Smartphone-Controlled Aptasensor for Voltammetric Detection of Patulin in Apple Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
2.3. Procedure
2.4. Selectivity Studies
2.5. Application of PAT Aptasensor to Apple Juice Samples
2.6. Applications of PAT Aptasensor with Smartphone-Integrated Portable Device
3. Results and Discussion
3.1. Optimization of the Experimental Parameters for PAT Aptasensor
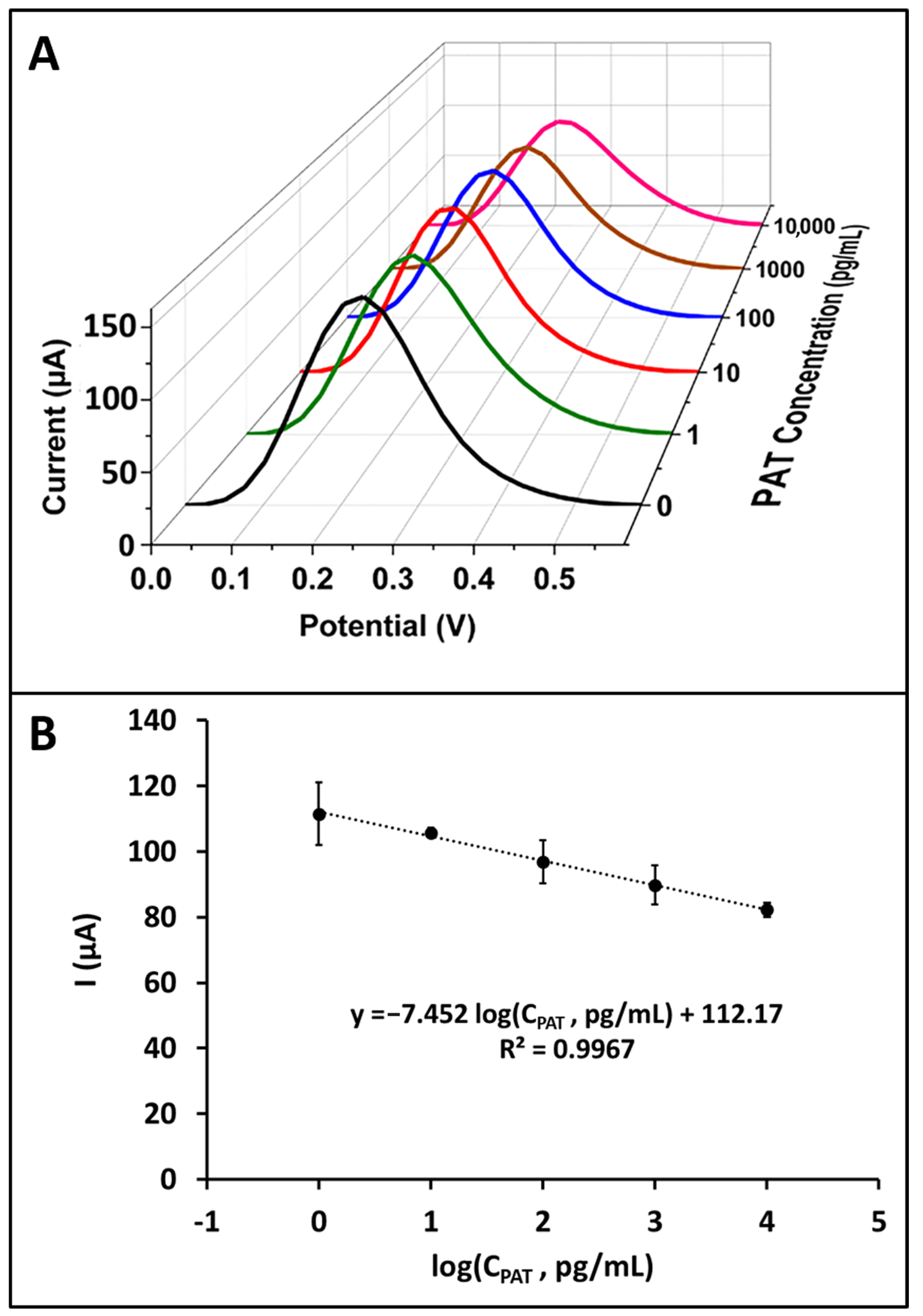
3.2. Analytical Performance of PAT Aptasensor
3.3. Selectivity of PAT Aptasensor
3.4. Application of Smartphone-Connected PAT Aptasensor in Apple Juice Samples
3.5. Recovery and Reproducibility of PAT Aptasensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zain, M.E. Impact of Mycotoxins on Humans and Animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- da Rocha, M.E.; Freire, F.D.; Maia, F.E.; Guedes, M.I.; Rondina, D. Mycotoxins and Their Effects on Human and Animal Health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical Methods for Determination of Mycotoxins: A Review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Kesici, E.; Erdem, A. Impedimetric Detection of Fumonisin B1 and Its Biointeraction with FsDNA. Int. J. Biol. Macromol. 2019, 139, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Muti, M.; Karadeniz, H.; Congur, G.; Canavar, E. Electrochemical Biosensors for Screening of Toxins and Pathogens. In NATO Science for Peace and Security Series A: Chemistry and Biology: Portable Chemical Sensors: Weapons Against Bioterrorism; Nikolelis, D.P., Ed.; Springer: Dordrecht, The Nertherland, 2012; pp. 323–334. ISBN 978-94-007-2871-4. [Google Scholar]
- Erdem, A.; Eksin, E.; Kesici, E.; Yaralı, E.; Kanat, E. Single-Use Sensor Technology for Monitoring of Zearalenone in Foods: ZentoSens. Microchem. J. 2019, 147, 37–42. [Google Scholar] [CrossRef]
- Moake, M.M.; Padilla-Zakour, O.I.; Worobo, R.W. Comprehensive Review of Patulin Control Methods in Foods. Compr. Rev. Food Sci. Food Saf. 2005, 4, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Beretta, B.; Gaiaschi, A.; Galli, C.L.; Restani, P. Patulin in Apple-Based Foods: Occurrence and Safety Evaluation. Food Addit. Contam. 2000, 17, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.L.; Harrison, M.A.; Koehler, P.E. Presence and Stability of Patulin in Pasteurized Apple Cider. J. Food Sci. 1987, 52, 479–480. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EC). COMMISSION REGULATION (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Food Stuffs. Off. J. Eur. Union 2006, 364, 1–20. [Google Scholar]
- Zhang, B.; Peng, X.; Li, G.; Xu, Y.; Xia, X.; Wang, Q. Oxidative Stress Is Involved in Patulin Induced Apoptosis in HEK293 Cells. Toxicon 2015, 94, 1–7. [Google Scholar] [CrossRef]
