ruban Kumar, A. Manganese doped nickel oxide as room temperature gas sensor for formaldehyde detection. Ceram. Int. 2022, 48, 17654–17667. [Google Scholar] [CrossRef]
- Venkatraman, M.; Kadian, A.; Choudhary, S.; Subramanian, A.; Singh, A.; Sikarwar, S. Ultra-Fast Benzene Gas (C6H6) Detection Characteristics of Cobalt-Doped Aluminum Oxide Sensors. ChemistrySelect 2023, 8, e202204531. [Google Scholar] [CrossRef]
- Qin, W.; Yuan, Z.; Gao, H.; Zhang, R.; Meng, F. Perovskite-structured LaCoO3 modified ZnO gas sensor and investigation on its gas sensing mechanism by first principle. Sens. Actuators B Chem. 2021, 341, 130015. [Google Scholar] [CrossRef]
- John, R.A.B.; Shruthi, J.; Ramana Reddy, M.; Ruban Kumar, A. Hole concentration modulated gas sensor for selective detection of 2-methoxy ethanol. Ceram. Int. 2023, 49, 9122–9129. [Google Scholar] [CrossRef]
- Tong, X.; Shen, W.; Chen, X.; Corriou, J.P. A fast response and recovery H2S gas sensor based on free-standing TiO2 nanotube array films prepared by one-step anodization method. Ceram. Int. 2017, 43, 14200–14209. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent advancement of nanostructured carbon for energy applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef]
- Freddi, S.; Achilli, S.; Soave, R.; Pagliara, S.; Drera, G.; De Poli, A.; De Nicola, F.; De Crescenzi, M.; Castrucci, P.; Sangaletti, L. Dramatic efficiency boost of single-walled carbon nanotube-silicon hybrid solar cells through exposure to ppm nitrogen dioxide in air: An ab-initio assessment of the measured device performances. J. Colloid Interface Sci. 2020, 566, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef]
- Kumar, R.; Joanni, E.; Sahoo, S.; Shim, J.J.; Tan, W.K.; Matsuda, A.; Singh, R.K. An overview of recent progress in nanostructured carbon-based supercapacitor electrodes: From zero to bi-dimensional materials. Carbon 2022, 193, 298–338. [Google Scholar] [CrossRef]
