Maximizing the Reliability and Precision of Measures of Prefrontal Cortical Oxygenation Using Frequency-Domain Near-Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics
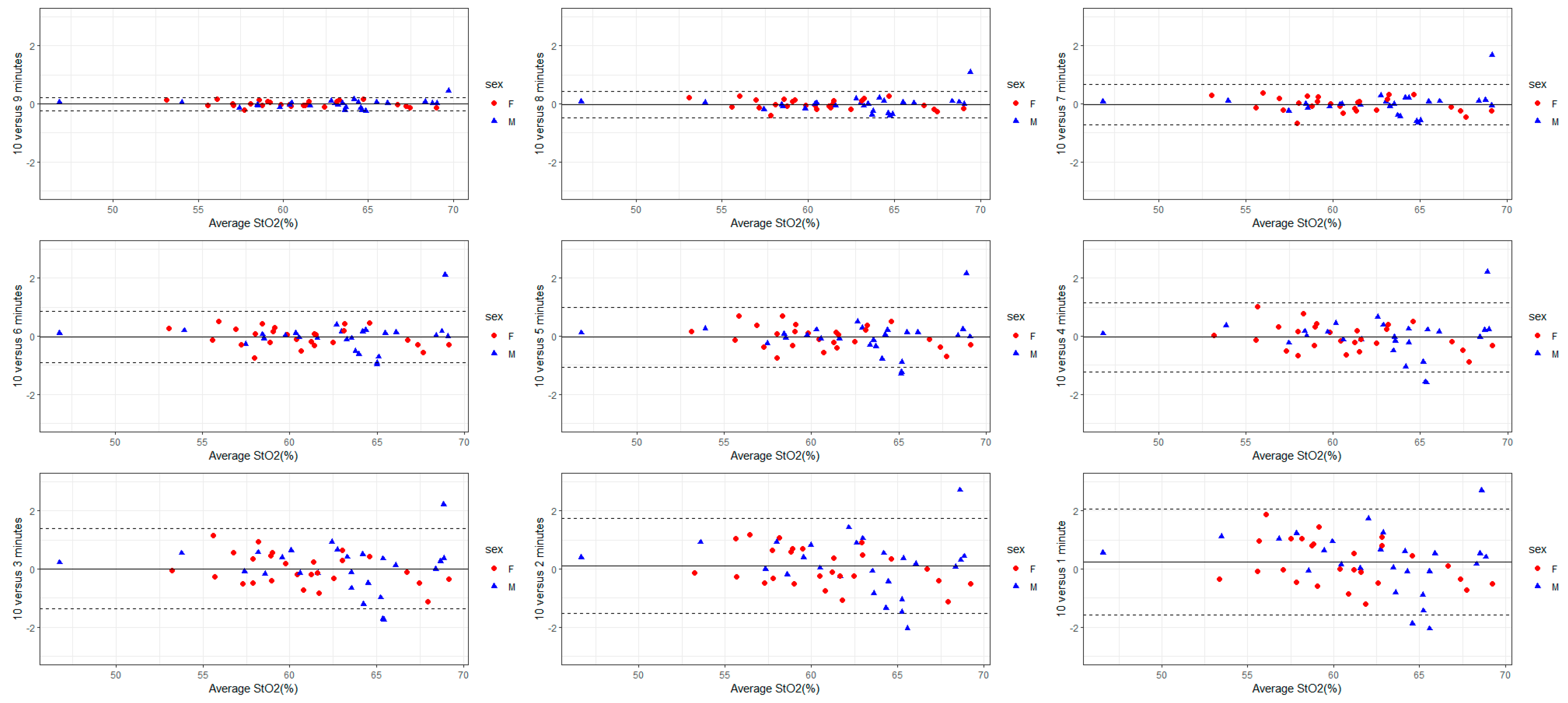
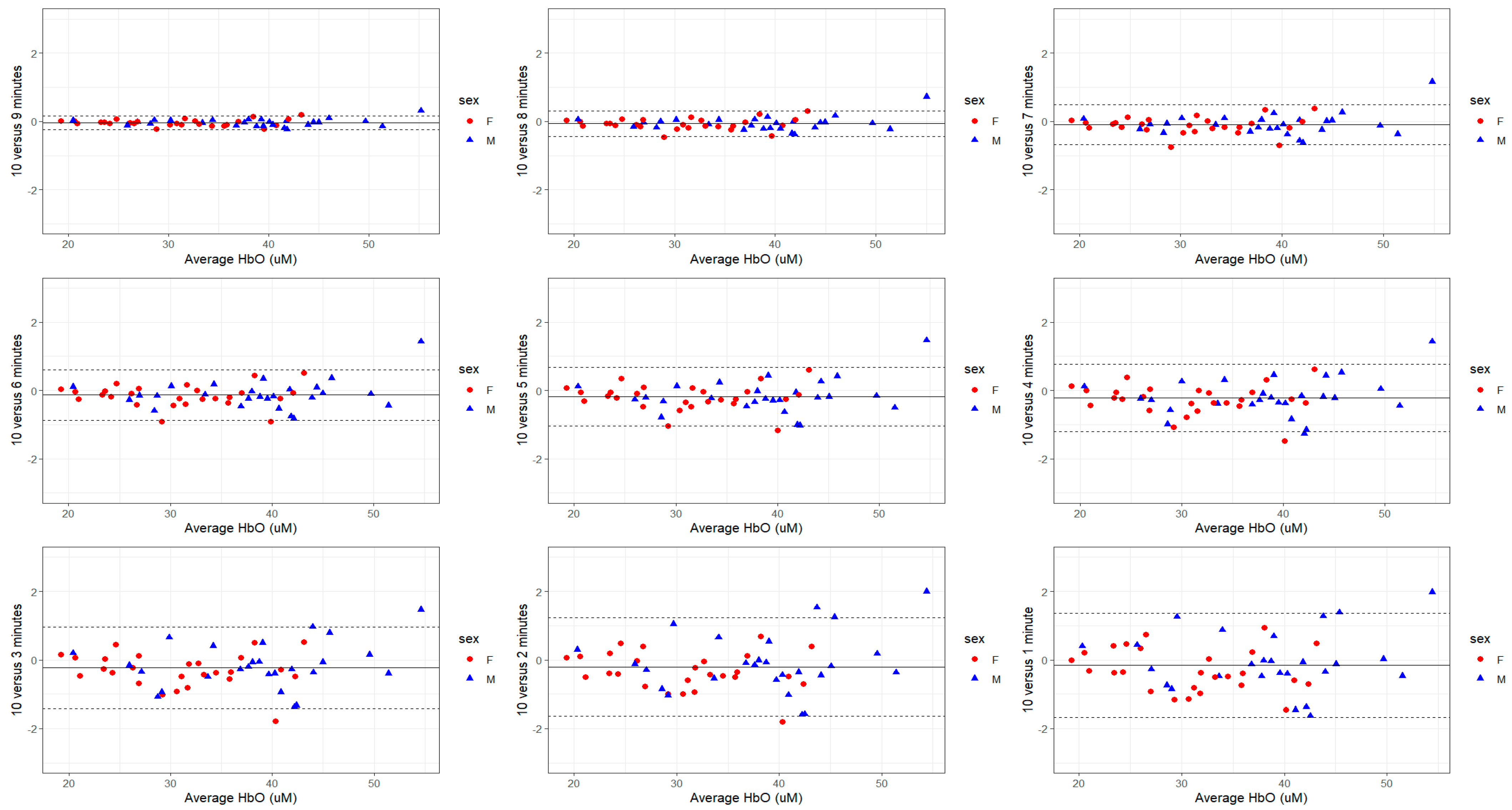
3.2. Variability Based on Recording Duration
3.3. Within- and Between-Day Reproducibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willie, C.K.; Tzeng, Y.-C.; Fisher, J.A.; Ainslie, P.N. Integrative Regulation of Human Brain Blood Flow. J. Physiol. 2014, 592, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Sakurai, T.; Hamaoka, T.; Esaki, K.; Ueya, K.; Toshinai, K.; Miyazaki, H.; Ogasawara, J.; Shirato, K.; Hashimoto, N.; et al. Cerebral Artery Blood Flow and Oxygenation in the Frontal Lobe Region in Response to a Judo Chokehold (Shimewaza). J. Exerc. Sports Orthop. 2016, 3, 1–8. [Google Scholar]
- Giza, C.C.; Hovda, D.A. The New Neurometabolic Cascade of Concussion. Neurosurgery 2014, 75 (Suppl. S4), S24–S33. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Arnulphi, M.; Alaraj, A.; Slavin, K.V. Near Infrared Technology in Neuroscience: Past, Present and Future. Neurol. Res. 2009, 31, 605–614. [Google Scholar] [CrossRef]
- Fantini, S.; Sassaroli, A. Frequency-Domain Techniques for Cerebral and Functional Near-Infrared Spectroscopy. Front. Neurosci. 2020, 14, 519087. [Google Scholar] [CrossRef]
- Hueber, D.M.; Fantini, S.; Cerussi, A.E.; Barbieri, B.B. New Optical Probe Designs for Absolute (Self-Calibrating) NIR Tissue Hemoglobin Measurements. In Optical Tomography and Spectroscopy of Tissue III; SPIE: Bellingham, WA, USA, 1999; pp. 618–631. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, Y.; Uchitel, J.; Collins-Jones, L.; Yang, S.; Loureiro, R.; Cooper, R.J.; Zhao, H. Review of Recent Advances in Frequency-Domain near-Infrared Spectroscopy Technologies [Invited]. Biomed. Opt. Express 2023, 14, 3234–3258. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Suzuki, H.; Yamashita, Y. Time-Domain Near-Infrared Spectroscopy and Imaging: A Review. Appl. Sci. 2019, 9, 1127. [Google Scholar] [CrossRef]
