A New Approach to Vascular Screening: Identification of Impaired Vascular Function Using the FMSF Technique
Abstract
:1. Introduction
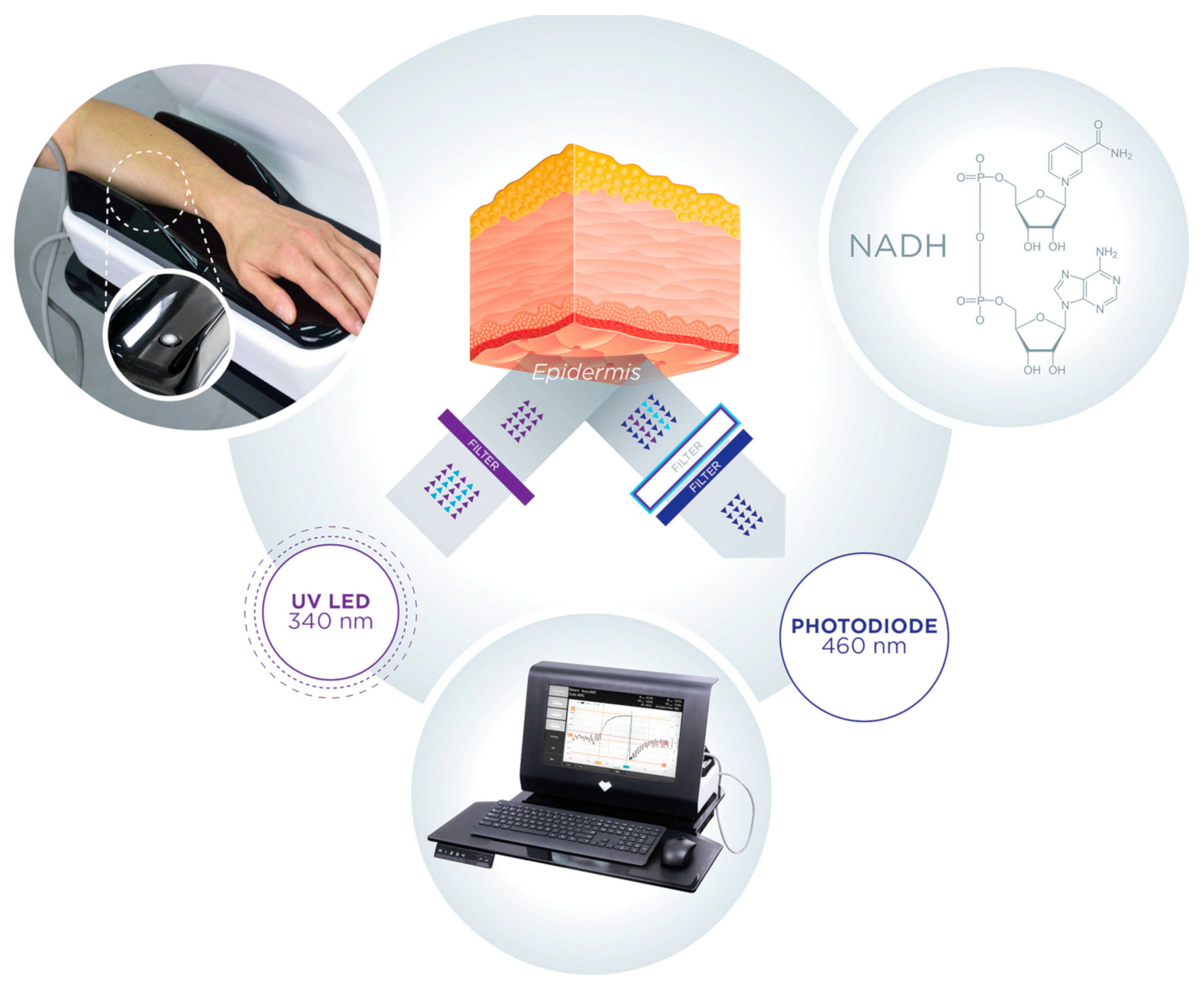
2. Brief Description of the FMSF Methodology
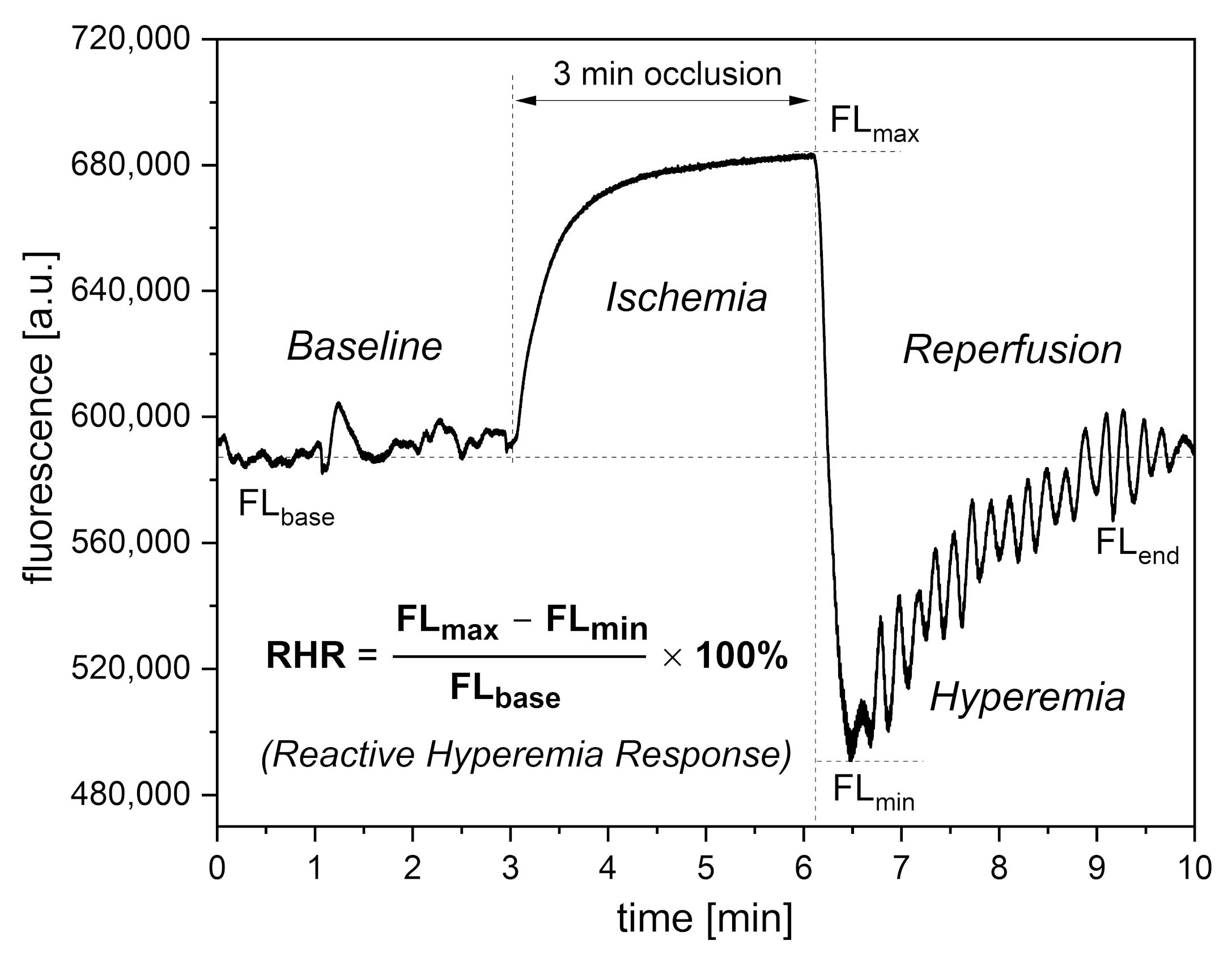
3. Interpretation of Key FMSF Parameters for Vascular Screening
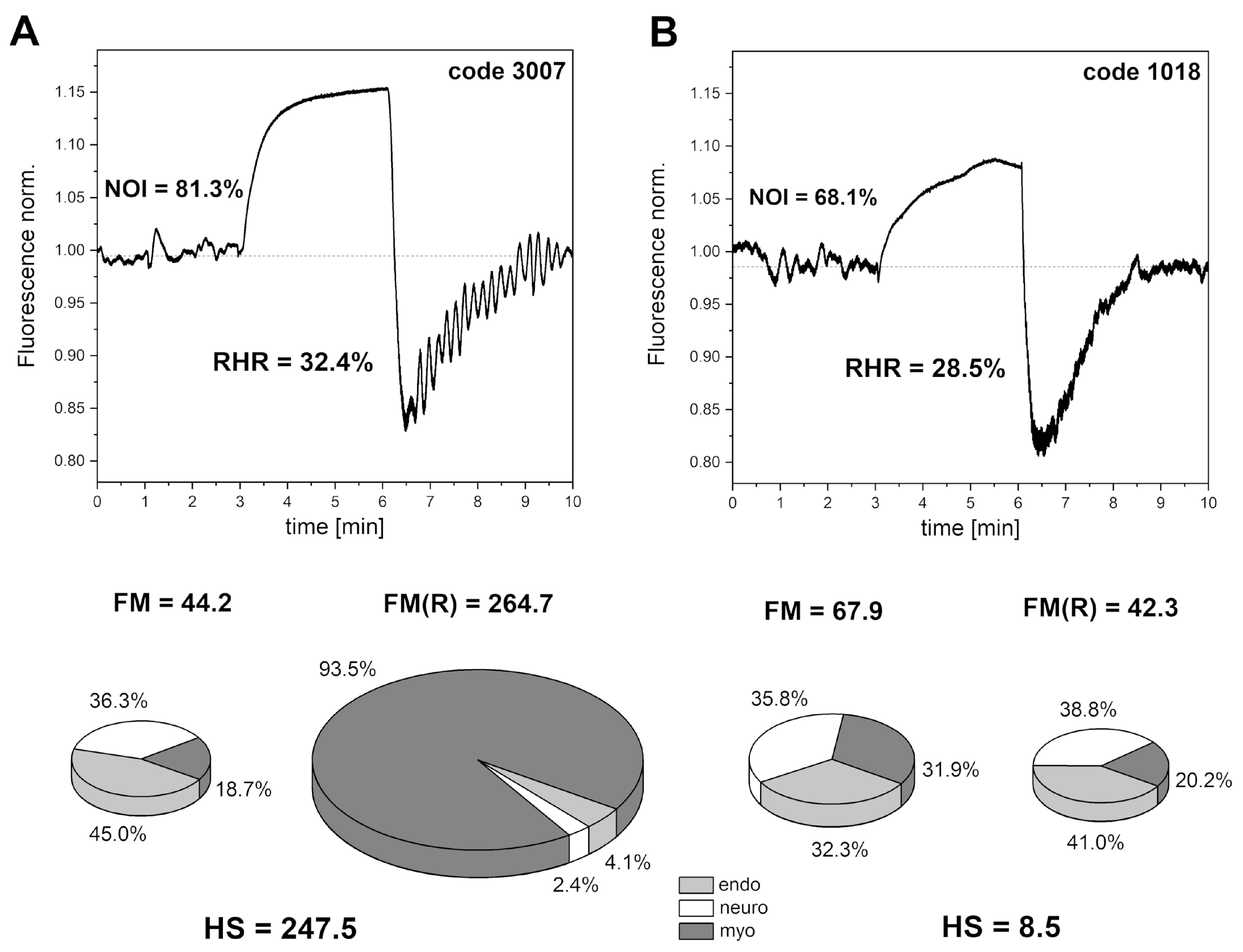
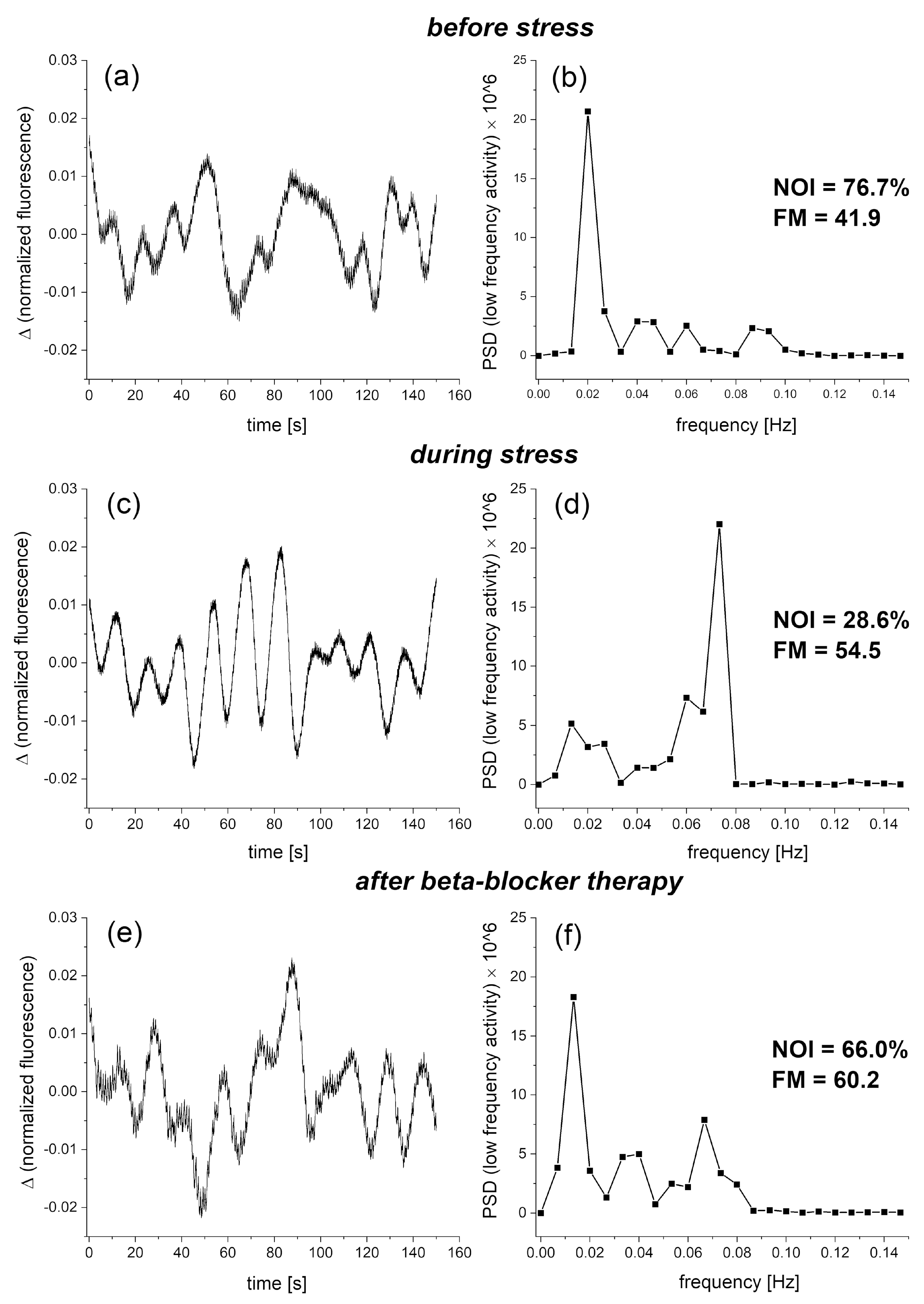
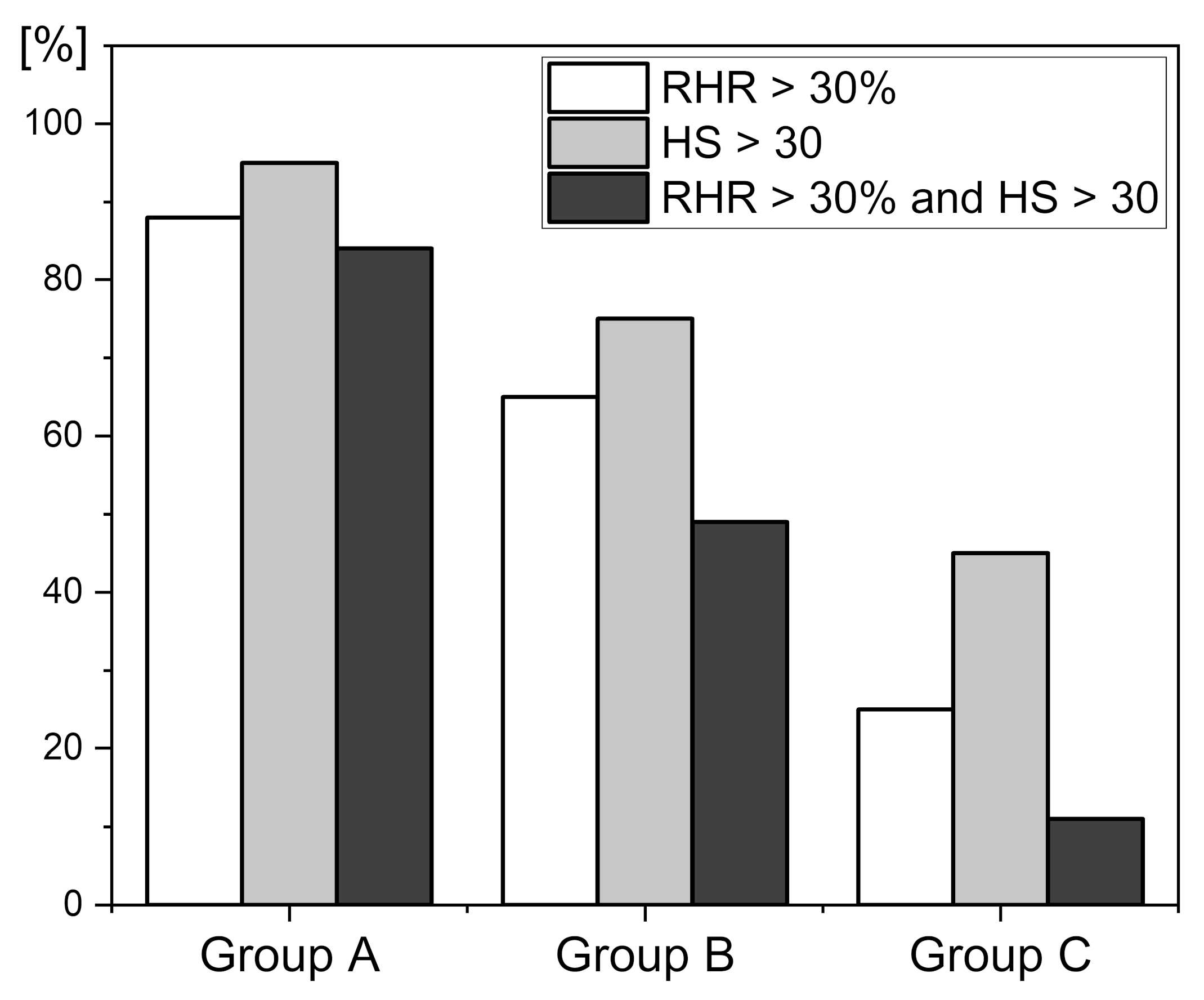
4. Selected Examples of FMSF Measurements Indicating Impaired Vascular Function
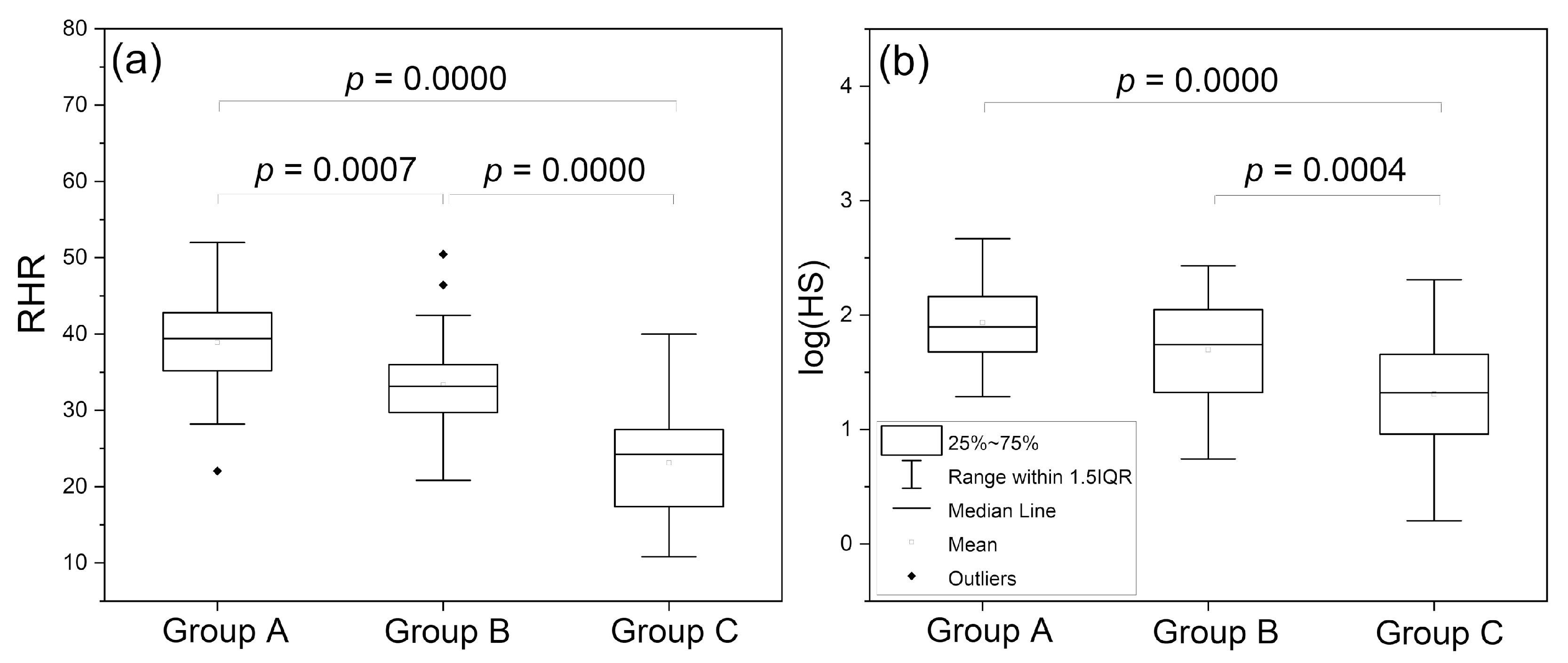
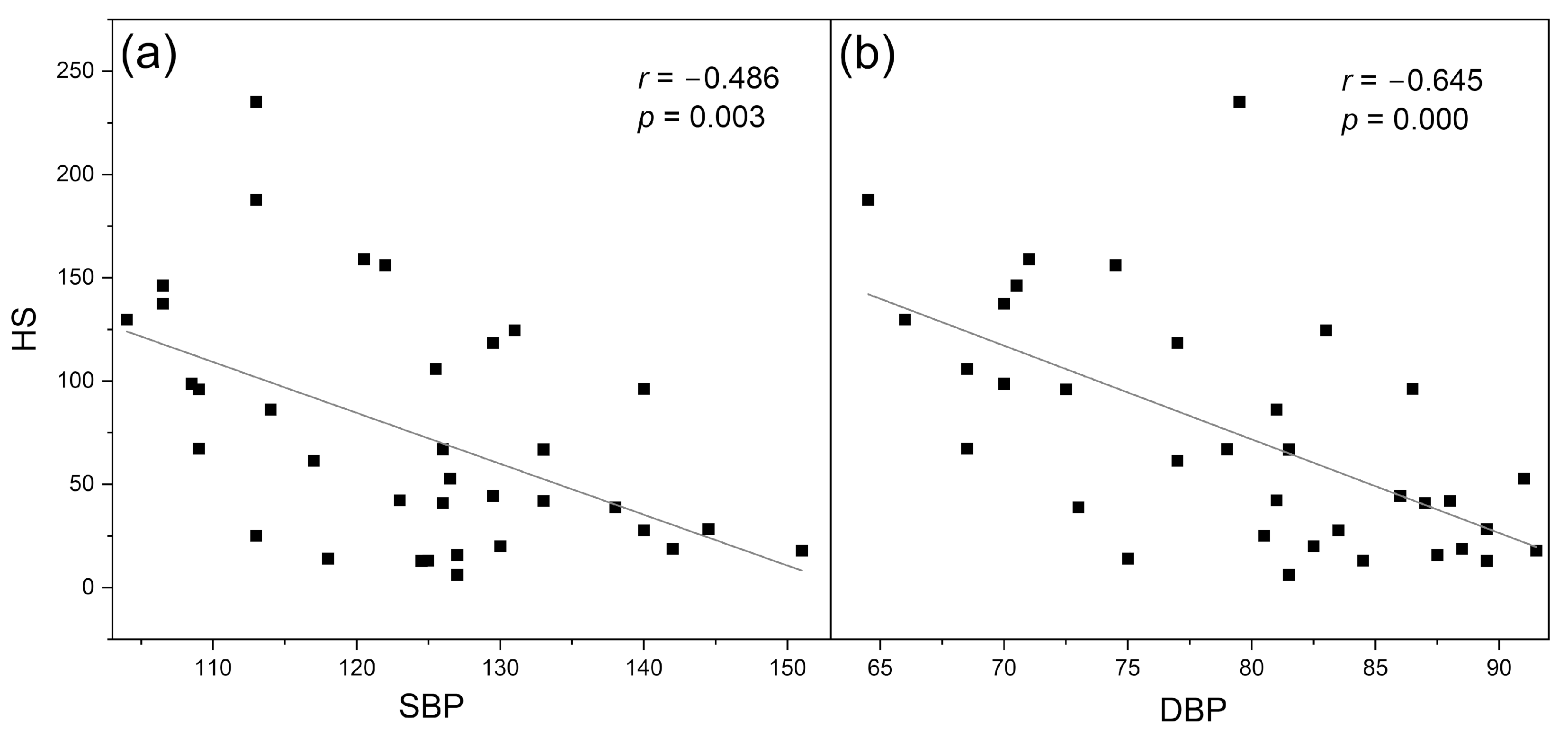
4.1. Identification of Cases with Dysfunctional Vascular Circulation
4.2. Selection of Cases with No Contradictions for Amateur Physical Activity
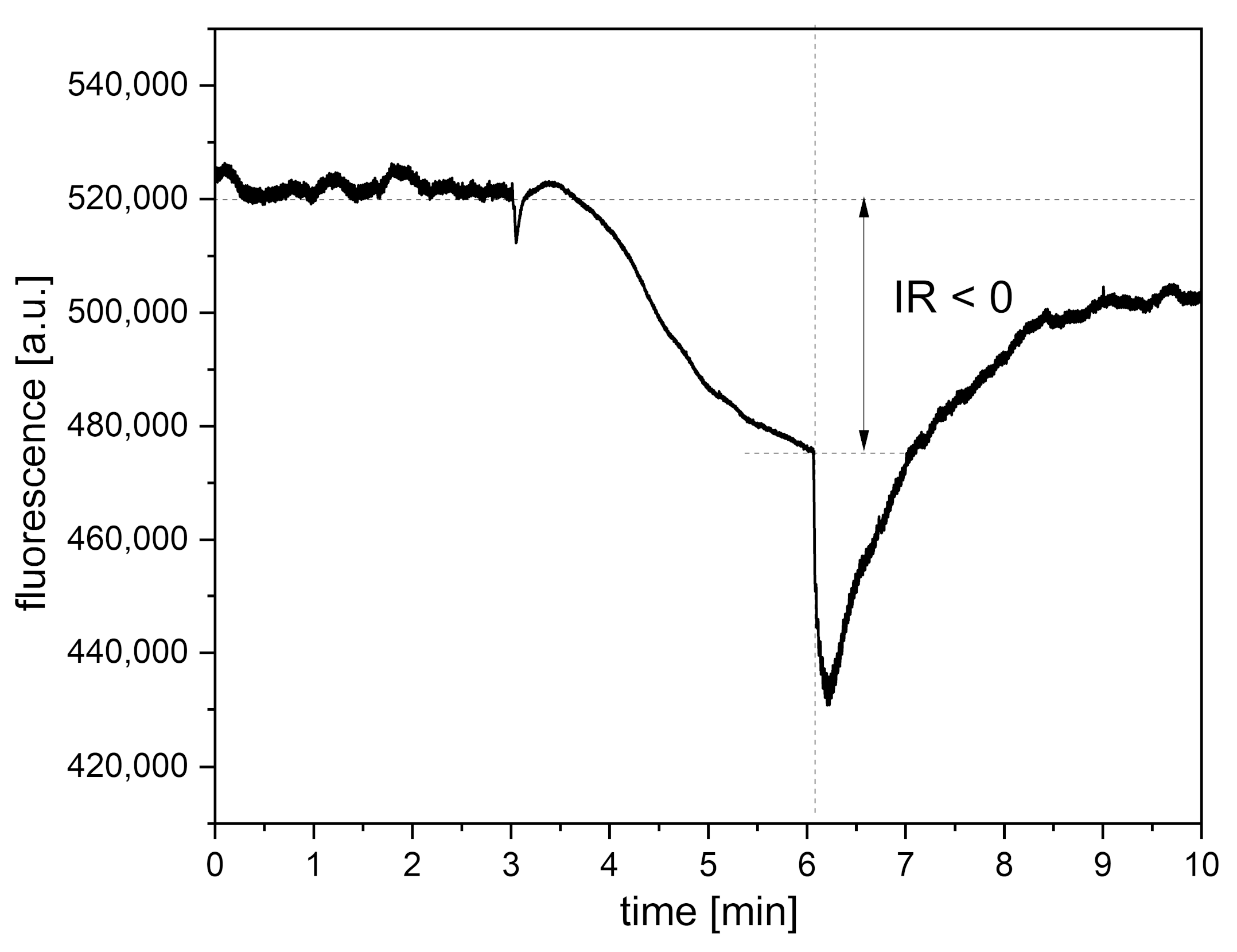
4.3. Presentation of Cases with Atypical Ischemic Response
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelm, M. Flow-mediated dilatation in human circulation: Diagnostic and therapeutic aspects. Am. J. Physiol. Circ. Physiol. 2002, 282, H1–H5. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, J.C.; Pyke, K.E. Flow-mediated dilation stimulated by sustained increases in shear stress: A useful tool for assessing endothelial function in humans? Am. J. Physiol. Circ. Physiol. 2017, 314, H508–H520. [Google Scholar] [CrossRef] [PubMed]
- Mućka, S.; Miodońska, M.; Jakubiak, G.K.; Starzak, M.; Cieślar, G.; Stanek, A. Endothelial Function Assessment by Flow-Mediated Dilation Method: A Valuable Tool in the Evaluation of the Cardiovascular System. Int. J. Environ. Res. Public Health 2022, 19, 11242. [Google Scholar] [CrossRef] [PubMed]
