Effect of Three-Dimensional-Printed Thermoplastics Used in Sensor Housings on Common Atmospheric Trace Gasses
Abstract
1. Introduction
2. Materials and Methods
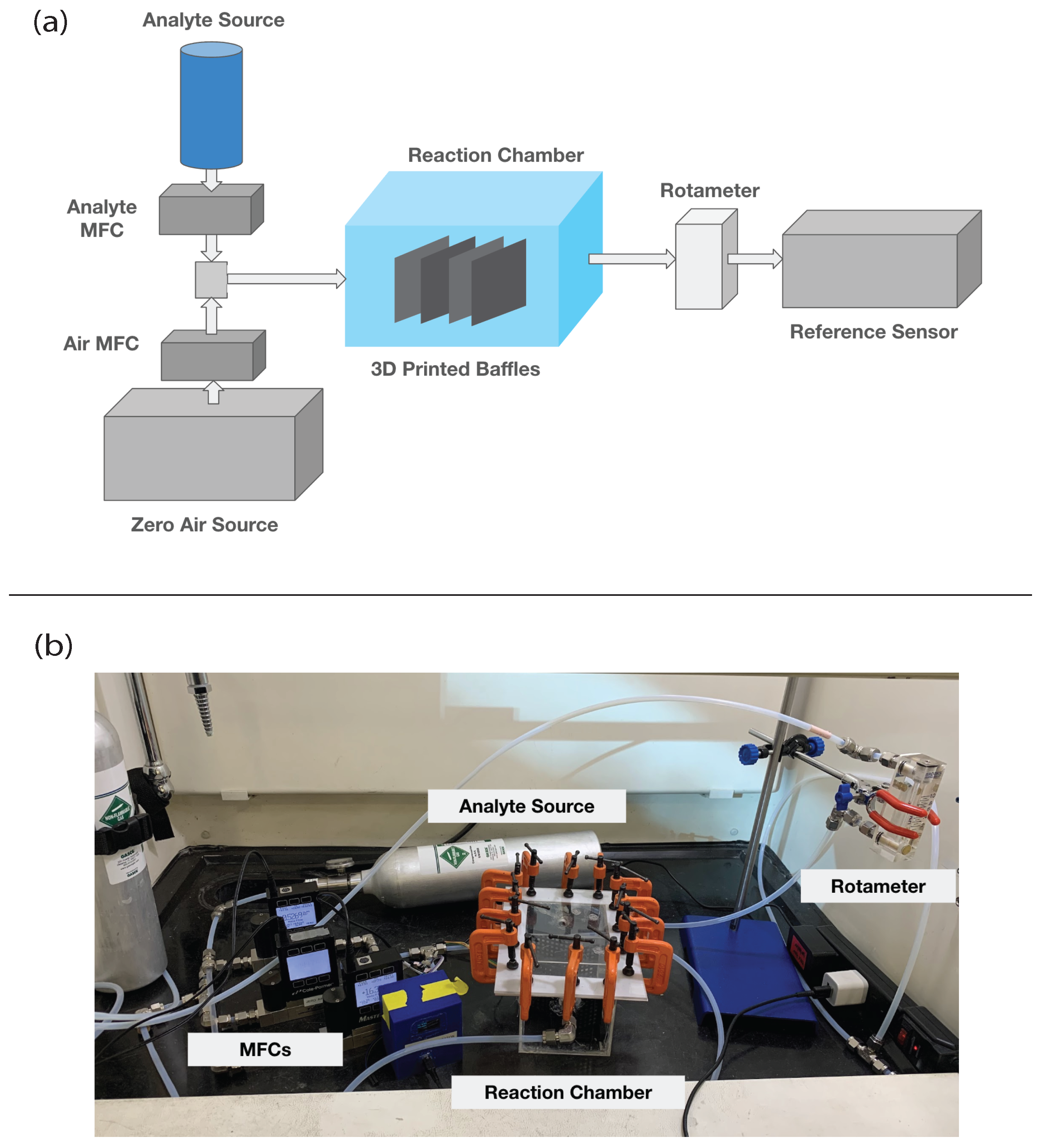
2.1. Experimental Setup
2.2. Experimental Design
2.3. Off-Gassing Data Analysis
2.4. Reaction Data Analysis
3. Results and Discussion
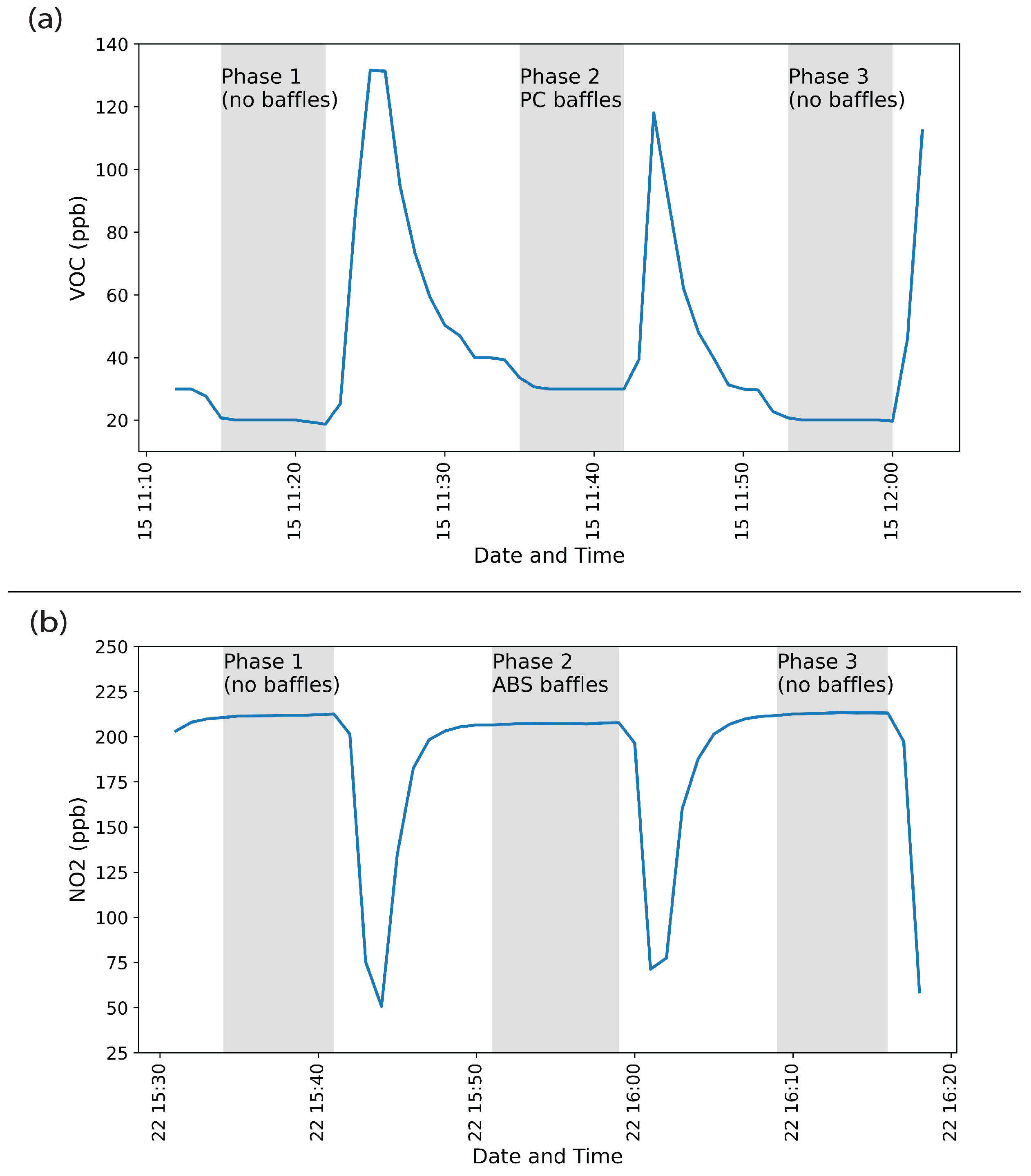
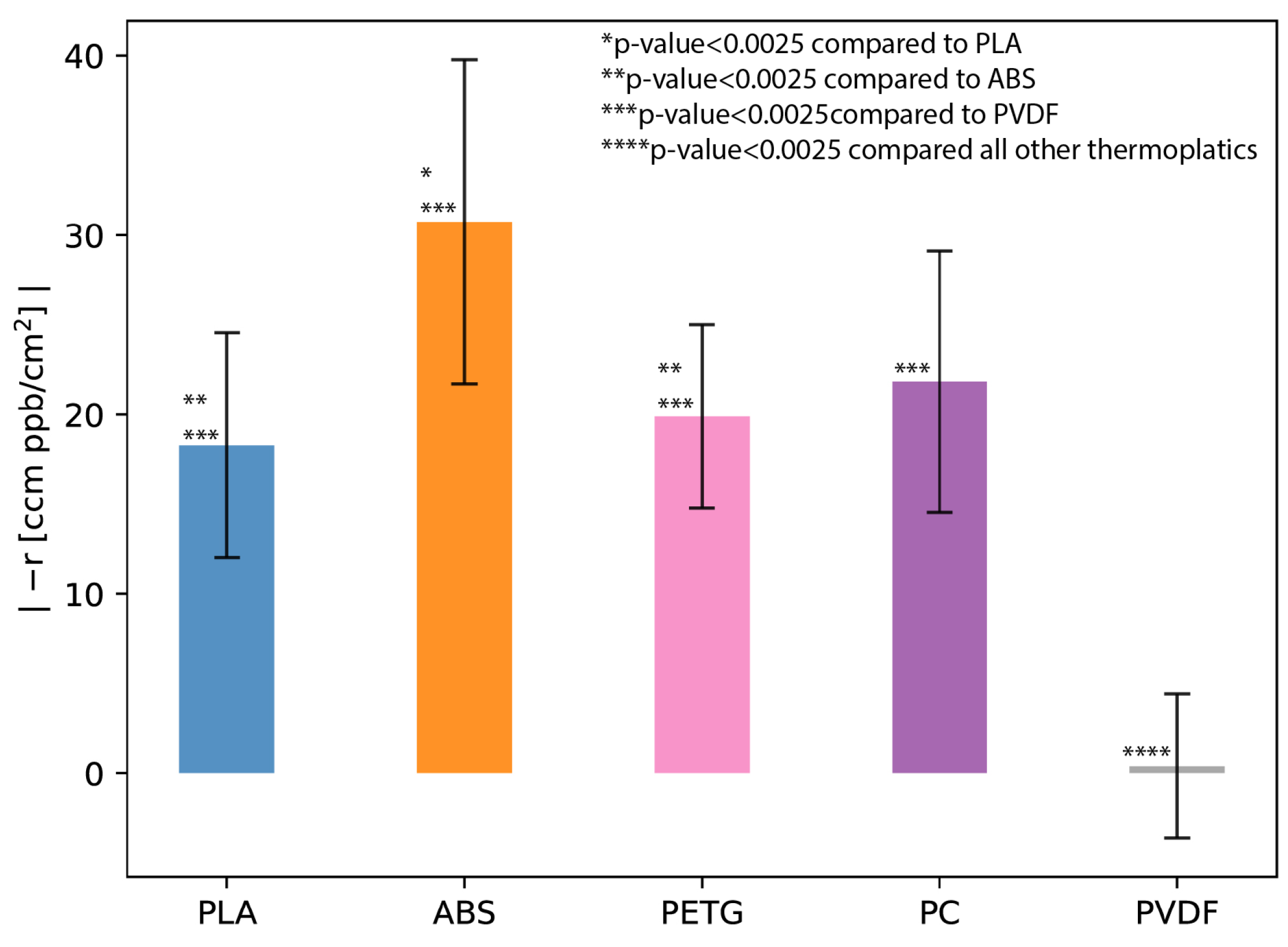
3.1. Off-Gassing Experiments
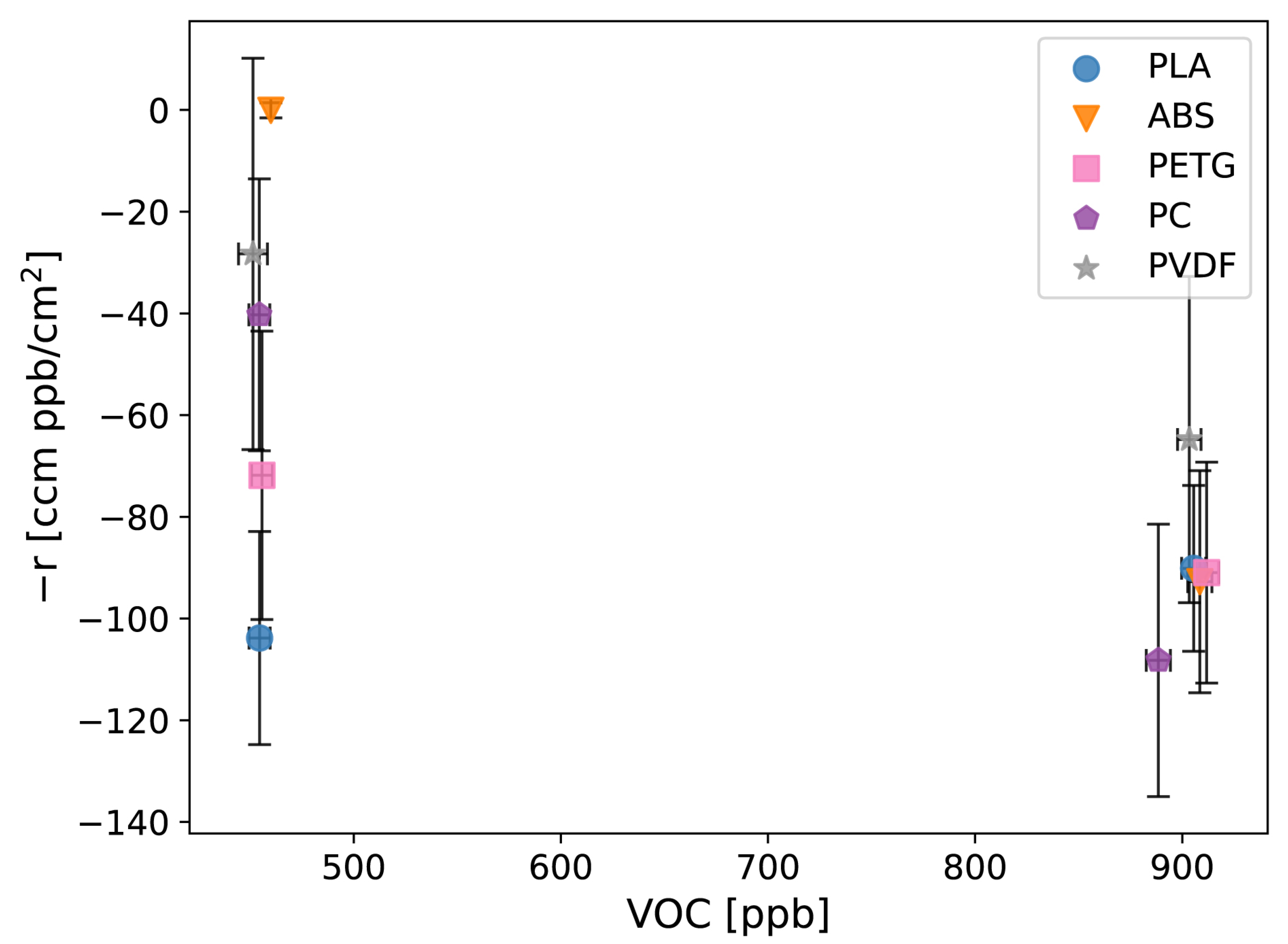
3.2. Reaction Experiments
3.3. Implications for Air Quality Measurements
3.4. Limitations and Future Work
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Concas, F.; Mineraud, J.; Lagerspetz, E.; Varjonen, S.; Liu, X.; Puolamäki, K.; Nurmi, P.; Tarkoma, S. Low-Cost Outdoor Air Quality Monitoring and Sensor Calibration: A Survey and Critical Analysis. ACM Trans. Sens. Netw. 2021, 17, 1–44. [Google Scholar] [CrossRef]
- Chojer, H.; Branco, P.T.; Martins, F.G.; Alvim-Ferraz, M.C.; Sousa, S.I. Development of low-cost indoor air quality monitoring devices: Recent advancements. Sci. Total Environ. 2020, 727, 138385. [Google Scholar] [CrossRef] [PubMed]
- Karagulian, F.; Barbiere, M.; Kotsev, A.; Spinelle, L.; Gerboles, M.; Lagler, F.; Redon, N.; Crunaire, S.; Borowiak, A. Review of the performance of low-cost sensors for air quality monitoring. Atmosphere 2019, 10, 506. [Google Scholar] [CrossRef]
- Clements, A.L.; Griswold, W.G.; Abhijit, R.S.; Johnston, J.E.; Herting, M.M.; Thorson, J.; Collier-Oxandale, A.; Hannigan, M. Low-cost air quality monitoring tools: From research to practice (A workshop summary). Sensors 2017, 17, 2478. [Google Scholar] [CrossRef] [PubMed]
- Tancev, G.; Toro, F.G. Stochastic Online Calibration of Low-Cost Gas Sensor Networks with Mobile References. IEEE Access 2022, 10, 13901–13910. [Google Scholar] [CrossRef]
- Delaria, E.R.; Kim, J.; Fitzmaurice, H.L.; Newman, C.; Wooldridge, P.J.; Worthington, K.; Cohen, R.C. The Berkeley Environmental Air-quality and CO2 Network: Field calibrations of sensor temperature dependence and assessment of network scale CO2 accuracy. Atmos. Meas. Tech. 2021, 14, 5487–5500. [Google Scholar] [CrossRef]