- Yin, F.; Yue, W.; Li, Y.; Gao, S.; Zhang, C.; Kan, H.; Niu, H.; Wang, W.; Guo, Y. Carbon-based nanomaterials for the detection of volatile organic compounds: A review. Carbon 2021, 180, 274–297. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Bogue, R. Nanomaterials for gas sensing: A review of recent research. Sens. Rev. 2014, 34, 1–8. [Google Scholar] [CrossRef]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Liu, J.; Bao, S.; Wang, X. Applications of graphene-based materials in sensors: A review. Micromachines 2022, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Freddi, S.; Sangaletti, L. Trends in the Development of Electronic Noses Based on Carbon Nanotubes Chemiresistors for Breathomics. Nanomaterials 2022, 12, 2992. [Google Scholar] [CrossRef]
- Bogue, R. Graphene sensors: A review of recent developments. Sens. Rev. 2014, 34, 233–238. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Freddi, S.; Gonzalez, M.C.R.; Carro, P.; Sangaletti, L.; De Feyter, S. Chemical defect-driven response on graphene-based chemiresistors for sub-ppm ammonia detection. Angew. Chem. Int. Ed. 2022, 61, e202200115. [Google Scholar] [CrossRef]
- Tang, X.; Debliquy, M.; Lahem, D.; Yan, Y.; Raskin, J.P. A review on functionalized graphene sensors for detection of ammonia. Sensors 2021, 21, 1443. [Google Scholar] [CrossRef]
- Kamran, U.; Heo, Y.J.; Lee, J.W.; Park, S.J. Functionalized carbon materials for electronic devices: A review. Micromachines 2019, 10, 234. [Google Scholar] [CrossRef]
- Ansari, N.; Lone, M.Y.; Shumaila; Ali, J.; Zulfequar, M.; Husain, M.; Islam, S.S.; Husain, S. Trace level toxic ammonia gas sensing of single-walled carbon nanotubes wrapped polyaniline nanofibers. J. Appl. Phys. 2020, 127, 044902. [Google Scholar] [CrossRef]
- Wang, X.; Wei, M.; Li, X.; Shao, S.; Ren, Y.; Xu, W.; Li, M.; Liu, W.; Liu, X.; Zhao, J. Large-Area Flexible Printed Thin-Film Transistors with Semiconducting Single-Walled Carbon Nanotubes for NO2 Sensors. ACS Appl. Mater. Interfaces 2020, 12, 51797–51807. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Tang, K.; Wang, H.; Chen, T.; Jiang, K.; Zhou, T.; Quan, H.; Guo, R. Ultralow detection limit and ultrafast response/recovery of the H2 gas sensor based on Pd-doped rGO/ZnO-SnO2 from hydrothermal synthesis. Microsyst. Nanoeng. 2022, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xiao, M.; Ning, Y.; Zhou, S.; He, J.; Lin, Y.; Zhang, Z. Toward practical gas sensing with rapid recovery semiconducting carbon nanotube film sensors. Sci. China Inf. Sci. 2022, 65, 162402. [Google Scholar] [CrossRef]