- Adingupu, D.D.; Evans, T.; Soroush, A.; Jarvis, S.; Brown, L.; Dunn, J.F. Non-Invasive Detection of Persistent Cortical Hypoxia in Multiple Sclerosis Using Frequency Domain Near-Infrared Spectroscopy (fdNIRS). Adv. Exp. Med. Biol. 2022, 1395, 89–93. [Google Scholar] [CrossRef]
- Kurth, C.D.; Thayer, W.S. A Multiwavelength Frequency-Domain near-Infrared Cerebral Oximeter. Phys. Med. Biol. 1999, 44, 727. [Google Scholar] [CrossRef]
- Fantini, S.; Franceschini, M.A.; Maier, J.S.; Walker, S.A.; Barbieri, B.B.; Gratton, E. Frequency-Domain Multichannel Optical Detector for Noninvasive Tissue Spectroscopy and Oximetry. Opt. Eng. 1995, 34, 32–42. [Google Scholar] [CrossRef]
- Franceschini, M.A.; Gratton, E.; Fantini, S. Noninvasive Optical Method of Measuring Tissue and Arterial Saturation: An Application to Absolute Pulse Oximetry of the Brain. Opt. Lett. OL 1999, 24, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Kleiser, S.; Ostojic, D.; Andresen, B.; Nasseri, N.; Isler, H.; Scholkmann, F.; Karen, T.; Greisen, G.; Wolf, M. Comparison of Tissue Oximeters on a Liquid Phantom with Adjustable Optical Properties: An Extension. Biomed. Opt. Express 2018, 9, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Davie, S.N.; Grocott, H.P. Impact of Extracranial Contamination on Regional Cerebral Oxygen Saturation: A Comparison of Three Cerebral Oximetry Technologies. Anesthesiology 2012, 116, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Dehaes, M.; Grant, P.E.; Sliva, D.D.; Roche-Labarbe, N.; Pienaar, R.; Boas, D.A.; Franceschini, M.A.; Selb, J. Assessment of the Frequency-Domain Multi-Distance Method to Evaluate the Brain Optical Properties: Monte Carlo Simulations from Neonate to Adult. Biomed. Opt. Express BOE 2011, 2, 552–567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ding, H.S.; Hou, X.L.; Zhou, C.L.; Chance, B. In Vivo Determination of the Optical Properties of Infant Brain Using Frequency-Domain near-Infrared Spectroscopy. J. Biomed. Opt. 2005, 10, 024028. [Google Scholar] [CrossRef] [PubMed]