- Jain, S.; Presto, A.A.; Zimmerman, N. Spatial Modeling of Daily PM2.5, NO2, and CO Concentrations Measured by a Low-Cost Sensor Network: Comparison of Linear, Machine Learning, and Hybrid Land Use Models. Environ. Sci. Technol. 2021, 55, 8631–8641. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Hagler, G.; Williams, R.; Sharpe, R.; Brown, R.; Garver, D.; Judge, R.; Caudill, M.; Rickard, J.; Davis, M.; et al. Community Air Sensor Network (CAIRSENSE) project: Evaluation of low-cost sensor performance in a suburban environment in the southeastern United States. Atmos. Meas. Tech. 2016, 9, 5281–5292. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Ginn, M.; Rastogi, V. A review of 3D printing techniques for environmental applications. Curr. Opin. Chem. Eng. 2020, 28, 173–178. [Google Scholar] [CrossRef]
- Salamone, F.; Sibilio, S.; Masullo, M. Low-cost carbon dioxide concentration sensors for assessing air quality in the built environment: An on-site evaluation of their measurement performance. J. Phys. Conf. Ser. 2023, 2600, 102019. [Google Scholar] [CrossRef]
- Salamone, F.; Belussi, L.; Danza, L.; Ghellere, M.; Meroni, I. Design and development of nEMoS, an all-in-one, low-cost, web-connected and 3D-printed device for environmental analysis. Sensors 2015, 15, 13012–13027. [Google Scholar] [CrossRef] [PubMed]
- Tryner, J.; Phillips, M.; Quinn, C.; Neymark, G.; Wilson, A.; Jathar, S.H.; Carter, E.; Volckens, J. Design and testing of a low-cost sensor and sampling platform for indoor air quality. Build. Environ. 2021, 206, 108398. [Google Scholar] [CrossRef] [PubMed]
- Ollé, E.P.; Farré-Lladós, J.; Casals-Terré, J.; Martínez, J.A.L. Enhanced selectivity of a 3D-printed microfluidic gas detector towards different volatile organic compounds (VOCs) for the effective monitoring of indoor air quality in vehicles. Environ. Technol. Innov. 2024, 33, 103481. [Google Scholar] [CrossRef]