- Roustit, M.; Cracowski, J.L. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol. Sci. 2013, 34, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.; Roustit, M.; Cracowski, J.L. Skin microvascular endothelial function as a biomarker in cardiovascular diseases? Pharmacol. Rep. 2015, 67, 803–810. [Google Scholar] [CrossRef]
- Cracowski, J.-L.; Roustit, M. Human Skin Microcirculation. In Comprehensive Physiology; Prakash, Y.S., Ed.; Wiley: New York, NY, USA, 2020; Chapter 3; Volume 10, pp. 1105–1154. [Google Scholar] [CrossRef]
- IJzerman, R.G.; De Jongh, R.T.; Beijk, M.A.M.; Van Weissenbruch, M.M.; Delemarre-van de Waal, H.A.; Serné, E.H.; Stehouwer, C.D.A. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur. J. Clin. Investig. 2003, 33, 536–542. [Google Scholar] [CrossRef]
- Rossi, M.; Matteucci, E.; Pesce, M.; Consani, C.; Franzoni, F.; Santoro, G.; Giampietro, O. Peripheral microvascular dysfunction as an independent predictor of atherosclerotic damage in type 1 diabetes patients: A preliminary study. Clin. Hemorheol. Microcirc. 2013, 54, 381–391. [Google Scholar] [CrossRef]
- Katarzynska, J.; Lipinski, Z.; Cholewinski, T.; Piotrowski, L.; Dworzynski, W.; Urbaniak, M.; Borkowska, A.; Cypryk, K.; Purgal, R.; Marcinek, A.; et al. Non-invasive evaluation of microcirculation and metabolic regulation using flow mediated skin fluorescence (FMSF): Technical aspects and methodology. Rev. Sci. Instrum. 2019, 90, 104104. [Google Scholar] [CrossRef]
- Hellmann, M.; Tarnawska, M.; Dudziak, M.; Dorniak, K.; Roustit, M.; Cracowski, J.L. Reproducibility of flow mediated skin fluorescence to assess microvascular function. Microvasc. Res. 2017, 113, 60–64. [Google Scholar] [CrossRef]