- Jia, X.; Cheng, C.; Yu, S.; Yang, J.; Li, Y.; Song, H. Preparation and enhanced acetone sensing properties of flower-like α-Fe2O3/multi-walled carbon nanotube nanocomposites. Sens. Actuators B Chem. 2019, 300, 127012. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Z.; Cao, Y.; Guo, J.; Long, M.; Duan, H.; Jia, D. Chemiresistive sensor arrays based on noncovalently functionalized multi-walled carbon nanotubes for ozone detection. Sens. Actuators B Chem. 2019, 297, 126689. [Google Scholar] [CrossRef]
- Hussain, A.; Lakhan, M.N.; Soomro, I.A.; Ahmed, M.; Hanan, A.; Maitlo, A.A.; Zehra, I.; Liu, J.; Wang, J. Preparation of reduced graphene oxide decorated two-dimensional WSe2 nanosheet sensor for efficient detection of ethanol gas. Phys. E Low-Dimens. Syst. Nanostruct. 2023, 147, 115574. [Google Scholar] [CrossRef]
- Basu, A.K.; Chauhan, P.S.; Awasthi, M.; Bhattacharya, S. α-Fe2O3 loaded rGO nanosheets based fast response/recovery CO gas sensor at room temperature. Appl. Surf. Sci. 2019, 465, 56–66. [Google Scholar] [CrossRef]
- Seekaew, Y.; Wisitsoraat, A.; Phokharatkul, D.; Wongchoosuk, C. Room temperature toluene gas sensor based on TiO2 nanoparticles decorated 3D graphene-carbon nanotube nanostructures. Sens. Actuators B Chem. 2019, 279, 69–78. [Google Scholar] [CrossRef]
- Chaitongrat, B.; Chaisitsak, S. Novel Preparation and Characterization of Fe2O33/CNT Thin Films for Flammable Gas Sensors. Mater. Sci. Forum 2019, 947, 47–51. [Google Scholar] [CrossRef]
- Gai, S.; Wang, B.; Wang, X.; Zhang, R.; Miao, S.; Wu, Y. Ultrafast NH3 gas sensor based on phthalocyanine-optimized non-covalent hybrid of carbon nanotubes with pyrrole. Sens. Actuators B Chem. 2022, 357, 131352. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Gottam, S.R.; Tsai, C.T.; Wang, L.W.; Wang, C.T.; Lin, C.C.; Chu, S.Y. Highly sensitive hydrogen gas sensor based on a MoS2-Pt nanoparticle composite. Appl. Surf. Sci. 2020, 506, 144981. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, X.; Yang, A.; Zong, X. Hierarchical assembly of urchin-like alpha-iron oxide hollow microspheres and molybdenum disulphide nanosheets for ethanol gas sensing. J. Colloid Interface Sci. 2018, 523, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gan, L.; Li, H.; Zhai, T. Two-dimensional layered nanomaterials for gas-sensing applications. Inorg. Chem. Front. 2016, 3, 433–451. [Google Scholar] [CrossRef]
