Improvement of Phased Antenna Array Applied in Focused Microwave Breast Hyperthermia
Abstract
:1. Introduction
2. Materials and Methods
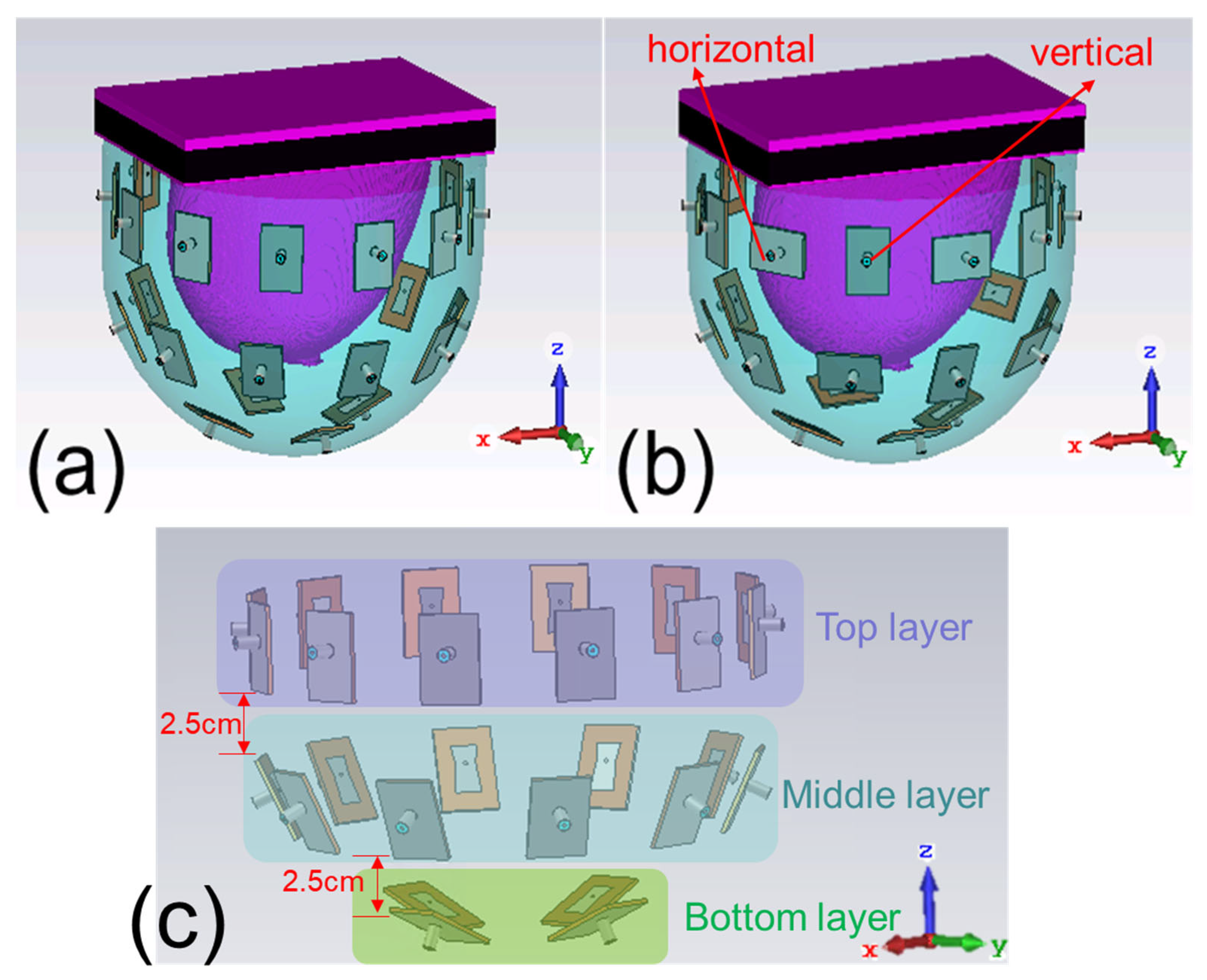
