A Novel Biosensor for the Detection of Glucose Concentration Using the Dual-Peak Long Period Grating in the Near- to Mid-Infrared
Abstract
:1. Introduction
2. Theory, Properties, Fabrication and Sensing Measurements of DPLPG
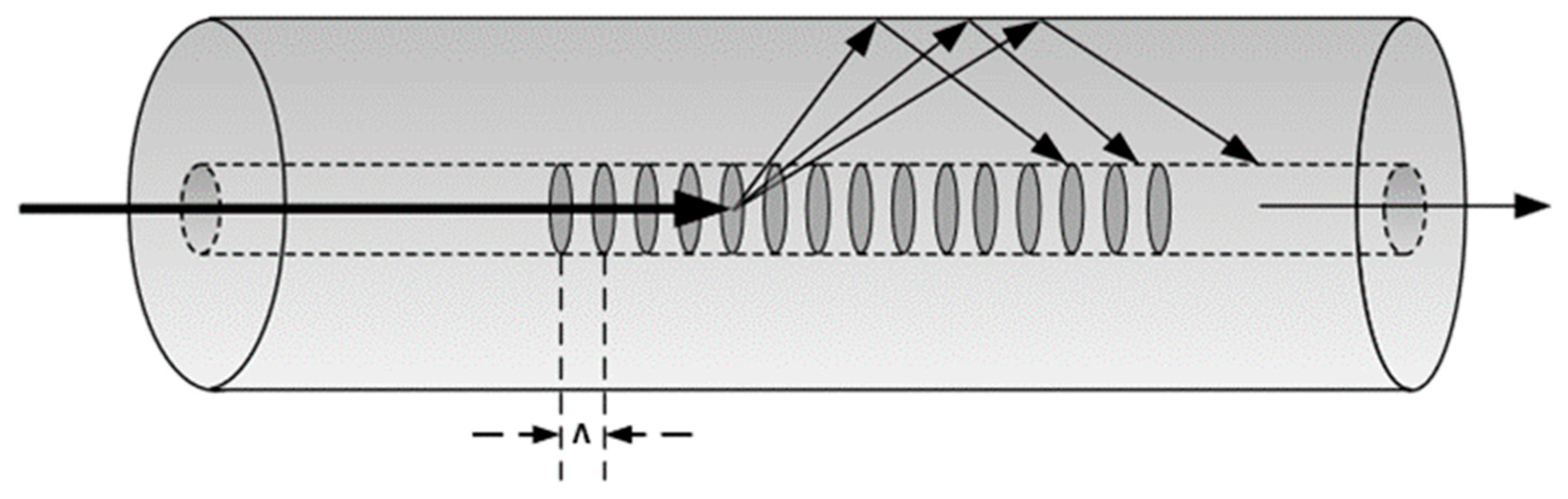
2.1. Theory
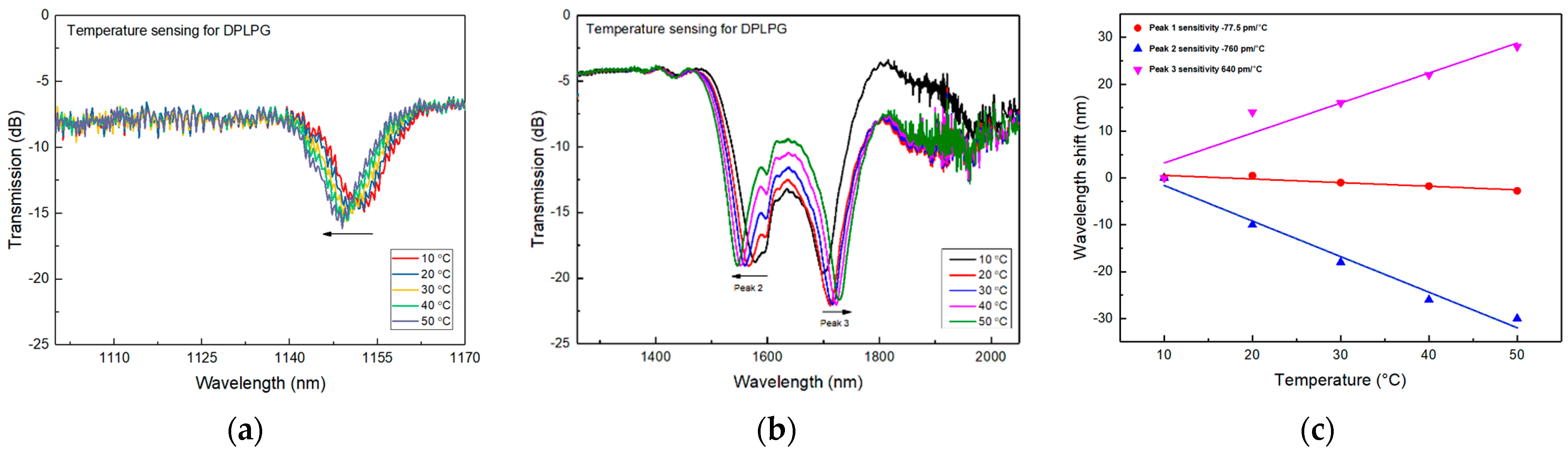
2.1.1. Thermal Sensing Property
2.1.2. Surrounding Refractive Index (SRI) Sensing Property
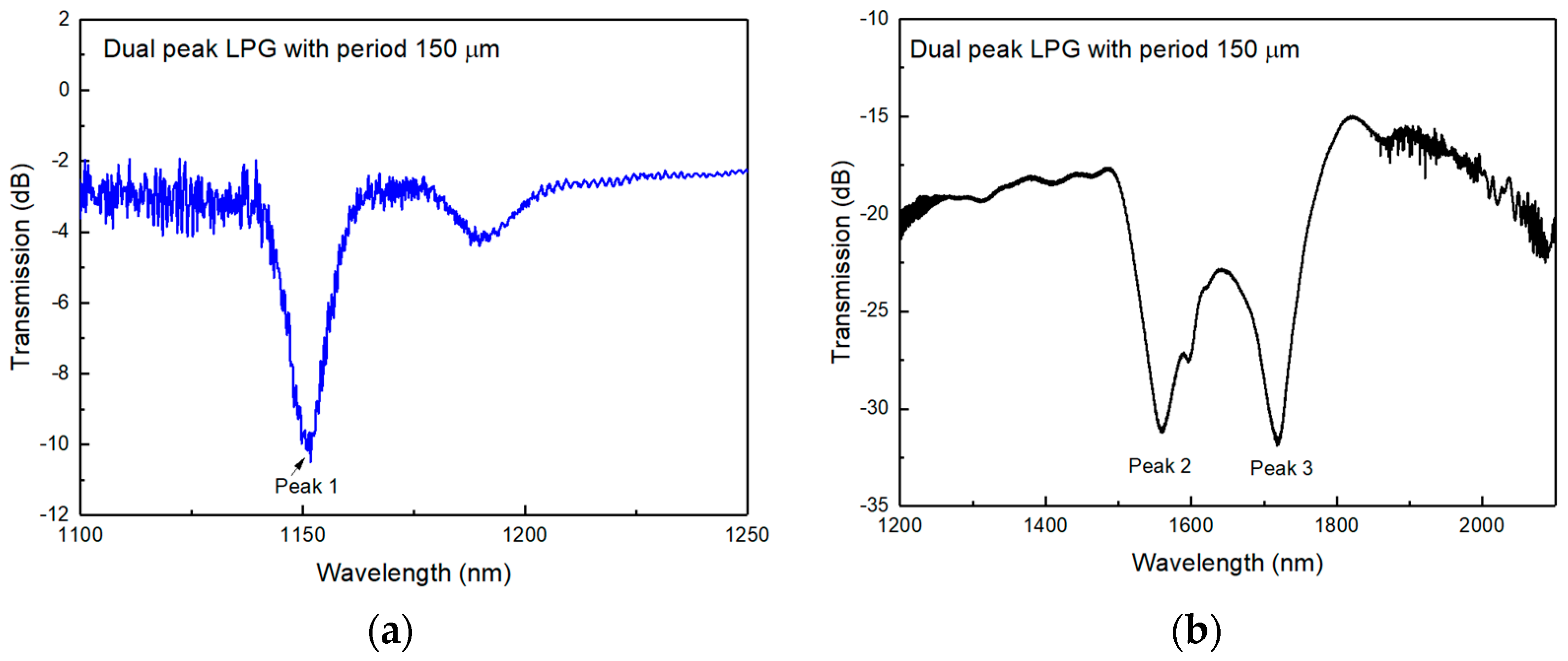
2.2. Fabrication of the DPLPG
2.3. Temperature Sensing and SRI Sensing and Analysing Results
3. Surface Modification for DPLPG and Glucose Detection
3.1. Surface Modification for DPLPG Sensor
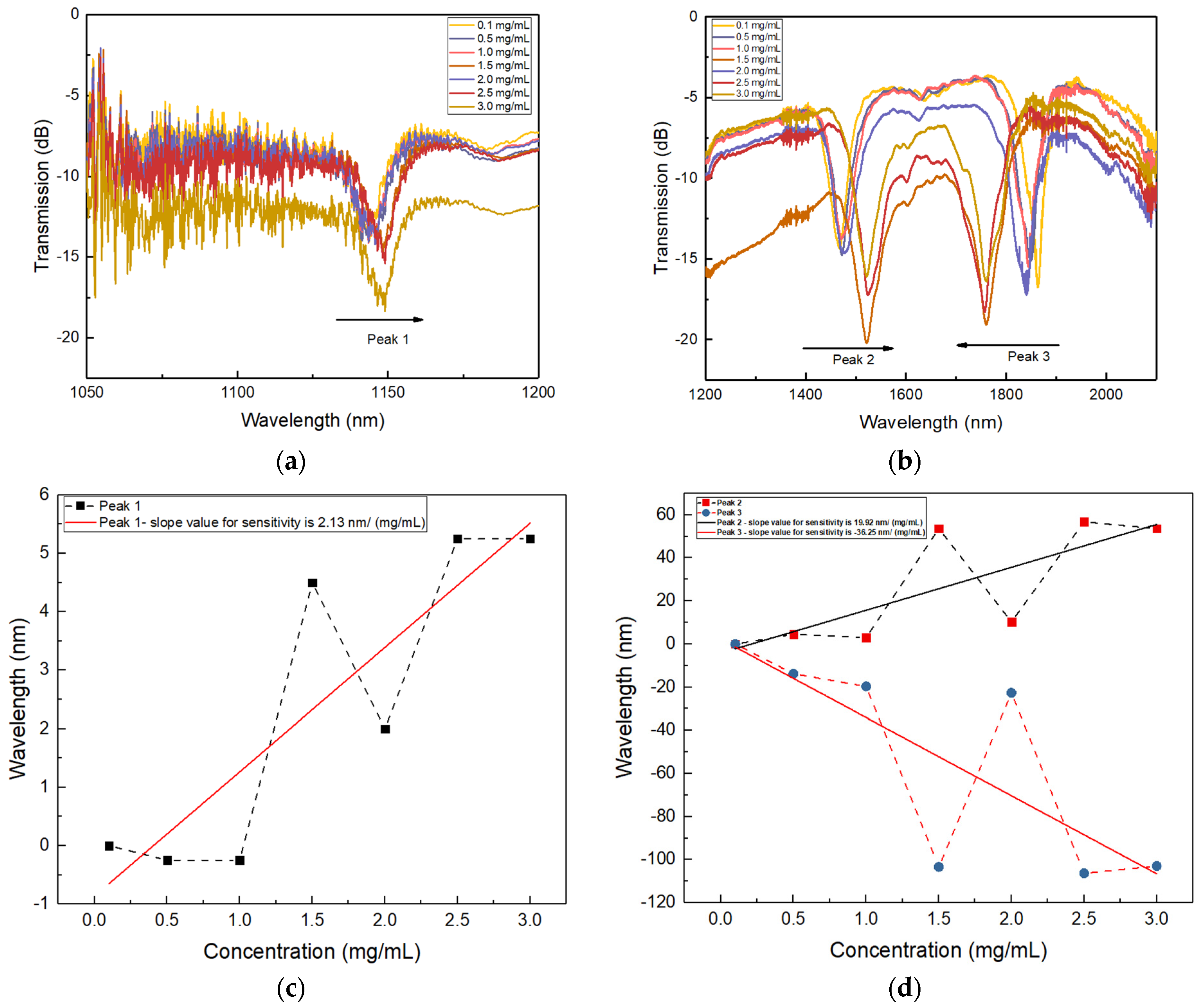
3.2. Glucose Detection by the GOD-Immobilised DPLPG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cano Perez, J.L.; Gutiérrez-Gutiérrez, J.; Perezcampos Mayoral, C.; Pérez-Campos, E.L.; Pina Canseco, M.D.; Tepech Carrillo, L.; Mayoral, L.P.; Vargas Treviño, M.; Apreza, E.L.; Rojas Laguna, R. Fiber Optic Sensors: A Review for Glucose Measurement. Biosensors 2021, 11, 61. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Marquardt, L.A.; Arnold, M.A.; Small, G.W. Near-infrared spectroscopic measurement of glucose in a protein matrix. Anal. Chem. 1993, 65, 3271–3278. [Google Scholar] [CrossRef]
- Kang, J.W.; Park, Y.S.; Chang, H.; Lee, W.; Singh, S.P.; Choi, W.; Galindo, L.H.; Dasari, R.R.; Nam, S.H.; Park, J.; et al. Direct observation of glucose fingerprint using in vivo Raman spectroscopy. Sci. Adv. 2020, 6, eaay5206. [Google Scholar] [CrossRef]