- Wang, W.; Zhen, Y.; Zhang, J.; Li, Y.; Zhong, H.; Jia, Z.; Xiong, Y.; Xue, Q.; Yan, Y.; Alharbi, N.S.; et al. SnO2 nanoparticles-modified 3D-multilayer MoS2 nanosheets for ammonia gas sensing at room temperature. Sens. Actuators B Chem. 2020, 321, 128471. [Google Scholar] [CrossRef]
- You, C.W.; Fu, T.; Li, C.B.; Song, X.; Tang, B.; Song, X.; Yang, Y.; Deng, Z.P.; Wang, Y.Z.; Song, F. A Latent-Fire-Detecting Olfactory System Enabled by Ultra-Fast and Sub-ppm Ammonia-Responsive Ti3C2Tx MXene/MoS2 Sensors. Adv. Funct. Mater. 2022, 32, 2208131. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, H.; Xie, Y.; Wang, J.; Fan, J.; Sun, B.; Jiang, L.; Zhang, Y.; Wang, R.; Shi, K. A 2D/2D/2D Ti3C2Tx@TiO2@ MoS2 heterostructure as an ultrafast and high-sensitivity NO2 gas sensor at room-temperature. J. Mater. Chem. A 2022, 10, 11980–11989. [Google Scholar] [CrossRef]
- Gorthala, G.; Ghosh, R. Ultra-Fast NO2 Detection by MoS2 Nanoflakes at Room Temperature. IEEE Sens. J. 2022, 22, 14727–14735. [Google Scholar] [CrossRef]
- Ikram, M.; Liu, L.; Liu, Y.; Ma, L.; Lv, H.; Ullah, M.; He, L.; Wu, H.; Wang, R.; Shi, K. Fabrication and characterization of a high-surface area MoS2@WS2 heterojunction for the ultra-sensitive NO2 detection at room temperature. J. Mater. Chem. A 2019, 7, 14602–14612. [Google Scholar] [CrossRef]
- Ikram, M.; Liu, L.; Liu, Y.; Ullah, M.; Ma, L.; Bakhtiar, S.u.H.; Wu, H.; Yu, H.; Wang, R.; Shi, K. Controllable synthesis of MoS2@MoO2 nanonetworks for enhanced NO2 room temperature sensing in air. Nanoscale 2019, 11, 8554–8564. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Long, X.; Cao, J.; Xin, X.; Guan, X.; Peng, J.; Zheng, X. Gas sensors based on mechanically exfoliated MoS2 nanosheets for room-temperature NO2 detection. Sensors 2019, 19, 2123. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Hu, J.Y.; Liu, C.; Tan, Y.M.; Peng, X.; Zhang, Y. Mechanically exfoliated MoS2 nanosheets decorated with SnS2 nanoparticles for high-stability gas sensors at room temperature. Rare Met. 2021, 40, 1536–1544. [Google Scholar] [CrossRef]