- Alves, I.; Oliveira, N.G.; Laires, A.; Rodrigues, A.S.; Rueff, J. Induction of Micronuclei and Chromosomal Aberrations by the Mycotoxin Patulin in Mammalian Cells: Role of Ascorbic Acid as a Modulator of Patulin Clastogenicity. Mutagenesis 2000, 15, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Showker, J.L. The Mechanism of Patulin’s Cytotoxicity and the Antioxidant Activity of Indole Tetramic Acids. Toxicol. Appl. Pharmacol. 1991, 109, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.; Goktepe, I. The Characteristics, Occurrence, and Toxicological Effects of Patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Leggott, N.L. Chromatographic Determination of the Mycotoxin Patulin in Fruit and Fruit Juices. J. Chromatogr. A 2000, 882, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, A.; Chrustek, A.; Olszewska-Słonina, D.; Koba, M.; Kruszewski, S. Determination of Patulin in Products Containing Dried Fruits by Enzyme-Linked Immunosorbent Assay Technique Patulin in Dried Fruits. Food Sci. Nutr. 2021, 9, 4211–4220. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Isabel Luque, M.; Andrade, M.J.; Rodríguez, M.; Asensio, M.A.; Córdoba, J.J. Development of Real-Time PCR Methods to Quantify Patulin-Producing Molds in Food Products. Food Microbiol. 2011, 28, 1190–1199. [Google Scholar] [CrossRef]
- Khan, R.; Ben Aissa, S.; Sherazi, T.A.; Catanante, G.; Hayat, A.; Marty, J.L. Development of an Impedimetric Aptasensor for Label Free Detection of Patulin in Apple Juice. Molecules 2019, 24, 1017. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lu, X. An Electrochemical Aptasensor Based on Tetrahedral DNA Nanostructures as a Signal Probe Carrier Platform for Sensitive Detection of Patulin. Anal. Chim. Acta 2020, 1138, 123–131. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Li, Z.; Zhou, Y.; Jin, P.; Zhang, F.; Sun, Q.; Le, T. Jirimutu Chromium Hydroxide Nanoparticles-Based Fluorescent Aptameric Sensing for Sensitive Patulin Detection: The Significance of Nanocrystal and Morphology Modulation. Talanta 2023, 257, 124296. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, L.; Jiang, S.; El-Seedi, H.R.; El-Garawani, I.M.; Zou, X. Sensitive Determination of Patulin by Aptamer Functionalized Magnetic Surface Enhanced Raman Spectroscopy (SERS) Sensor. J. Food Compos. Anal. 2023, 115, 104985. [Google Scholar] [CrossRef]
- He, B.; Dong, X. Aptamer Based Voltammetric Patulin Assay Based on the Use of ZnO Nanorods. Microchim. Acta 2018, 185, 462. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Dong, X.N. BbvCI Powered DNA Walking Machine-Based Zr-MOFs-Labeled Electrochemical Aptasensor Using Pt@AuNRs/Fe-MOFs/PEI-RGO as Electrode Modification Material for Patulin Detection. Chem. Eng. J. 2021, 405, 126642. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Li, W.; Wei, Y.; Sheng, Q.; Shang, Y. A Dual-Signaling Electrochemical Aptasensor Based on an In-Plane Gold Nanoparticles–Black Phosphorus Heterostructure for the Sensitive Detection of Patulin. Foods 2023, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qiao, X.; Wang, Y.; Sheng, Q.; Yue, T.; Zheng, J.; Zhou, M. Electrostatic Assembly of Gold Nanoparticles on Black Phosphorus Nanosheets for Electrochemical Aptasensing of Patulin. Microchim. Acta 2019, 186, 238. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, Q.; Pividori, M.I.; Zhang, Z.; Marty, J.L.; Catanante, G. A Sensitive Aptasensor Using Biotin-Streptavidin System for Patulin Detection in Apple Juice. Biosensors 2022, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Lavaee, P.; Emrani, A.S.; Abnous, K.; Taghdisi, S.M. A Rapid and Simple Ratiometric Fluorescent Sensor for Patulin Detection Based on a Stabilized DNA Duplex Probe Containing Less Amount of Aptamer-Involved Base Pairs. Talanta 2019, 204, 641–646. [Google Scholar] [CrossRef]