- Spandonidis, C.; Tsantilas, S.; Giannopoulos, F.; Giordamlis, C.; Zyrichidou, I.; Syropoulou, P. Design and Development of a New Cost-Effective Internet of Things Sensor Platform for Air Quality Measurements. J. Eng. Sci. Technol. Rev. 2020, 13, 81–91. [Google Scholar] [CrossRef]
- Pei, Z.; Balitskiy, M.; Thalman, R.; Kelly, K.E. Laboratory Performance Evaluation of a Low-Cost Electrochemical Formaldehyde Sensor. Sensors 2023, 23, 7444. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.; Pitarma, R. A cost-effective air quality supervision solution for enhanced living environments through the internet of things. Electronics 2019, 8, 170. [Google Scholar] [CrossRef]
- Rebeiro-Hargrave, A.; Motlagh, N.H.; Varjonen, S.; Lagerspetz, E.; Nurmi, P.; Tarkoma, S. MegaSense: Cyber-Physical System for Real-time Urban Air Quality Monitoring. In Proceedings of the 2020 15th IEEE Conference on Industrial Electronics and Applications, Kristiansand, Norway, 9–13 November 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Hartings, M.R.; Ahmed, Z. Chemistry from 3D printed objects. Nat. Rev. Chem. 2019, 3, 305–314. [Google Scholar] [CrossRef]
- Scheirs, J. Compositional and Failure Analysis of Polymer A Practical Approach; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; pp. 636–645. [Google Scholar]
- Jensen, N. Vapor pressure of plastic materials. J. Appl. Phys. 1956, 27, 1460–1462. [Google Scholar] [CrossRef]
- Kwon, J.S.; Jung, H.; Yeo, I.S.; Song, T.H. Outgassing characteristics of a polycarbonate core material for vacuum insulation panels. Vacuum 2011, 85, 839–846. [Google Scholar] [CrossRef]
- Dinh, L.N.; Sze, J.; Schildbach, M.A.; Chinn, S.C.; Maxwell, R.S.; Raboin, P.; McLean, W. Vacuum outgassing of high density polyethylene. J. Vac. Sci. Technol. A Vacuum Surfaces Film. 2009, 27, 376–380. [Google Scholar] [CrossRef]