- Hou, H.; Du, G.; Wang, Y.; Su, C.; Guo, L.; Chen, X. Noninvasive in vivo study of NADH fluorescence and its real-time intrinsic dynamical changes: Experiments and seven-layered skin model Monte Carlo simulations. J. Innov. Opt. Health Sci. 2022, 15, 2230006. [Google Scholar] [CrossRef]
- Katarzynska, J.; Cholewinski, T.; Sieron, L.; Marcinek, A.; Gebicki, J. Flowmotion Monitored by Flow Mediated Skin Fluorescence (FMSF): A Tool for Characterization of Microcirculatory Status. Front. Physiol. 2020, 11, 702. [Google Scholar] [CrossRef]
- Gebicki, J.; Marcinek, A.; Zielinski, J. Assessment of Microcirculatory Status Based on Stimulation of Myogenic Oscillations by Transient Ischemia: From Health to Disease. Vasc. Health Risk Manag. 2021, 17, 33–36. [Google Scholar] [CrossRef]
- Katarzyńska, J.; Zieliński, J.; Marcinek, A.; Gebicki, J. New Approach to Non-Invasive Assessment of Vascular Circulation Based on the Response to Transient Ischemia. Vasc. Health Risk Manag. 2022, 18, 113–116. [Google Scholar] [CrossRef]
- Gebicki, J.; Katarzynska, J.; Marcinek, A. Effect of Psychological Stress on Microcirculation Oscillations: Diagnostic Aspects. Vasc. Health Risk Manag. 2023, 19, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.; Filipiak, T.; Marcinek, A.; Wozniacka, A. Assessment of NADH/NAD+ Redox Imbalance in Psoriatic Lesions Using the FMSF Technique: Therapeutic Aspects. Sensors 2023, 23, 8718. [Google Scholar] [CrossRef]
- Marcinek, A.; Katarzynska, J.; Sieron, L.; Skokowski, R.; Zielinski, J.; Gebicki, J. Non-Invasive Assessment of Vascular Circulation Based on Flow Mediated Skin Fluorescence (FMSF). Biology 2023, 12, 385. [Google Scholar] [CrossRef]