- Arri, S.J.; Muehlemann, T.; Biallas, M.; Bucher, H.U.; Wolf, M. Precision of Cerebral Oxygenation and Hemoglobin Concentration Measurements in Neonates Measured by Near-Infrared Spectroscopy. J. Biomed. Opt. 2011, 16, 047005. [Google Scholar] [CrossRef]
- Burma, J.S.; Miutz, L.N.; Newel, K.T.; Labrecque, L.; Drapeau, A.; Brassard, P.; Copeland, P.; Macaulay, A.; Smirl, J.D. What Recording Duration Is Required to Provide Physiologically Valid and Reliable Dynamic Cerebral Autoregulation Transfer Functional Analysis Estimates? Physiol. Meas. 2021, 42, 044002. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, O.M.; Gjedde, A.; Vang, K.; Law, I.; Aanerud, J.; Rostrup, E. Regional and Interindividual Relationships between Cerebral Perfusion and Oxygen Metabolism. J. Appl. Physiol. 2021, 130, 1836–1847. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Hallacoglu, B.; Sassaroli, A.; Fantini, S.; Wysocki, M.; Guerrero-Berroa, E.; Beeri, M.S.; Haroutunian, V.; Shaul, M.; Rosenberg, I.H.; Troen, A. Absolute Measurement of Cerebral Optical Coefficients, Hemoglobin Concentration and Oxygen Saturation in Old and Young Adults with near-Infrared Spectroscopy. J. Biomed. Opt. 2012, 17, 081406. [Google Scholar] [CrossRef]
- Tanji, J.; Hoshi, E. Behavioral Planning in the Prefrontal Cortex. Curr. Opin. Neurobiol. 2001, 11, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.P. Quantifying Test-Retest Reliability Using the Intraclass Correlation Coefficient and the SEM. J. Strength. Cond. Res. 2005, 19, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bonett, D.G. Sample Size Requirements for Estimating Intraclass Correlations with Desired Precision. Stat. Med. 2002, 21, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Burma, J.S.; Graver, S.; Miutz, L.N.; Macaulay, A.; Copeland, P.V.; Smirl, J.D. The Validity and Reliability of Ultra-Short-Term Heart Rate Variability Parameters and the Influence of Physiological Covariates. J. Appl. Physiol. 2021, 130, 1848–1867. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Shih, W.J. Assessing Reproducibility by the within-Subject Coefficient of Variation with Random Effects Models. Biometrics 1996, 52, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, A.N.V.; Salimi-Khorshidi, G.; Lai, M.-C.; Baron-Cohen, S.; Lombardo, M.V.; Tait, R.J.; Suckling, J. A Meta-Analysis of Sex Differences in Human Brain Structure. Neurosci. Biobehav. Rev. 2014, 39, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Hamer, J.; Churchill, N.W.; Hutchison, M.G.; Graham, S.J.; Schweizer, T.A. Sex Differences in Cerebral Blood Flow Associated with a History of Concussion. J. Neurotrauma 2020, 37, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Gur, R.E.; Gur, R.C. Gender Differences in Regional Cerebral Blood Flow. Schizophr. Bull. 1990, 16, 247–254. [Google Scholar] [CrossRef]
- Mazzucco, S.; Li, L.; Tuna, M.A.; Rothwell, P.M. Age-Specific Sex-Differences in Cerebral Blood Flow Velocity in Relation to Haemoglobin Levels. Eur. Stroke J. 2024, 1–9. [Google Scholar] [CrossRef]
- Davies, D.J.; Clancy, M.; Lighter, D.; Balanos, G.M.; Lucas, S.J.E.; Dehghani, H.; Su, Z.; Forcione, M.; Belli, A. Frequency-Domain vs. Continuous-Wave near-Infrared Spectroscopy Devices: A Comparison of Clinically Viable Monitors in Controlled Hypoxia. J. Clin. Monit. Comput. 2017, 31, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, T.; Goos, T.G.; Van Ballegooijen, L.; Pichler, G.; Urlesberger, B.; Reiss, I.K.M.; De Jonge, R.C.J. Comparison of Frequency-Domain and Continuous-Wave near-Infrared Spectroscopy Devices during the Immediate Transition. BMC Pediatr. 2020, 20, 94. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, M.A.; Fantini, S.; Paunescu, L.A.; Maier, J.S.; Gratton, E. Influence of a Superficial Layer in the Quantitative Spectroscopic Study of Strongly Scattering Media. Appl. Opt. 1998, 37, 7447–7458. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, H.; Delpy, D.T. Near-Infrared Spectroscopy of the Adult Head: Effect of Scattering and Absorbing Obstructions in the Cerebrospinal Fluid Layer on Light Distribution in the Tissue. Appl. Opt. 2000, 39, 4721–4729. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Wolf, M.; Toronov, V.; Wolf, U.; Polzonetti, C.; Hueber, D.; Safonova, L.P.; Gupta, R.; Michalos, A.; Mantulin, W.; et al. Noninvasive Determination of the Optical Properties of Adult Brain: Near-Infrared Spectroscopy Approach. J. Biomed. Opt. 2004, 9, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Gatto, R.; Hoffman, W.; Mueller, M.; Flores, A.; Valyi-Nagy, T.; Charbel, F.T. Frequency Domain Near-Infrared Spectroscopy Technique in the Assessment of Brain Oxygenation: A Validation Study in Live Subjects and Cadavers. J. Neurosci. Methods 2006, 157, 274–277. [Google Scholar] [CrossRef]
- Wassenaar, E.B.; Van den Brand, J.G.H. Reliability of Near-Infrared Spectroscopy in People with Dark Skin Pigmentation. J. Clin. Monit. Comput. 2005, 19, 195–199. [Google Scholar] [CrossRef]


| Physiological Parameter | Mean ± Standard Deviation | |
|---|---|---|
| Female | Male | |
| Heart rate (BPM) | 70.3 ± 11.7 | 66.0 ± 9.20 |
| Mean Blood Pressure (mmHg) | 69.3 ± 8.59 | 81.4 ± 6.59 |
| PETCO2 (mmHg) | 42.8 ± 6.81 | 35.6 ± 3.39 |
| Duration (Minutes) | StO2 (%) | THb (µM) | HbO (µM) | HHb (µM) | ||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | |
| 1 | 60.58 ± 4.20 | 62.34 ± 5.16 | 50.98 ± 9.65 | 61.44 ± 11.53 | 31.08 ± 7.08 | 38.44 ± 8.20 | 19.91 ± 3.50 | 23.00 ± 4.76 |
| 2 | 60.73 ± 4.17 | 62.46 ± 5.06 | 50.92 ± 9.64 | 61.42 ± 11.46 | 31.10 ± 7.06 | 38.49 ± 8.12 | 19.82 ± 3.51 | 22.92 ± 4.73 |
| 3 | 60.80 ± 4.13 | 62.57 ± 5.01 | 50.84 ± 9.61 | 61.41 ± 11.44 | 31.09 ± 7.03 | 38.56 ± 8.12 | 19.75 ± 3.51 | 22.85 ± 4.69 |
| 4 | 60.82 ± 4.09 | 62.64 ± 4.95 | 50.75 ± 9.60 | 61.39 ± 11.45 | 31.04 ± 7.00 | 38.59 ± 8.14 | 19.71 ± 3.50 | 22.80 ± 4.65 |
| 5 | 60.81 ± 4.05 | 62.65 ± 4.93 | 50.67 ± 9.58 | 61.33 ± 11.43 | 30.98 ± 6.96 | 38.56 ± 8.17 | 19.69 ± 3.51 | 22.77 ± 4.60 |
| 6 | 60.82 ± 4.02 | 62.63 ± 4.90 | 50.60 ± 9.58 | 61.27 ± 11.42 | 30.94 ± 6.95 | 38.52 ± 8.17 | 19.66 ± 3.52 | 22.75 ± 4.56 |