- Battes, K.; Day, C.; Hauer, V. Outgassing behavior of different high-temperature resistant polymers. J. Vac. Sci. Technol. A Vacuum Surfaces Film. 2018, 36, 021602. [Google Scholar] [CrossRef]
- Aeronautics, N.; Space Administration, G.S.F.C. Outgassing Data for Selecting Spacecraft Materials 10th Compilation. Available online: https://outgassing.nasa.gov/Home (accessed on 28 March 2024).
- Gale, P.J.; Duncan, M.W.; Yergey, A.L. Quantifying Small Molecules by Mass Spectrometry. LCGC North Am. 2015, 33, 34–41. [Google Scholar]
- The suitability of 3D printed plastic parts for laboratory use. Am. J. Phys. 2015, 83, 281–285. [CrossRef]
- Budde, K.J.; Holzapfel, W.J.; Beyer, M.M. Application of Ion Mobility Spectrometry to Semiconductor Technology: Outgassing of Advanced Polymers under Thermal Stress. J. Electrochem. Soc. 1995, 142, 888–897. [Google Scholar] [CrossRef]
- Wojnowski, W.; Kalinowska, K.; Majchrzak, T.; Zabiegała, B. Real-time monitoring of the emission of volatile organic compounds from polylactide 3D printing filaments. Sci. Total Environ. 2022, 805, 150181. [Google Scholar] [CrossRef] [PubMed]
- Wojnowski, W.; Marć, M.; Kalinowska, K.; Kosmela, P.; Zabiegała, B. Emission Profiles of Volatiles during 3D Printing with ABS, ASA, Nylon, and PETG Polymer Filaments. Molecules 2022, 27, 3814. [Google Scholar] [CrossRef] [PubMed]
- Floyd, E.L.; Wang, J.; Regens, J.L. Fume emissions from a low-cost 3-D printer with various filaments. J. Occup. Environ. Hyg. 2017, 14, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, A.B.; Lebouf, R.F.; Yi, J.; Ham, J.; Nurkewicz, T.; Schwegler-Berry, D.E.; Chen, B.T.; Wells, J.R.; Duling, M.G.; Lawrence, R.B.; et al. Characterization of chemical contaminants generated by a desktop fused deposition modeling 3-dimensional printer. J. Occup. Environ. Hyg. 2017, 14, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yoon, C.; Ham, S.; Park, J.; Kim, S.; Kwon, O.; Tsai, P.J. Emissions of Nanoparticles and Gaseous Material from 3D Printer Operation. Environ. Sci. Technol. 2015, 49, 12044–12053. [Google Scholar] [CrossRef]