- McMullen, R.L.; Chen, S.; Moore, D.J. Spectrofluorescence of skin and hair. Int. J. Cosmet. Sci. 2012, 34, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Cicchi, R.; Vogler, N.; Kapsokalyvas, D.; Dietzek, B.; Popp, J.; Pavone, F.S. From molecular structure to tissue architecture: Collagen organization probed by SHG microscopy. J. Biophotonics 2013, 6, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Dunaev, A.V.; Dremin, V.V.; Zherebtsov, E.A.; Rafailov, I.E.; Litvinova, K.S.; Palmer, S.G.; Stewart, N.A.; Sokolovski, S.G.; Rafailov, E.U. Individual variability analysis of fluorescence parameters measured in skin with different levels of nutritive blood flow. Med. Eng. Phys. 2015, 37, 574–583. [Google Scholar] [CrossRef]
- Mayevsky, A.; Rogatsky, G.G. Mitochondrial function in vivo evaluated by NADH fluorescence: From animal models to human studies. Am. J. Physiol. 2007, 292, C615–C640. [Google Scholar] [CrossRef]
- Mayevsky, A.; Barbiro-Michaely, E. Use of NADH fluorescence to determine mitochondrial function in vivo. Int. J. Biochem. Cell Biol. 2009, 41, 1977–1988. [Google Scholar] [CrossRef]
- Balu, M.; Mazhar, A.; Hayakawa, C.K.; Mittal, R.; Krasieva, T.B.; König, K.; Venugopalan, V.; Tromberg, B.J. In vivo multiphoton NADH fluorescence reveals depth-dependent keratinocyte metabolism in human skin. Biophys. J. 2013, 104, 258–267. [Google Scholar] [CrossRef]