| 7 | 60.82 ± 4.01 | 62.62 ± 4.88 | 50.54 ± 9.58 | 61.21 ± 11.41 | 30.90 ± 6.95 | 38.48 ± 8.21 | 19.64 ± 3.52 | 22.73 ± 4.51 |
| 8 | 60.81 ± 3.97 | 62.62 ± 4.90 | 50.48 ± 9.58 | 61.17 ± 11.39 | 30.86 ± 6.94 | 38.46 ± 8.23 | 19.62 ± 3.51 | 22.70 ± 4.48 |
| 9 | 60.79 ± 3.96 | 62.62 ± 4.92 | 50.42 ± 9.59 | 61.12 ± 11.37 | 30.82 ± 6.95 | 38.44 ± 8.26 | 19.60 ± 3.50 | 22.68 ± 4.46 |
| 10 | 60.79 ± 3.93 | 62.60 ± 4.95 | 50.37 ± 9.59 | 61.08 ± 11.34 | 30.79 ± 6.95 | 38.40 ± 8.28 | 19.58 ± 3.48 | 22.67 ± 4.45 |
| Variables | StO2 | THb | HbO | HHb |
|---|---|---|---|---|
| 9 min | 0.2 (95%CI: −1.5, 2.0) p = 0.790 | −0.5 (95%CI: −4.5, 3.5) p = 0.811 | −0.2 (95%CI: 3.1, 2.7) p = 0.912 | −0.3 (95% CI: −1.9, 1.2) p = 0.679 |
| 8 min | 0.1 (95% CI: −1.6, 1.9) p = 0.879 | 0.0 (95% CI: −4.1, 4.0) p = 0.982 | (95% CI: −2.9, 2.9) p = 0.979 | −0.1 (95% CI: −1.6, 1.5) p = 0.915 |
| 7 min | 0.2 (95% CI: −1.5, 2.0) p = 0.795 | −0.1 (95% CI: −4.1, 3.9) p = 0.965 | 0.1 (95% CI: −2.8, 3.0) p = 0.964 | −0.2 (95% CI: −1.7, 1.4) p = 0.845 |
| 6 min | 0.3 (95% CI: −1.5, 2.0) p = 0.756 | −0.1 (95% CI: −4.2, 3.9) p = 0.945 | 0.1 (95% CI: −2.8, 3.0) p = 0.968 | −0.2 (95% CI: −1.8, 1.4) p = 0.799 |
| 5 min | 0.3 (95% CI: −1.5, 2.0) p = 0.760 | −0.2 (95% CI: −4.2, 3.8) p = 0.917 | 0.0 (95% CI: −2.9, 2.9) p = 0.992 | −0.2 (95% CI: −1.8, 1.3) p = 0.774 |
| 4 min | 0.3 (95% CI: −1.5, 2.0) p = 0.766 | −0.3 (95% CI: −4.3, 3.7) p = 0.891 | −0.0 (95% CI: −2.9, 2.9) p = 0.984 | −0.3 (95% CI: −1.8, 1.3) p = 0.752 |
| 3 min | 0.3 (95% CI: −1.5, 2.0) p = 0.770 | −0.3 (95% CI: −4.4, 3.7) p = 0.868 | −0.1 (95% CI: −3.0, 2.8) p = 0.965 | −0.3 (95% CI: −1.8, 1.3) p = 0.730 |
| 2 min | 0.3 (95% CI: −1.5, 2.0) p = 0.772 | −0.4 (95% CI: −4.4, 3.6) p = 0.848 | −0.1 (95% CI: −3.0, 2.8) p = 0.948 | −0.3 (95% CI: −1.9, 1.3) p = 0.709 |
| 1 min | 0.2 (95% CI: −1.5, 2.0) p = 0.781 | −0.4 (95% CI: −4.5, 3.6) p = 0.829 | −0.1 (95% CI: −3.0, 2.8) p = 0.930 | −0.3 (95% CI: −1.9, 1.2) p = 0.693 |