- Pors, A.; Rasmussen, K.G.; Inglev, R.; Jendrike, N.; Philipps, A.; Ranjan, A.G.; Vestergaard, V.; Henriksen, J.E.; Nørgaard, K.; Freckmann, G.; et al. Accurate Post-Calibration Predictions for Noninvasive Glucose Measurements in People Using Confocal Raman Spectroscopy. ACS Sens. 2023, 8, 1272–1279. [Google Scholar] [CrossRef]
- Bilal, H.M.; Gerard, L.C. Real-time, closed-loop dual-wavelength optical polarimetry for glucose monitoring. J. Biomed. Opt. 2010, 15, 017002. [Google Scholar] [CrossRef]
- MacKenzie, H.A.; Ashton, H.S.; Spiers, S.; Shen, Y.; Freeborn, S.S.; Hannigan, J.; Lindberg, J.; Rae, P. Advances in Photoacoustic Noninvasive Glucose Testing. Clin. Chem. 1999, 45, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Hussain, F.; Ikram, M. Optical coherence tomography for glucose monitoring in blood. Appl. Phys. B 2015, 120, 355–366. [Google Scholar] [CrossRef]
- Gabriely, I.; Wozniak, R.; Mevorach, M.; Kaplan, J.; Aharon, Y.; Shamoon, H. Transcutaneous glucose measurement using near-infared spectroscopy during hypoglycemia. Diabetes Care 2000, 22, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Eaton, R.; Haaland, D.; Koepp, G.; Thomas, E.; Stallard, B.; Robinson, P. Noninvasive Glucose Monitoring in Diabetic Patients: A Preliminary Evaluation. Clin. Chem. 1992, 38, 1618–1622. [Google Scholar] [CrossRef]
- Maier, J.S.; Walker, S.A.; Fantini, S.; Franceschini, M.A.; Gratton, E. Possible correlation between blood glucose concentration and the reduced scattering coefficient of tissues in the near infrared. Opt. Lett. 1994, 19, 2062–2064. [Google Scholar] [CrossRef]