- Pouli, D.; Balu, M.; Alonzo, C.A.; Liu, Z.; Quinn, K.P.; Rius-Diaz, F.; Harris, R.M.; Kelly, K.M.; Tromberg, B.J.; Georgakoudi, I. Imaging mitochondrial dynamics in human skin reveals depth-dependent hypoxia and malignant potential for diagnosis. Sci. Transl. Med. 2016, 8, 367ra169. [Google Scholar] [CrossRef]
- Nilsson, H.; Aalkjaer, C. Vasomotion: Mechanisms and physiological importance. Mol. Interv. 2003, 3, 79–89. [Google Scholar] [CrossRef]
- Kvandal, P.; Landsverk, S.A.; Bernjak, A.; Stefanovska, A.; Kvernmo, H.D.; Kirkebøen, K.A. Low-frequency oscillations of the laser Doppler perfusion signal in human skin. Microvasc. Res. 2006, 72, 120–127. [Google Scholar] [CrossRef]
- Bernjak, A.; Clarkson, P.B.M.; McClintock, P.V.E.; Stefanovska, A. Low-frequency blood flow oscillations in congestive heart failure and after β1-blockade treatment. Microvasc. Res. 2008, 76, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Ticcinelli, V.; Stankovski, T.; McClintock, P.V.E.; Stefanovska, A. Ageing of the couplings between cardiac; respiratory and myogenic activity in humans. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 7366–7369. [Google Scholar] [CrossRef]
- Clough, G.F.; Kuliga, K.Z.; Chipperfield, A.J. Flow motion dynamics of microvascular blood flow and oxygenation: Evidence of adaptive changes in obesity and type 2 diabetes mellitus/insulin resistance. Microcirculation 2017, 24, e12331. [Google Scholar] [CrossRef]
- Mikosiński, J.; Mikosiński, P.; Kwapisz, A.; Katarzynska, J.; Gebicki, J. Conclusions from an Observational Study of Patients with Vascular Diseases Using the FMSF Technique. Vasc. Health Risk Manag. 2023, 19, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.; Katarzynska, J.; Marcinek, A. Can efficient stimulation of myogenic microcirculatory oscillations by transient ischemia predict low incidence of COVID-19 infection? Respir. Physiol. Neurobiol. 2021, 286, 103618. [Google Scholar] [CrossRef] [PubMed]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar]