- Davis, A.Y.; Zhang, Q.; Wong, J.P.; Weber, R.J.; Black, M.S. Characterization of volatile organic compound emissions from consumer level material extrusion 3D printers. Build. Environ. 2019, 160, 106209. [Google Scholar] [CrossRef]
- Weber, R.J.; Zhang, Q.; Wong, J.P.; Davis, A.; Black, M. Fine particulate and chemical emissions from desktop 3D printers. In NIP & Digital Fabrication Conference; Society for Imaging Science and Technology: Springfield, VA, USA, 2016; pp. 121–123. [Google Scholar] [CrossRef]
- Wilkins, D.F.; Traum, M.J.; Wilkins-Earley, J.G. Teaching space-borne recycling to middle school students via 3d printing—Managing classroom air quality. In Proceedings of the AIAA Scitech 2020 Forum, Orlando, FL, USA, 6–10 January 2020; Volume 1. [Google Scholar] [CrossRef]
- Davydov, E.Y.; Gaponova, I.S.; Pariiskii, G.B.; Pokholok, T.V.; Zaikov, G.E. Classification of Polymers in Reactivity Toward Nitrogen Oxides; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010. [Google Scholar]
- Pariiskii, G.B.; Gaponova, I.S.; Davydov, E.Y. Reactions of nitrogen oxides with polymers. Russ. Chem. Rev. 2000, 69, 985–999. [Google Scholar] [CrossRef]
- Jellinek, H.H.G. Polymer-Gas Reactions (Air Pollutants: NO2 and SO2) as Function of Pressure, UV Light, Temperature, and Morphology—A Survey; National Bureau of Standards Special Publication: Gaithersburg, MD, USA, 1970; Volume 357, pp. 101–110. [Google Scholar]
- Version 3.0.0, N.C.C. Chemical Datasheet Nitrogen Dioxide. 2023. Available online: https://cameochemicals.noaa.gov/chemical/4072 (accessed on 1 January 2024).
- Version 3.0.0, N.C.C. Chemical Datasheet Nitric Oxide. 2023. Available online: https://cameochemicals.noaa.gov/chemical/1192 (accessed on 1 January 2024).
- Takeuchi, K. 5.16—Polycarbonates; Elsevier: Amsterdam, The Netherlands, 2012; Volume 5, pp. 363–376. [Google Scholar] [CrossRef]
- Nivedhitha, D.M.; Jeyanthi, S. Polyvinylidene fluoride, an advanced futuristic smart polymer material: A comprehensive review. Polym. Adv. Technol. 2023, 34, 474–505. [Google Scholar] [CrossRef]