- Hong, H.S.; Phuong, N.H.; Huong, N.T.; Nam, N.H.; Hue, N.T. Highly sensitive and low detection limit of resistive NO2 gas sensor based on a MoS2/graphene two-dimensional heterostructures. Appl. Surf. Sci. 2019, 492, 449–454. [Google Scholar] [CrossRef]
- Bai, X.; Lv, H.; Liu, Z.; Chen, J.; Wang, J.; Sun, B.; Zhang, Y.; Wang, R.; Shi, K. Thin-layered MoS2 nanoflakes vertically grown on SnO2 nanotubes as highly effective room-temperature NO2 gas sensor. J. Hazard. Mater. 2021, 416, 125830. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, L.; He, S.; Zhou, L.; Wang, M.; Wang, J.; Komarneni, S. UV-activated WS2/SnO2 2D/0D heterostructures for fast and reversible NO2 gas sensing at room temperature. Sens. Actuators B Chem. 2022, 364, 131903. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.; Su, C.; Wang, S.; Li, H.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; et al. Interface engineered WS2/ZnS heterostructures for sensitive and reversible NO2 room temperature sensing. Sens. Actuators B Chem. 2019, 296, 126666. [Google Scholar] [CrossRef]
- Ikram, M.; Liu, Y.; Lv, H.; Liu, L.; Rehman, A.U.; Kan, K.; Zhang, W.; He, L.; Wang, Y.; Wang, R.; et al. 3D-multilayer MoS2 nanosheets vertically grown on highly mesoporous cubic In2O3 for high-performance gas sensing at room temperature. Appl. Surf. Sci. 2019, 466, 1–11. [Google Scholar] [CrossRef]
- Saravanan, A.; Huang, B.R.; Chu, J.P.; Prasannan, A.; Tsai, H.C. Interface engineering of ultrananocrystalline diamond/MoS2-ZnO heterostructures and its highly enhanced hydrogen gas sensing properties. Sens. Actuators B Chem. 2019, 292, 70–79. [Google Scholar] [CrossRef]
- Kathiravan, D.; Huang, B.R.; Saravanan, A.; Prasannan, A.; Hong, P.D. Highly enhanced hydrogen sensing properties of sericin-induced exfoliated MoS2 nanosheets at room temperature. Sens. Actuators B Chem. 2019, 279, 138–147. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Guo, J.; Hu, Y.; Zhang, D. Two-step hydrothermal fabrication of CeO2-loaded MoS2 nanoflowers for ethanol gas sensing application. Appl. Surf. Sci. 2021, 568, 150942. [Google Scholar] [CrossRef]
- Chen, W.Y.; Jiang, X.; Lai, S.N.; Peroulis, D.; Stanciu, L. Nanohybrids of a MXene and transition metal dichalcogenide for selective detection of volatile organic compounds. Nat. Commun. 2020, 11, 1302. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Puri, N.K. Zinc oxide incorporated molybdenum diselenide nanosheets for chemiresistive detection of ethanol gas. J. Alloys Compd. 2023, 955, 170178. [Google Scholar] [CrossRef]