- Chryssis, A.N.; Saini, S.S.; Lee, S.M.; Yi, H.M.; Bentley, W.E.; Dagenais, M. Detecting hybridization of DNA by highly sensitive evanescent field etched core fiber bragg grating sensors. IEEE J. Sel. Top. Quantum Electron. 2005, 11, 864–872. [Google Scholar] [CrossRef]
- Du, C.; Wang, Q.; Zhao, S.; Deng, X. Biological sensors based on long period fiber grating. Opt. Laser Technol. 2023, 158, 108936. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Li, P.; Bi, S.; Zhang, X.; Chen, S.; Peng, W. Nanopatterned evanescent-field fiber-optic interferometer as a versatile platform for gas sensing. Sens. Actuators B Chem. 2019, 301, 127136. [Google Scholar] [CrossRef]
- Ye, M.-Y.; Shen, M.-X.; Lin, X.-M. Ringing phenomenon based whispering-gallery-mode sensing. Sci. Rep. 2016, 6, 19597. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.W.; Hale, C.P.; Magee, J.R.; Kavaya, M.J.; Huffaker, A.V. Eye-safe coherent laser radar system at 2.1 μm using Tm,Ho:YAG lasers. Opt. Lett. 1991, 16, 773–775. [Google Scholar] [CrossRef]
- Johnson, D.E. Use of the holmium-yag (Ho:YAG) laser for treatment of superficial bladder-carcinoma. Lasers Surg. Med. 1994, 14, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Futao, S.; Wentong, Z.; Yan, Z.; Qingyu, D.; Aiwu, L. Application of endoscopic Ho:YAG laser incision technique treating urethral strictures and urethral atresias in pediatric patients. Pediatr. Surg. Int. 2006, 22, 514–518. [Google Scholar] [CrossRef]