- Dallaev, R.; Pisarenko, T.; Sobola, D.; Orudzhev, F.; Ramazanov, S.; Trčka, T. Brief Review of PVDF Properties and Applications Potential. Polymers 2022, 14, 4793. [Google Scholar] [CrossRef] [PubMed]
- TSI Inc. Q-TRAK Indoor Air Quality Monitor Model 7575 Operation and Service Manual Revision H. 2022. Available online: https://tsi.com/getmedia/a9ce2668-b27b-41d1-961b-a01fc8e7933d/7575-Q-Trak-Op_Svc_Mnl_6004850?ext=.pdf (accessed on 18 April 2024).
- TSI Inc. Q-TRAK Multi-Function Indoor Air Quality Monitor Model 7575 Specification Sheet, P/N 5001355 Revision G. 2022. Available online: https://tsi.com/getmedia/d2a8d1d1-7551-47fe-8a0f-3c14b09b494b/7575_QTrak_A4_UK_5001356-web?ext=.pdf (accessed on 18 April 2024).
- TSI Inc. Volative Organic Compound Probes Models 984, 985, 986, and 987 Operation and Service Manual Revision F. 2022. Available online: https://tsi.com/getmedia/19a70324-ce9b-4b0f-b158-cfb3b81a480a/VOC_Probe_OpSvcMnl_6007661_web?ext=.pdf (accessed on 4 January 2024).
- TSI Inc. Volatile Organic Compounds (VOC) Indoor Air Quality Probes Models 984, 985, 986 & 987, P/N 5001365 Revision E. 2022. Available online: https://tsi.com/getmedia/06aaa1ab-9e79-48d7-ae37-ed1352794f1d/VOC_Probes_US_5001365-web?ext=.pdf (accessed on 18 April 2024).
- Alphasense, Ametek. PID-AH2 Photo Ionisation Detector Technical Specifications Version 1.0. 2022. Available online: https://www.alphasense.com/products (accessed on 7 February 2024).
- Thermo Fisher Scientific Inc. Model 42i Instruction Manual Chemiluminescence NO-NO2-NOx Analyzer. 2015. Available online: https://tools.thermofisher.com/content/sfs/manuals/EPM-manual-Model%2042inox.pdf (accessed on 15 April 2024).
- Thermo Fisher Scientific Inc. Thermo Scientific Model 42i NO-NO2-NOx Chemiluminescent Gas Analyzer Product Specification, EPM_42i_DS_0921. 2021. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/Specification-Sheets/EPM-42i-Datasheet.pdf (accessed on 15 April 2024).