- Chang, X.; Li, X.; Qiao, X.; Li, K.; Xiong, Y.; Li, X.; Guo, T.; Zhu, L.; Xue, Q. Metal-organic frameworks derived ZnO@MoS2 nanosheets core/shell heterojunctions for ppb-level acetone detection: Ultra-fast response and recovery. Sens. Actuators B Chem. 2020, 304, 127430. [Google Scholar] [CrossRef]
- Lee, J.H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B Chem. 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Hu, K.; Zhao, Q.; Guo, Y.; Zhang, J.; Bao, J.; Yang, Y.; He, T.; Su, Y. Dual mechanisms of Pd-doped In2O3/CeO2 nanofibers for hydrogen gas sensing. ACS Appl. Nano Mater. 2022, 5, 6232–6240. [Google Scholar] [CrossRef]
- Katoch, A.; Kim, J.H.; Kwon, Y.J.; Kim, H.W.; Kim, S.S. Bifunctional sensing mechanism of SnO2–ZnO composite nanofibers for drastically enhancing the sensing behavior in H2 gas. ACS Appl. Mater. Interfaces 2015, 7, 11351–11358. [Google Scholar] [CrossRef]

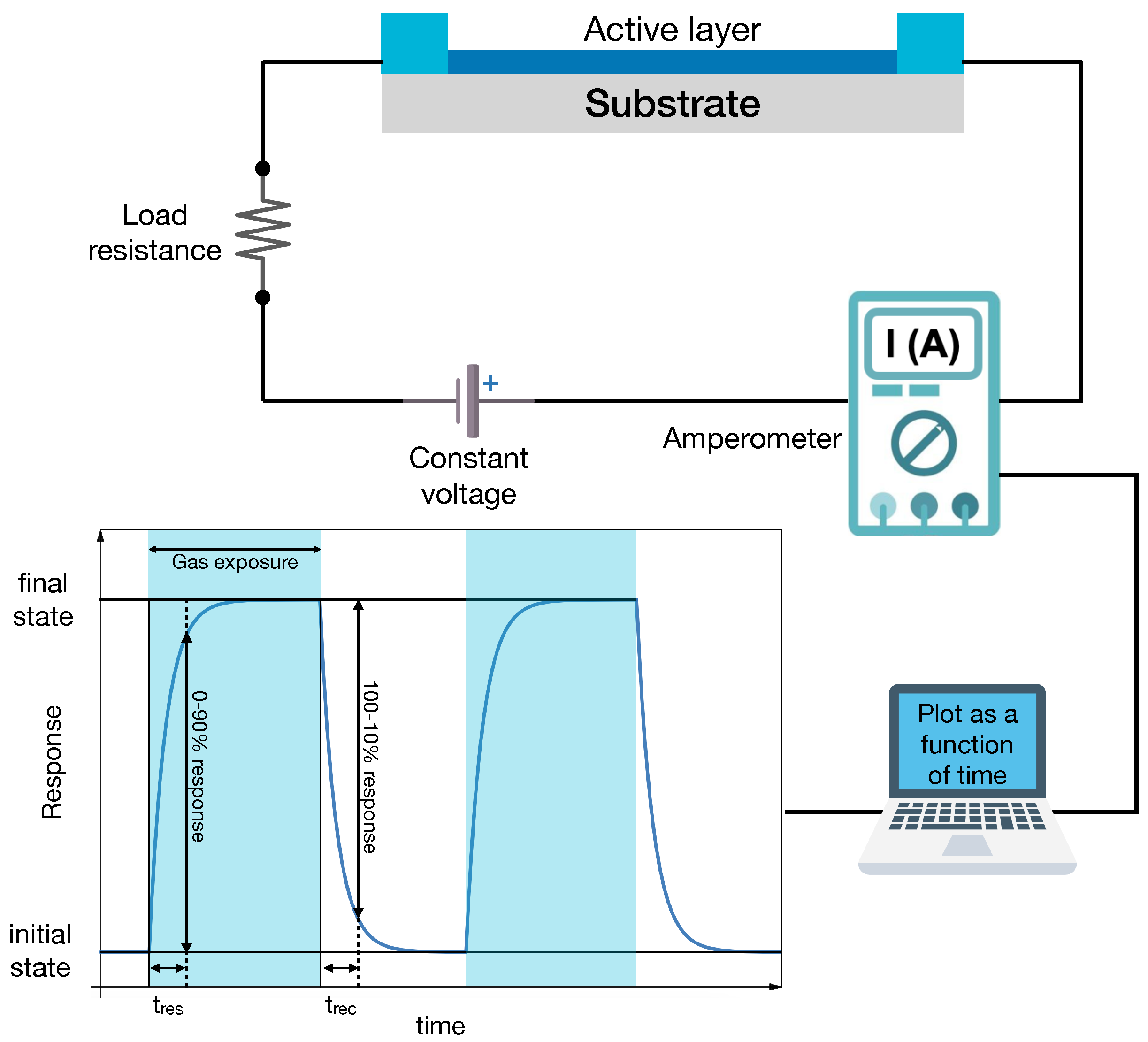
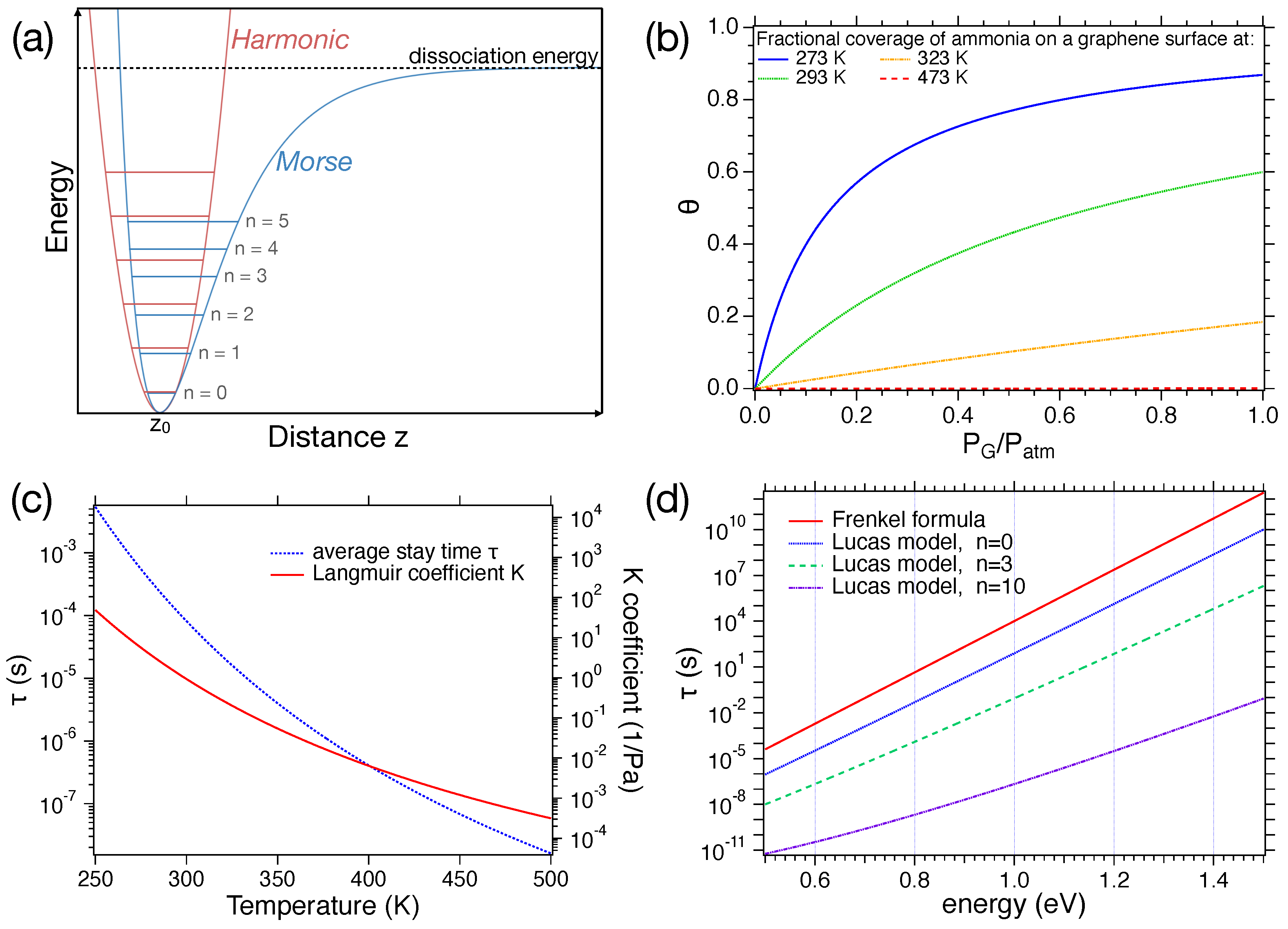
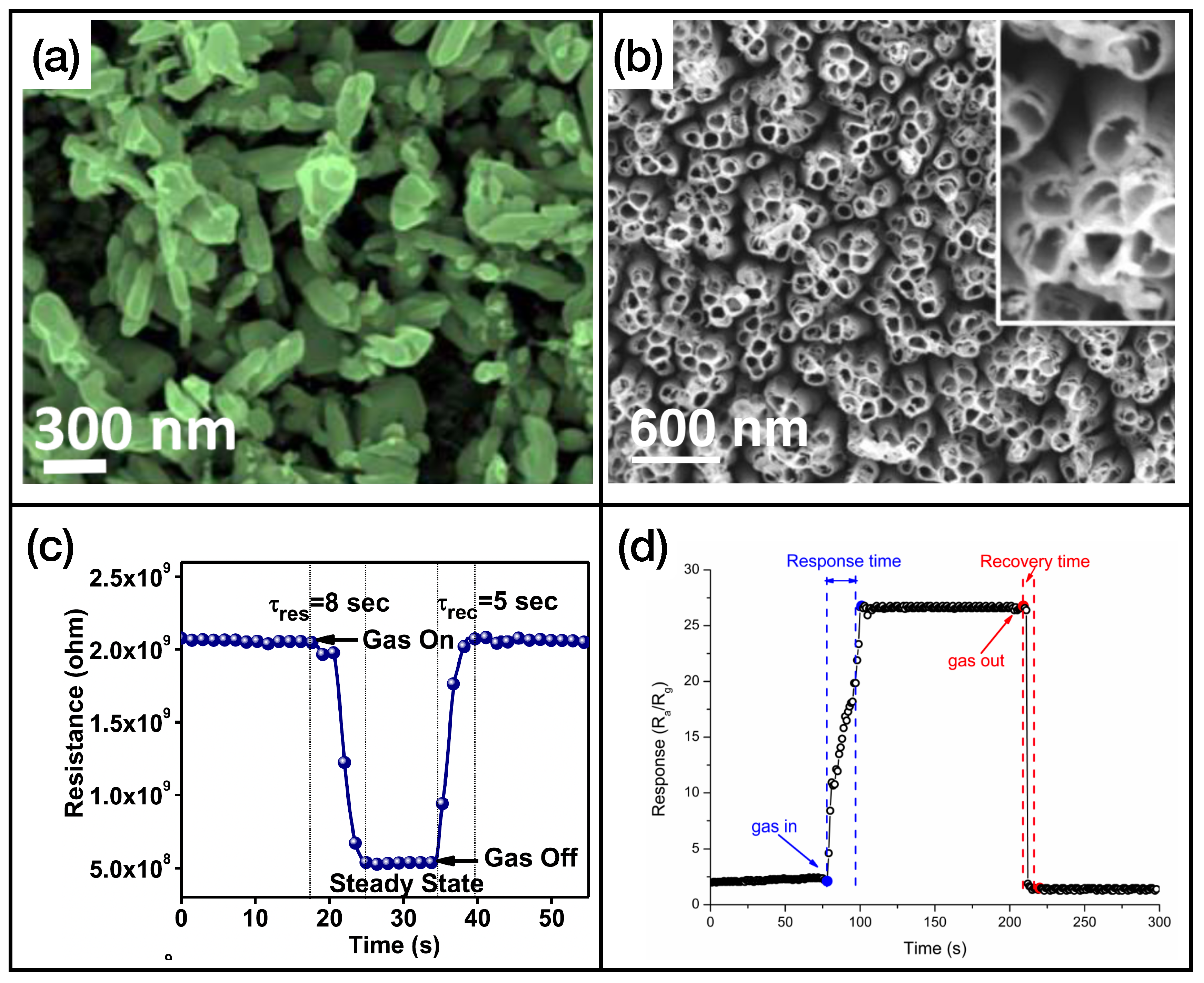


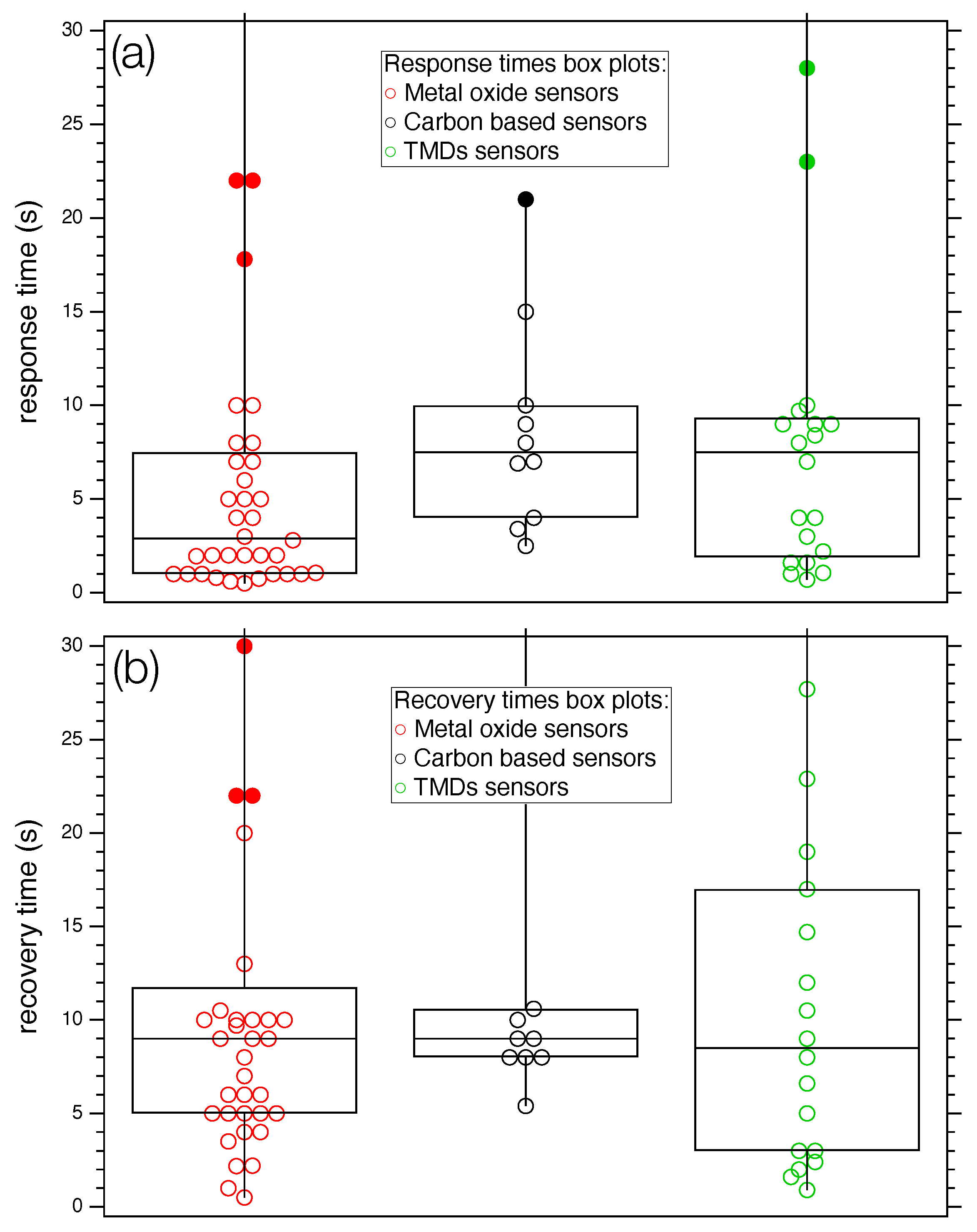
| Ref. | Year | Active Layer | Gas | Conc. (ppm) | T (°) | Response | t/t (s) |
|---|---|---|---|---|---|---|---|
| [52] | 2020 | ZnO NFs | 3 | 250 | 0.8 | 3/5 s | |
| [53] | 2020 | NBs | 100 | RT | 0.75 | 8/5 s | |
| [54] | 2022 | nanofibers + NPs | 1 | RT | 0.56 | 7/7 s | |
| [55] | 2023 | Ag-doped nanostructures | 1 | 150 | 316 | 0.5/3.5 s | |
| [56] | 2021 | Nanohybrid of MXene-derived | 1000 | RT | 115 | 64/10 s | |
| [57] | 2022 | + MXene NSs | 20 | RT | 3.68 | 22/10 s | |
| [58] | 2021 | Au-functionalized CuO NRs | 20 | RT | 3.0 | 8/176 s | |
| [59] | 2023 | /NiO heterojunction | 60 | 180 | 13 | 5/53 s | |
| [60] | 2020 | Three-dimensional flower-like / (NAS) | 50 | RT | 18.76 | 1.06/40.26 s | |
| [61] | 2019 | - nano-ellipsoids | 50 | 260 | 8 | 0.8/2.2 s | |
| [62] | 2019 | Cadmium sulfide + ultrathin porous layer of hollow microspheres | 100 | RT | 12.78 | 0.6/1 s | |
| [63] | 2022 | Carbon modification on coral-like | 50 | 275 | 25.5 | 1/20 s | |
| 50 | 275 | 25.5 | 7/9 s | ||||