- Poyton, R.; Hendrickson, M. Crosstalk between nitric oxide and hypoxia-inducible factor signaling pathways: An update. Res. Rep. Biochem. 2015, 5, 147–161. [Google Scholar] [CrossRef]
- Cowburn, A.S.; Takeda, N.; Boutin, A.T.; Kim, J.W.; Sterling, J.C.; Nakasaki, M.; Southwood, M.; Goldrath, A.W.; Jamora, C.; Nizet, V.; et al. HIF isoforms in the skin differentially regulate systemic arterial pressure. Proc. Natl. Acad. Sci. USA 2013, 110, 17570–17575. [Google Scholar] [CrossRef]
- Huang, Y.; Di Lorenzo, A.; Jiang, W.; Cantalupo, A.; Sessa, W.C.; Giordano, F.J. Hypoxia-inducible factor-1α in vascular smooth muscle regulates blood pressure homeostasis through a peroxisome proliferator-activated receptor-γ-angiotensin II receptor type 1 axis. Hypertension 2013, 62, 634–640. [Google Scholar] [CrossRef]
- Salvi, P.; Faini, A.; Castiglioni, P.; Brunacci, F.; Montaguti, L.; Severi, F.; Gautier, S.; Pretolani, E.; Benetos, A.; Parati, G. Increase in slow-wave vasomotion by hypoxia and ischemia in lowlanders and highlanders. J. Appl. Physiol. 2018, 125, 780–789. [Google Scholar] [CrossRef]
- Katarzynska, J.; Borkowska, A.; Los, A.; Marcinek, A.; Cypryk, K.; Gebicki, J. Flow-Mediated Skin Fluorescence (FMSF) Technique for Studying Vascular Complications in Type 2 Diabetes. J. Diabetes Sci. Technol. 2020, 14, 693–694. [Google Scholar] [CrossRef]