- Yan, X.; Du, G.; Chen, H.; Zhao, Q.; Guo, Q.; Wang, J.; Wang, Z.; Song, W.; Sheng, Q.; Luo, Y.; et al. Label-Free Fluorescence Aptasensor for the Detection of Patulin Using Target-Induced DNA Gates and TCPP/BDC-NH2 Mixed Ligands Functionalized Zr-MOF Systems. Biosens. Bioelectron. 2022, 217, 114723. [Google Scholar] [CrossRef]
- Pang, H.; Li, H.; Zhang, W.; Mao, J.; Zhang, L.; Zhang, Z.; Zhang, Q.; Wang, D.; Jiang, J.; Li, P. Fullerenol Quantum Dots-Based Highly Sensitive Fluorescence Aptasensor for Patulin in Apple Juice. Toxins 2022, 14, 272. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Biosensors: Towards Point-of-Care Cancer Diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef]
- Bakker, E.; Telting-Diaz, M. Electrochemical Sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Zhang, W.; Zhao, S.; Wang, Z. Screening and Development of DNA Aptamers as Capture Probes for Colorimetric Detection of Patulin. Anal. Biochem. 2016, 508, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.G.; Ferreira, A.A.P.; Yamanaka, H. Label-Free Impedimetric Immunosensor for Detection of the Textile Azo Dye Disperse Red 1 in Treated Water. Sens. Actuators B Chem. 2016, 236, 52–59. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Davis, J.J.; Luo, X. Ultrasensitive and Selective Voltammetric Aptasensor for Dopamine Based on a Conducting Polymer Nanocomposite Doped with Graphene Oxide. Microchim. Acta 2015, 182, 1123–1129. [Google Scholar] [CrossRef]
- Senturk, H.; Eksin, E.; Işık, Ö.; İlaslan, Z.; Mısırlı, F.; Erdem, A. Impedimetric Aptasensor for Lysozyme Detection Based on Carbon Nanofibres Enriched Screen-Printed Electrodes. Electrochim. Acta 2021, 377, 138078. [Google Scholar] [CrossRef]
- Geleta, G.S.; Zhao, Z.; Wang, Z. A Sensitive Electrochemical Aptasensor for Detection of Aflatoxin B2 Based on a Polyacrylamide/Phytic Acid/Polydopamine Hydrogel Modified Screen Printed Carbon Electrode. Anal. Methods 2018, 10, 4689–4694. [Google Scholar] [CrossRef]
- Roushani, M.; Dizajdizi, B.Z.; Rahmati, Z.; Azadbakht, A. Development of an Electrochemical Aptasensor Based on the Gold Nanorods and Its Application for Detection of Aflatoxin B1 in Rice and Blood Serum Samples. Nanochem. Res. 2019, 4, 35–42. [Google Scholar] [CrossRef]
- Ahmadi, S.F.; Hojjatoleslamy, M.; Kiani, H.; Molavi, H. Monitoring of Aflatoxin M1 in Milk Using a Novel Electrochemical Aptasensor Based on Reduced Graphene Oxide and Gold Nanoparticles. Food Chem. 2022, 373, 131321. [Google Scholar] [CrossRef]
- Negahdary, M.; Behjati-Ardakani, M.; Heli, H. An Electrochemical Troponin T Aptasensor Based on the Use of a Macroporous Gold Nanostructure. Microchim. Acta 2019, 186, 377. [Google Scholar] [CrossRef]
- IUPAC. Nomenclature, Symbols, Units and Their Usage in Spectrochemical Analysis—III. Analytical Flame Spectroscopy and Associated Non-Flame Procedures. Pure Appl. Chem. 1976, 45, 105–123. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of Detection A Closer Look at the IUPAC Definition. Anal. Chem. 1983, 55, 712–714. [Google Scholar]