- Pang, X.; Shaw, M.D.; Gillot, S.; Lewis, A.C. The impacts of water vapour and co-pollutants on the performance of electrochemical gas sensors used for air quality monitoring. Sens. Actuators B Chem. 2018, 266, 674–684. [Google Scholar] [CrossRef]
- Fleming, L.; Gibson, D.; Song, S.; Li, C.; Reid, S. Reducing N2O induced cross-talk in a NDIR CO2 gas sensor for breath analysis using multilayer thin film optical interference coatings. Surf. Coat. Technol. 2018, 336, 9–16. [Google Scholar] [CrossRef]
- TSI Inc. Photo-ionization Detection (PID) Technology, Application Note TSI-147 Revision B. 2013. Available online: https://tsi.com/getmedia/c2814d77-a757-4269-9682-ff6fab17ed2b/TSI-147_Photo_Ionization_Detection_Technology-A4?ext=.pdf (accessed on 2 April 2024).
- Yamaguchi, S.; Kishikawa, N.; Ohyama, K.; Ohba, Y.; Kohno, M.; Masuda, T.; Takadate, A.; Nakashima, K.; Kuroda, N. Evaluation of chemiluminescence reagents for selective detection of reactive oxygen species. Anal. Chim. Acta 2010, 665, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Pandas Development Team. pandas-dev/pandas: Pandas, 2020. [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Manufacturing, D.A. Safety Data Sheet FLUORXTM PVDF [POLYVINYLIDINE FLUORIDE POLYMER] Revision V1.1. 2022. Available online: https://www.3dxtech.com/tech-data-sheets-safety-data-sheets/ (accessed on 22 December 2023).
- Creutz, C.; Fujita, E. Carbon Dioxide as a Feedstock; National Academies Press (US): Washington, DC, USA, 2001; Volume 5. [Google Scholar]
- Version 3.0.0, N.C.C. Chemical Datasheet Carbon Monoxide. 2023. Available online: https://cameochemicals.noaa.gov/chemical/335 (accessed on 1 January 2024).
- Chong, S.; Chiu, H.L.; Liao, Y.C.; Hung, S.T.; Pan, G.T. Cradle to Cradle® design for 3D printing. Chem. Eng. Trans. 2015, 45, 1669–1674. [Google Scholar] [CrossRef]
- Rumantri, R.; Khakim, M.Y.; Iskandar, I. Design and characterization of low-cost sensors for air quality monitoring system. J. Pendidik. IPA Indones. 2018, 7, 347–354. [Google Scholar] [CrossRef][Green Version]
- Russell, H.S.; Frederickson, L.B.; Kwiatkowski, S.; Emygdio, A.P.M.; Kumar, P.; Schmidt, J.A.; Hertel, O.; Johnson, M.S. Enhanced Ambient Sensing Environment—A New Method for Calibrating Low-Cost Gas Sensors. Sensors 2022, 22, 7238. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A. Chapter 2—Adsorption technology and surface science. In Surface Science of Adsorbents and Nanoadsorbents; Interface Science and Technology; Saleh, T.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 34, pp. 39–64. [Google Scholar] [CrossRef]
- FlashForge. Creator Pro 2 Specification. Available online: https://www.flashforge.com/product-detail/flashforgecreator-pro-2-3d-printer (accessed on 22 December 2023).
- Hagen, R. Polylactic Acid; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 231–236. ISBN 9780080878621. [Google Scholar] [CrossRef]
- Liu, B.; Zimmerman, N. Fleet-based vehicle emission factors using low-cost sensors: Case study in parking garages. Transp. Res. Part Transp. Environ. 2021, 91, 102635. [Google Scholar] [CrossRef]
- Lulzbot. Lulzbot Taz6 Desktop 3D Printer Complete Technical Specifications. Available online: https://lulzbot.com/store/taz-6 (accessed on 22 December 2023).
- McKeen, L.W. Styrenic Plastics; Elsevier: Amsterdam, The Netherlands, 2010; pp. 51–71. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Turku, I.; Kasala, S.; Kärki, T. Characterization of Feedstock Filament Extruded from Secondary Sources of PS, ABS and PVC. 2018. Available online: https://api.semanticscholar.org/CorpusID:53317722 (accessed on 22 December 2023).