- Katarzynska, J.; Borkowska, A.; Czajkowski, P.; Los, A.; Szczerbinski, L.; Milewska-Kranc, A.; Marcinek, A.; Kretowski, A.; Cypryk, K.; Gebicki, J. Flow Mediated Skin Fluorescence technique reveals remarkable effect of age on microcirculation and metabolic regulation in type 1 diabetes. Microvasc. Res. 2019, 124, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Los-Stegienta, A.; Katarzynska, J.; Borkowska, A.; Marcinek, A.; Cypryk, K.; Gebicki, J. Differentiation of diabetic foot ulcers based on stimulation of myogenic oscillations by transient ischemia. Vasc. Health Risk Manag. 2021, 17, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Los-Stegienta, A.; Borkowska, A.; Cypryk, K. Assessment of microvascular function using a novel technique Flow Mediated Skin Fluorescence (FMSF) in patients with diabetic kidney disease: A preliminary study. Microvasc. Res. 2022, 144, 104417. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.S.; Toya, T.; Ahmad, A.; Clark, M.M.; Gilliam, W.P.; Lerman, L.O.; Lerman, A. Mental Stress and Its Effects on Vascular Health. Mayo Clin. Proc. 2022, 97, 951–990. [Google Scholar] [CrossRef] [PubMed]
- MacCormack, J.K.; Armstrong-Carter, E.L.; Gaudier-Diaz, M.M.; Meltzer-Brody, S.; Sloan, E.K.; Lindquist, K.A.; Muscatell, K.A. β-Adrenergic Contributions to Emotion and Physiology During an Acute Psychosocial Stressor. Psychosom. Med. 2021, 83, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Bugaj, O.; Zieliński, J.; Kusy, K.; Kantanista, A.; Wieliński, D.; Guzik, P. The effect of exercise on the skin content of the reduced form of NAD and its response to transient ischemia and reperfusion in highly trained athletes. Front. Physiol. 