- Song, D.; Liu, W.; Yin, Z.; Wang, Q.; Yan, X.; Zhang, X.; Wang, F.; Suzuki, T.; Ohishi, Y.; Cheng, T. Mid-infrared refractive index sensor with high sensitivity and wide detection range based on multimode interference in tellurite optical fibers. Results Opt. 2023, 12, 100458. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, L.; Bennion, I. Sensitivity characteristics near the dispersion turning points of long-period fiber gratings in B/Ge codoped fiber. Opt. Lett. 2001, 26, 1755–1757. [Google Scholar] [CrossRef] [PubMed]
- Disley, D.M.; Blyth, J.; Cullen, D.C.; You, H.X.; Eapen, S.; Lowe, C.R. Covalent coupling of immunoglobulin G to a poly(vinyl)alcohol-poly(acrylic acid) graft polymer as a method for fabricating the interfacial-recognition layer of a surface plasmon resonance immunosensor. Biosens. Bioelectron. 1998, 13, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Cesar, E.; Bariáin, C.; Matias, I.; Arregui, F.; Luquin, A.; Laguna, M. Volatile alcoholic compunds fibre optic nano sensor. Sens. Actuators B Chem. 2006, 115, 444–449. [Google Scholar] [CrossRef]
- Wang, Z.; Heflin, J.; Cott, K.; Stolen, R.; Ramachandran, S.; Ghalmi, S. Biosensors employing ionic self-assembled multilayers adsorbed on long-period fiber gratings. Sens. Actuators B Chem. 2009, 139, 618–623. [Google Scholar] [CrossRef]