| [64] | 2022 | Pd-modified NPs | 500 | 125 | 254 | 1/22 s | |
| [65] | 2023 | QDs- | 200 | 400 | 0.41 | 2/5 s | |
| [66] | 2023 | / | 1000 | 75 | 2843 | 1/13 s | |
| [67] | 2021 | Yolk shell / | acetone | 100 | 200 | 49.8 | 4/5 s |
| [68] | 2020 | Hierarchical-structured NSs | acetone | 200 | 400 | 21.6 | 0.75/0.5 s |
| [69] | 2021 | NSs | acetone | 50 | 350 | 14.7 | 5/8 s |
| [70] | 2019 | Pd-doped WO3 NSs | acetone | 100 | 300 | 107.29 | 1/9 s |
| [71] | 2022 | Flame-annealed porous NSs | CO | 500 | 300 | 0.39 | 2/6 s |
| [72] | 2023 | CO | 50 | 200 | 1.41 | 2/10 s | |
| [73] | 2019 | : | methane | 50 | 300 | 0.44 | 6/4 s |
| [74] | 2019 | NPs with porous nanostructure | toluene | 100 | 225 | 132 | 2/6 s |
| [75] | 2020 | CuO NPs + MXene | toluene | 50 | 250 | 11.4 | 270/10 s |
| [76] | 2020 | p-type | toluene | 200 | 180 | 8.5 | 10/30 s |
| [77] | 2019 | MOF-based ZnO/ | triethylamine | 100 | 170 | 7.6 | 1/9 s |
| [78] | 2023 | -ZnO heterojunction | triethylamine | 100 | 270 | 2553 | 1/3600 s |
| [79] | 2019 | Pt-decorated nanobelts | formaldehyde | 100 | RT | 0.39 | 17.8/10.5 s |
| [80] | 2022 | Mg-doped NiO | formaldehyde | 100 | RT | 12,593 | 5/5 s |
| [81] | 2023 | Co-doped | benzene | 5 | 100 | 1.66 | 1.95/2.18 s |
| [82] | 2021 | + ZnO | ethanol | 100 | 320 | 55 | 2.8/9.7 s |
| [83] | 2023 | Ag-NiO | 2-methoxy ethanol | 100 | RT | 6419.57 | 10/10 s |
| Ref. | Year | Active Layer | Gas | Conc. (ppm) | T (°) | Response | t/t (s) |
|---|---|---|---|---|---|---|---|
| [100] | 2020 | SWCNT-PANI composite | 10 | RT | 0.25 | 4/10 s | |
| [101] | 2020 | SWCNT | 16 | RT | 1.00 | 8/8 s | |
| [102] | 2022 | Pd-doped rGO + ZnO- | 100 | RT | 9.4 | 4/8 s | |
| [103] | 2022 | Pd-decorated CNT | 10 | RT | 0.08 | 9/50 s | |
| [104] | 2019 | flower-like - and MWCNT nanocomposites | Acetone | 50 | 220 | 20.32 | 2.3/10.6 s |
| [105] | 2019 | non-covalently functionalized MWCNT | 0.08 | RT | 0.013 | 6.9/5.4 s | |
| [106] | 2023 | rGO + | ethanol | 100 | 180 | 5.5 | 15/10 s |
| [107] | 2019 | - + rGO | CO | 10 | RT | 4 | 21/8 s |
| [108] | 2019 | 3D TiO2/G-CNT | Toluene | 500 | RT | 0.43 | 7/9 s |
| [109] | 2019 | /CNT | LPG | 50,000 | RT | 0.02 | 10/59 s |
| Ref. | Category | Gas | Conc. (ppm) | T (°) | t/t (s) | t + t s |
|---|---|---|---|---|---|---|