2019, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Chomicka, R.; Uruski, P.; Krauze, T.; Piskorski, J.; Tykarski, A.; Guzik, P. Arterial Blood Pressure Features of Hypertensive Patients with Typical and Atypical 460 nm Skin Fluorescence Response to Transient Ischaemia. J. Clin. Med. 2023, 12, 5886. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Chomicka, R.; Chomicki, W.; Krauze, T.; Uruski, P.; Guzik, M.; Piskorski, J.; Tykarski, A.; Guzik, P. Investigating the Ischaemic Phase of Skin NADH Fluorescence Dynamics in Recently Diagnosed Primary Hypertension: A Time Series Analysis. J. Clin. Med. 2023, 12, 1247. [Google Scholar] [CrossRef] [PubMed]
- Pabbidi, M.R.; Kuppusamy, M.; Didion, S.P.; Sanapureddy, P.; Reed, J.T.; Sontakke, S.P. Sex differences in the vascular function and related mechanisms: Role of 17β-estradiol. Am. J. Physiol. Circ. Physiol. 2018, 315, H1499–H1518. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinek, A.; Katarzynska, J.; Gebicki, J. A New Approach to Vascular Screening: Identification of Impaired Vascular Function Using the FMSF Technique. Sensors 2024, 24, 1721. https://doi.org/10.3390/s24061721
Marcinek A, Katarzynska J, Gebicki J. A New Approach to Vascular Screening: Identification of Impaired Vascular Function Using the FMSF Technique. Sensors. 2024; 24(6):1721. https://doi.org/10.3390/s24061721
Chicago/Turabian StyleMarcinek, Andrzej, Joanna Katarzynska, and Jerzy Gebicki. 2024. "A New Approach to Vascular Screening: Identification of Impaired Vascular Function Using the FMSF Technique" Sensors 24, no. 6: 1721. https://doi.org/10.3390/s24061721