- Luo, B.; Yan, Z.; Sun, Z.; Liu, Y.; Zhao, M.; Zhang, L. Biosensor based on excessively tilted fiber grating in thin-cladding optical fiber for sensitive and selective detection of low glucose concentration. Opt. Express 2015, 23, 32429–32440. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Tiwari, U.; Kumar, P.; Mishra, V.; Jain, S.C.; Singh, N.; Kapur, P.; Bharadwaj, L.M. Immobilization of enzyme on long period grating fibers for sensitive glucose detection. Biosens. Bioelectron. 2012, 33, 190–195. [Google Scholar] [CrossRef]
- Erdogan, T. Cladding-mode resonances in short- and long-period fiber grating filters. J. Opt. Soc. Am. A 1997, 14, 1760–1773. [Google Scholar] [CrossRef]
- Kashyap, R. Fiber Bragg Gratings; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Xuewen, S.; Lin, Z.; Bennion, I. Sensitivity characteristics of long-period fiber gratings. J. Light. Technol. 2002, 20, 255–266. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, N.; Sun, B.; Tan, Y.; Zhou, K.; Zhang, L. A Novel Biosensor for the Detection of Glucose Concentration Using the Dual-Peak Long Period Grating in the Near- to Mid-Infrared. Sensors 2024, 24, 1247. https://doi.org/10.3390/s24041247
Sahoo N, Sun B, Tan Y, Zhou K, Zhang L. A Novel Biosensor for the Detection of Glucose Concentration Using the Dual-Peak Long Period Grating in the Near- to Mid-Infrared. Sensors. 2024; 24(4):1247. https://doi.org/10.3390/s24041247
Chicago/Turabian StyleSahoo, Namita, Bing Sun, Yidong Tan, Kaiming Zhou, and Lin Zhang. 2024. "A Novel Biosensor for the Detection of Glucose Concentration Using the Dual-Peak Long Period Grating in the Near- to Mid-Infrared" Sensors 24, no. 4: 1247. https://doi.org/10.3390/s24041247