- Hong, S.A.; Kwon, J.; Kim, D.; Yang, S. A Rapid, Sensitive and Selective Electrochemical Biosensor with Concanavalin A for the Preemptive Detection of Norovirus. Biosens. Bioelectron. 2015, 64, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Qiao, X.; Zhang, X.; Niu, C.; Yue, T.; Sheng, Q. Simultaneous Electrochemical Aptasensing of Patulin and Ochratoxin A in Apple Juice Based on Gold Nanoparticles Decorated Black Phosphorus Nanomaterial. Anal. Bioanal. Chem. 2021, 413, 3131–3140. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Tested Values | Selected Value |
|---|---|---|
| Aptamer concentration | 0.25 µg/mL–1 µg/mL–2.5 µg/mL–5 µg/mL | 2.5 µg/mL |
| Aptamer immobilization time | 30 min and 60 min | 60 min |
| PAT interaction time | 15 min, 30 min, and 60 min | 30 min |
| Technique | Linear Range | LOD | Application | Smartphone Implementation | Reference |
|---|---|---|---|---|---|
| Electrochemical impedance spectroscopy | 1–25 pg/mL | 2.8 pg/mL | Apple juice samples | No | [18] |
| Differential pulse voltammetry | 0.05 pg/mL–500,000 pg/mL | 0.0304 pg/mL | Apple juice samples | No | [19] |
| Differential pulse voltammetry | 0.5 pg/mL–50,000 pg/mL | 0.27 pg/mL | Apple juice samples | No | [22] |
| Differential pulse voltammetry | 0.05 pg/mL–500 pg/mL | 0.0414 pg/mL | Apple juice and apple wine samples | No | [23] |
| Square wave voltammetry | 0.1 nM–100 µM | 0.043 nM | Apple, pear and tomato samples | No | [24] |
| Electrochemical impedance spectroscopy | 1 nM–1 µM | 0.3 µM | Apple juice samples | No | [25] |
| Lateral flow assay | 2700–139,800 pg/mL in buffer and 7070–359,500 pg/mL in apple juice sample | 190 pg/mL in buffer and 360 pg/mL apple juice sample | Apple juice samples | No | [26] |
| Fluorescent | 15 pg/mL–35,000 pg/mL | 6 pg/mL | Apple juice samples | No | [27] |
| Fluorescent | 1 pg/mL–100,000 pg/mL | 0.753 pg/mL | Apple juice samples | No | [28] |
| Fluorescent | 20–1000 pg/mL in buffer and 50–1000 pg/mL apple juice sample | 10 pg/mL in buffer and 30 pg/mL in apple juice sample | Apple juice samples | No | [29] |
| Fluorescent | 10–10,000 pg/mL and 1000–200,000 pg/mL | 7300 pg/mL | Apple juice samples | No | [20] |
| Surface-enhanced Raman spectroscopy | 0–70,000 pg/mL | 38.4 pg/mL | Apple samples | No | [21] |
| Differential pulse voltammetry | 1–10,000 pg/mL in buffer and in apple juice medium | 0.18 pg/mL in buffer and 0.47 pg/mL in apple juice medium | Apple juice samples | Yes | This work |
| PAT Added (pg/mL) | PAT Found (pg/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|
| 1 | 0.92 | 92.00 | 9.22 |
| 100 | 93.47 | 93.47 | 5.11 |
| 10,000 | 9124.12 | 91.24 | 2.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdem, A.; Senturk, H. Smartphone-Controlled Aptasensor for Voltammetric Detection of Patulin in Apple Juice. Sensors 2024, 24, 754. https://doi.org/10.3390/s24030754
Erdem A, Senturk H. Smartphone-Controlled Aptasensor for Voltammetric Detection of Patulin in Apple Juice. Sensors. 2024; 24(3):754. https://doi.org/10.3390/s24030754
Chicago/Turabian StyleErdem, Arzum, and Huseyin Senturk. 2024. "Smartphone-Controlled Aptasensor for Voltammetric Detection of Patulin in Apple Juice" Sensors 24, no. 3: 754. https://doi.org/10.3390/s24030754