- Turner, S.R.; Liu, Y. 5.14—Chemistry and Technology of Step-Growth Polyesters; Elsevier: Amsterdam, The Netherlands, 2012; Volume 5, pp. 311–331. ISBN 9780080878621. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Wang, H.; Wang, C. Flotation separation of acrylonitrile-butadiene-styrene and polystyrene in WEEE based on oxidation of active sites. Miner. Eng. 2020, 146, 106131. [Google Scholar] [CrossRef]



| Study | Thermoplastic Filaments 1 | VOC Detection Method (Off-Line) | VOC Detection Method (Real-Time) | Thermoplastic VOC Emission Ranking (Highest-Lowest) |
|---|---|---|---|---|
| Wojnowski et al. [28,29] | PLA, ABS, ASA, Nylon, and PETG | Proton transfer reaction time-of-flight mass spectrometry | ABS > PLA > ASA > Nylon > PETG | |
| Floyd et al. [30] | ABS, PLA, PVA, HIPS, PCABS, Nylon, Bronze-PLA, and PET | Tri-sorbent sampling tubes | Photoionization detector (PID) | Bronze-PLA > PCABS > ABS > PVA > PET > Nylon > PLA > HIPS |
| Stefaniak et al. [31] | ABS and PLA | Silonite-coated canisters | PID | ABS > PLA |
| Kim et al. [32] | ABS and PLA | 2,4-DNPH sorbent cartridges and absorbent charcoal tubes | PID | ABS > PLA |
| Davis et al. [33] | ABS, PLA, Nylon, HIPS, and PVA | Tenax TA mesh sorption tubes and 2,4-DNPH sorbent cartridges | Nylon > HIPS > ABS > PLA > PVA | |
| Wilkins et al. [35] | PLA, ABS, and PETG | Semiconductor VOC sensor | Each thermoplastic filament emitted VOC during print—no ranking available 2 | |
| Weber et al. [34] | Nylon, PLA, and ABS | Absorption columns | Nylon > ABS and PLA 3 |
| Trace Gas | Instrument | Specifications 1 |
|---|---|---|
| CO/CO2 | TSI Q-Track with probe 982 | Operating principle: electrochemical CO, NDIR 2 CO2 |
| Range: 0–500 ppm CO, 0–5000 ppm CO2 | ||
| Required flow rate: 500 ccm | ||
| Resolution: 0.1 ppm CO, 1 ppm CO2 | ||
| Response time: <60 s CO, 20 s CO2 | ||
| Reference: [44,45] | ||
| VOC | TSI Q-Track with probe 984 | Operating principle: PID 3 |
| Range: 10–20,000 ppb | ||
| Required flow rate: 500 ccm | ||
| Resolution: 10 ppb | ||
| Response time: <3 s | ||
| Reference(s): [44,45,46,47,48] | ||
| NO/NO2 | Thermo Fisher NOx Analyzer | Operating principle: chemiluminescence |
| Range: 0–2000 ppb NO, 0–200 ppb NO2 4 | ||
| Model: 42i | Limit of Detection: 0.40 ppb | |
| Required flow rate: 700 ccm (900 ccm recommended) | ||
| Resolution/Precision: ±0.4 ppb | ||
| Log interval: 60 s | ||
| Reference: [49,50] |
| Thermoplastic Filament 1 | Date Printed | Baffle Surface Area (±Error) | Extrusion Temperature Range | FDM 3D Printer |
|---|---|---|---|---|
| [] | [°C] | |||
| ABS | 5/9/2023 | 538 (±3) | 210–240 | FlashForge Creator Pro 2 |
| PLA | 5/10/2023 | 538 (±3) | 180–220 | FlashForge Creator Pro 2 |
| PETG | 5/11/2023 | 538 (±3) | 230–260 | FlashForge Creator Pro 2 |
| PC | 5/12/2023 | 538 (±6) | 250–270 | LulzBot TAZ6 |
| PVDF | 6/18/2023 | 538 (±6) | 245–265 | LulzBot TAZ6 |
| Gas (unit) | Flow Rate (±Error) | Off-Gassing Concentration | Reaction Target Concentrations 1 | Temperature 2 |
|---|---|---|---|---|
| [ccm] | [] | [] | [°C] | |
| CO (ppm) | 1100 (±1.12) | 0 (zero-air) | 2, 4 | 24.2 (±0.3) |
| CO2 (ppm) | 1100 (±1.12) | 0 (zero-air) | 1000, 2000 | 24.6 (±0.3) |
| NO (ppb) | 1100 (±1.12) | 0 (zero-air) | 500, 1000, 1500 | 25.2 (±0.4) |
| NO2 (ppb) | 1100 (±1.12) | 0 (zero-air) | 100, 150, 200 | 24.6 (±0.5) |
| Isobutylene (VOC) (ppb) | 1100 (±1.12) | 0 (zero-air) | 400, 800 | 24.3 (±0.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangin, T.; Blanchard, E.K.; Kelly, K.E. Effect of Three-Dimensional-Printed Thermoplastics Used in Sensor Housings on Common Atmospheric Trace Gasses. Sensors 2024, 24, 2610. https://doi.org/10.3390/s24082610
Mangin T, Blanchard EK, Kelly KE. Effect of Three-Dimensional-Printed Thermoplastics Used in Sensor Housings on Common Atmospheric Trace Gasses. Sensors. 2024; 24(8):2610. https://doi.org/10.3390/s24082610
Chicago/Turabian StyleMangin, Tristalee, Evan K. Blanchard, and Kerry E. Kelly. 2024. "Effect of Three-Dimensional-Printed Thermoplastics Used in Sensor Housings on Common Atmospheric Trace Gasses" Sensors 24, no. 8: 2610. https://doi.org/10.3390/s24082610
APA StyleMangin, T., Blanchard, E. K., & Kelly, K. E. (2024). Effect of Three-Dimensional-Printed Thermoplastics Used in Sensor Housings on Common Atmospheric Trace Gasses. Sensors, 24(8), 2610. https://doi.org/10.3390/s24082610