| [53] | MOX | 100 | RT | 8/5 s | ||
| [54] | MOX | 1 | RT | 7/7 s | ||
| [55] | MOX | 1 | 150 | 0.5/3.5 s | ✓ | |
| [61] | MOX | 50 | 260 | 0.8/2.2 s | ✓ | |
| [62] | MOX | 100 | RT | 0.6/1 s | ✓ | |
| MOX | 50 | 275 | 7/9 s | |||
| [65] | MOX | 200 | 400 | 2/5 s | ✓ | |
| [67] | MOX | acetone | 100 | 200 | 4/5 s | ✓ |
| [68] | MOX | acetone | 200 | 400 | 0.75/0.5 s | ✓ |
| [69] | MOX | acetone | 50 | 350 | 5/8 s | |
| [70] | MOX | acetone | 100 | 300 | 1/9 s | ✓ |
| [71] | MOX | CO | 500 | 300 | 2/6 s | ✓ |
| [72] | MOX | CO | 50 | 200 | 2/10 s | |
| [73] | MOX | methane | 50 | 300 | 6/4 s | ✓ |
| [74] | MOX | toluene | 100 | 225 | 2/6 s | ✓ |
| [77] | MOX | triethylamine | 100 | 170 | 1/9 s | ✓ |
| [80] | MOX | formaldehyde | 100 | RT | 5/5 s | ✓ |
| [81] | MOX | benzene | 5 | 100 | 1.95/2.18 s | ✓ |
| [82] | MOX | ethanol | 100 | 320 | 2.8/9.7 s | |
| [83] | MOX | 2-methoxy ethanol | 100 | RT | 10/10 s | |
| [100] | Carbon | 10 | RT | 4/10 s | ||
| [101] | Carbon | 16 | RT | 8/8 s | ||
| [102] | Carbon | 100 | RT | 4/8 s | ||
| [105] | Carbon | 0.08 | RT | 6.9/5.4 s | ||
| [108] | Carbon | Toluene | 500 | RT | 7/9 s | |
| [117] | TMD | 100 | RT | 3/2.4 s | ✓ | |
| [119] | TMD | 3 | RT | 9/3 s | ||
| [124] | TMD | 10 | 200 | 0.7/0.9 s | ✓ | |
| [126] | TMD | 0.5 | RT | 9/8 s | ||
| [130] | TMD | 100 | RT | 10/9 s | ||
| [131] | TMD | ethanol | 50 | RT | 7/5 s | |
| [132] | TMD | ethanol | 40 | RT | 9.7/6.6 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvani, M.; Freddi, S.; Sangaletti, L. Disclosing Fast Detection Opportunities with Nanostructured Chemiresistor Gas Sensors Based on Metal Oxides, Carbon, and Transition Metal Dichalcogenides. Sensors 2024, 24, 584. https://doi.org/10.3390/s24020584
Galvani M, Freddi S, Sangaletti L. Disclosing Fast Detection Opportunities with Nanostructured Chemiresistor Gas Sensors Based on Metal Oxides, Carbon, and Transition Metal Dichalcogenides. Sensors. 2024; 24(2):584. https://doi.org/10.3390/s24020584
Chicago/Turabian StyleGalvani, Michele, Sonia Freddi, and Luigi Sangaletti. 2024. "Disclosing Fast Detection Opportunities with Nanostructured Chemiresistor Gas Sensors Based on Metal Oxides, Carbon, and Transition Metal Dichalcogenides" Sensors 24, no. 2: 584. https://doi.org/10.3390/s24020584