| Sex | 1.8 (95% CI: 1.0, 2.5) p < 0.001 | 11 (95% CI: 8.9, 12) p < 0.001 | 7.6 (95% CI: 6.3, 8.9) p < 0.001 | 3.1 (95% CI: 2.4, 3.8) p < 0.001 |
| Concussion history | 0.8 (95% CI: −0.0, 1.6) p = 0.060 | −1.5 (95% CI: −3.3, 0.4) p = 0.125 | −0.7 (95% CI: −2.1, 0.6) p = 0.283 | −0.7 (95% CI: −1.4, −0.0) p = 0.049 |
| Duration (Minutes) | StO2 (% [95% CI]) | THb (% [95% CI]) | HbO (% [95% CI]) | HHb (% [95% CI]) | ||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | |
| 1 | 0.76 (0.53, 1.00) | 0.94 (0.63, 1.25) | 0.96 (0.70, 1.21) | 0.75 (0.52, 0.99) | 1.32 (1.01, 1.62) | 1.27 (0.88, 1.65) | 1.39 (0.96, 1.82) | 1.77 (1.16, 2.37) |
| 2 | 0.65 (0.49, 0.81) | 0.83 (0.54, 1.11) | 0.85 (0.62, 1.08) | 0.72 (0.52, 0.93) | 1.14 (0.82, 1.45) | 1.22 (0.85, 1.59) | 1.08 (0.74, 1.41) | 1.46 (0.88, 2.04) |
| 3 | 0.54 (0.40, 0.68) | 0.67 (0.43, 0.91) | 0.75 (0.54, 0.95) | 0.61 (0.46, 0.77) | 1.01 (0.70, 1.32) | 1.03 (0.72, 1.33) | 0.82 (0.55, 1.09) | 1.18 (0.69, 1.67) |
| 4 | 0.45 (0.32, 0.57) | 0.51 (0.27, 0.75) | 0.62 (0.43, 0.80) | 0.49 (0.38, 0.61) | 0.84 (0.55, 1.12) | 0.85 (0.60, 1.11) | 0.62 (0.40, 0.85) | 0.97 (0.50, 1.45) |
| 5 | 0.38 (0.28, 0.48) | 0.42 (0.20, 0.63) | 0.51 (0.35, 0.66) | 0.39 (0.30, 0.49) | 0.71 (0.47, 0.95) | 0.72 (0.50, 0.93) | 0.50 (0.32, 0.68) | 0.78 (0.35, 1.22) |
| 6 | 0.32 (0.24, 0.41) | 0.35 (0.16, 0.54) | 0.40 (0.28, 0.52) | 0.30 (0.23, 0.37) | 0.59 (0.38, 0.79) | 0.57 (0.38, 0.76) | 0.39 (0.25, 0.53) | 0.66 (0.25, 1.06) |
| 7 | 0.25 (0.18, 0.32) | 0.27 (0.12, 0.41) | 0.29 (0.19, 0.39) | 0.21 (0.16, 0.26) | 0.45 (0.29, 0.62) | 0.42 (0.27, 0.56) | 0.29 (0.18, 0.40) | 0.50 (0.18, 0.81) |
| 8 | 0.17 (0.13, 0.21) | 0.18 (0.09, 0.28) | 0.19 (0.13, 0.26) | 0.14 (0.10, 0.17) | 0.32 (0.22, 0.42) | 0.27 (0.18, 0.36) | 0.19 (0.12, 0.26) | 0.33 (0.12, 0.54) |
| 9 | 0.09 (0.07, 0.12) | 0.11 (0.07, 0.15) | 0.10 (0.07, 0.14) | 0.07 (0.05, 0.09) | 0.18 (0.13, 0.23) | 0.15 (0.10, 0.20) | 0.12 (0.07, 0.16) | 0.18 (0.09, 0.27) |
| Time of Measurement | Oxygen Saturation (%) | Total Hemoglobin Concentration (µM) | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Morning | 60.58 ± 0.95 | 61.80 ± 0.62 | 52.53 ± 2.75 | 62.06 ± 2.82 |
| Afternoon | 60.09 ± 0.67 | 59.21 ± 0.43 | 51.45 ± 2.37 | 58.62 ± 3.65 |
| Combined | 60.34 ± 0.58 | 60.51 ± 0.54 | 51.98 ± 2.17 | 60.34 ± 2.50 |
| StO2 (% [95% CI]) | THb (% [95% CI]) | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Morning | 3.60 (2.61–4.60) | 2.42 (0.90–3.93) | 9.42 (5.55–13.3) | 5.99 (0.00–12.6) |
| Afternoon | 3.31 (2.54–4.08) | 5.34 (4.14–6.54) | 6.03 (2.90–9.16) | 10.2 (0.00–20.9) |
| Combined | 3.55 (2.93–4.18) | 4.55 (3.27–5.82) | 8.27 (5.05–11.5) | 8.96 (2.10–15.8) |
| StO2 (ICC, [95% CI], p-Value) | THb (ICC, [95% CI], p-Value) | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Morning | 0.92, (0.811–0.975) p < 0.001 | 0.94 (0.74–1.00) p < 0.001 | 0.76 (0.44–0.93) p < 0.001 | 0.83 (0.18–0.99) p = 0.01 |
| Afternoon | 0.94 (0.85–0.98) p < 0.001 | 0.50 (0.00– 0.97) p = 0.18 | 0.92 (0.81–0.98) p < 0.001 | 0.69 (0.00–0.98) p = 0.05 |
| Combined | 0.96 (0.92–0.99) p < 0.001 | 0.87 (0.56–0.99) p < 0.001 | 0.93 (0.84–0.98) p < 0.001 | 0.86 (0.52–0.99) p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fletcher, E.K.S.; Burma, J.S.; Javra, R.M.; Friesen, K.B.; Emery, C.A.; Dunn, J.F.; Smirl, J.D. Maximizing the Reliability and Precision of Measures of Prefrontal Cortical Oxygenation Using Frequency-Domain Near-Infrared Spectroscopy. Sensors 2024, 24, 2630. https://doi.org/10.3390/s24082630
Fletcher EKS, Burma JS, Javra RM, Friesen KB, Emery CA, Dunn JF, Smirl JD. Maximizing the Reliability and Precision of Measures of Prefrontal Cortical Oxygenation Using Frequency-Domain Near-Infrared Spectroscopy. Sensors. 2024; 24(8):2630. https://doi.org/10.3390/s24082630
Chicago/Turabian StyleFletcher, Elizabeth K. S., Joel S. Burma, Raelyn M. Javra, Kenzie B. Friesen, Carolyn A. Emery, Jeff F. Dunn, and Jonathan D. Smirl. 2024. "Maximizing the Reliability and Precision of Measures of Prefrontal Cortical Oxygenation Using Frequency-Domain Near-Infrared Spectroscopy" Sensors 24, no. 8: 2630. https://doi.org/10.3390/s24082630