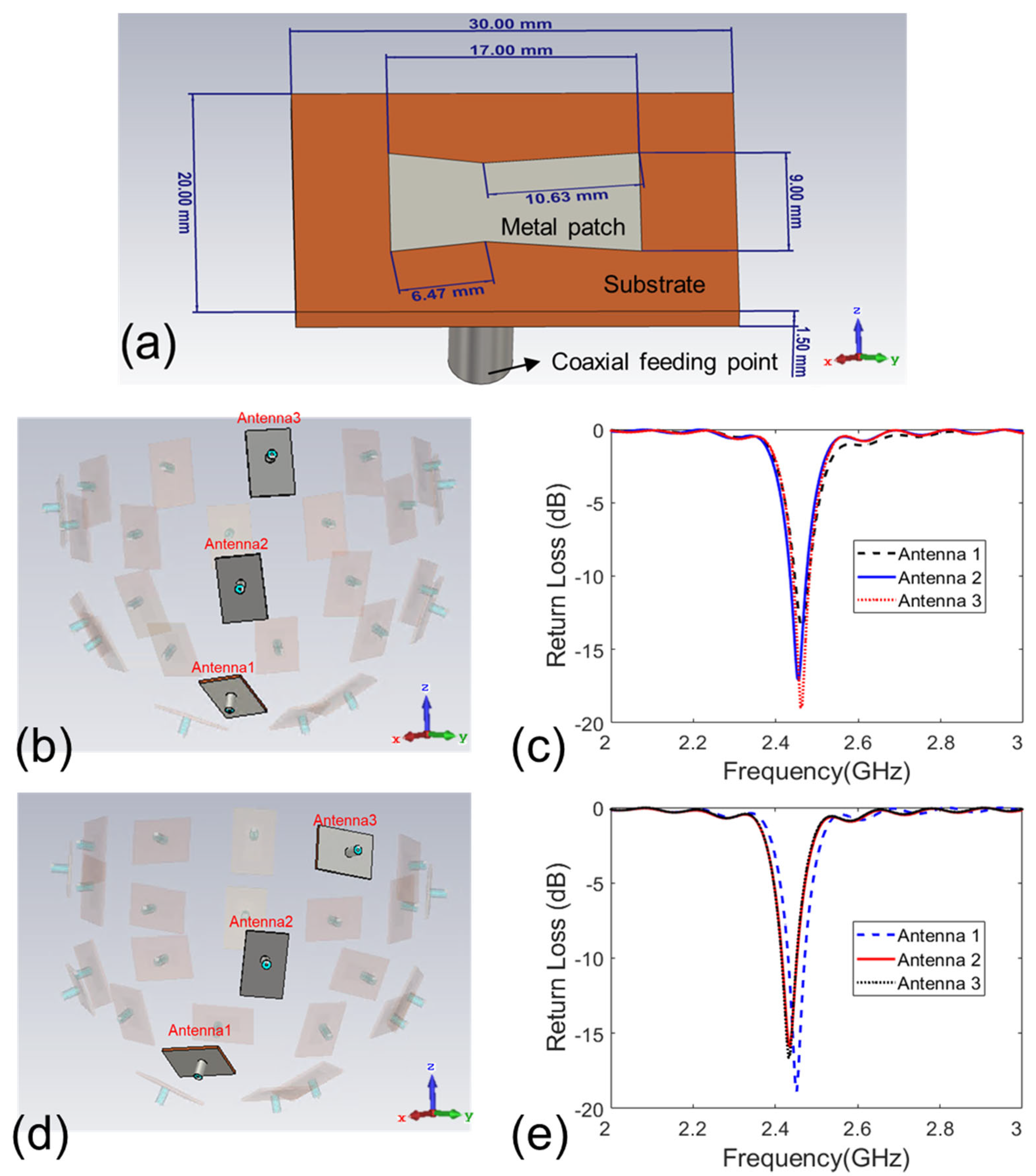
2.1. Design of Antenna Array
2.2. Digital Realistic Human Breast Phantoms
2.3. Optimization Algorithm for Microwave Focusing
2.4. Procedure of Computational Study
3. Results
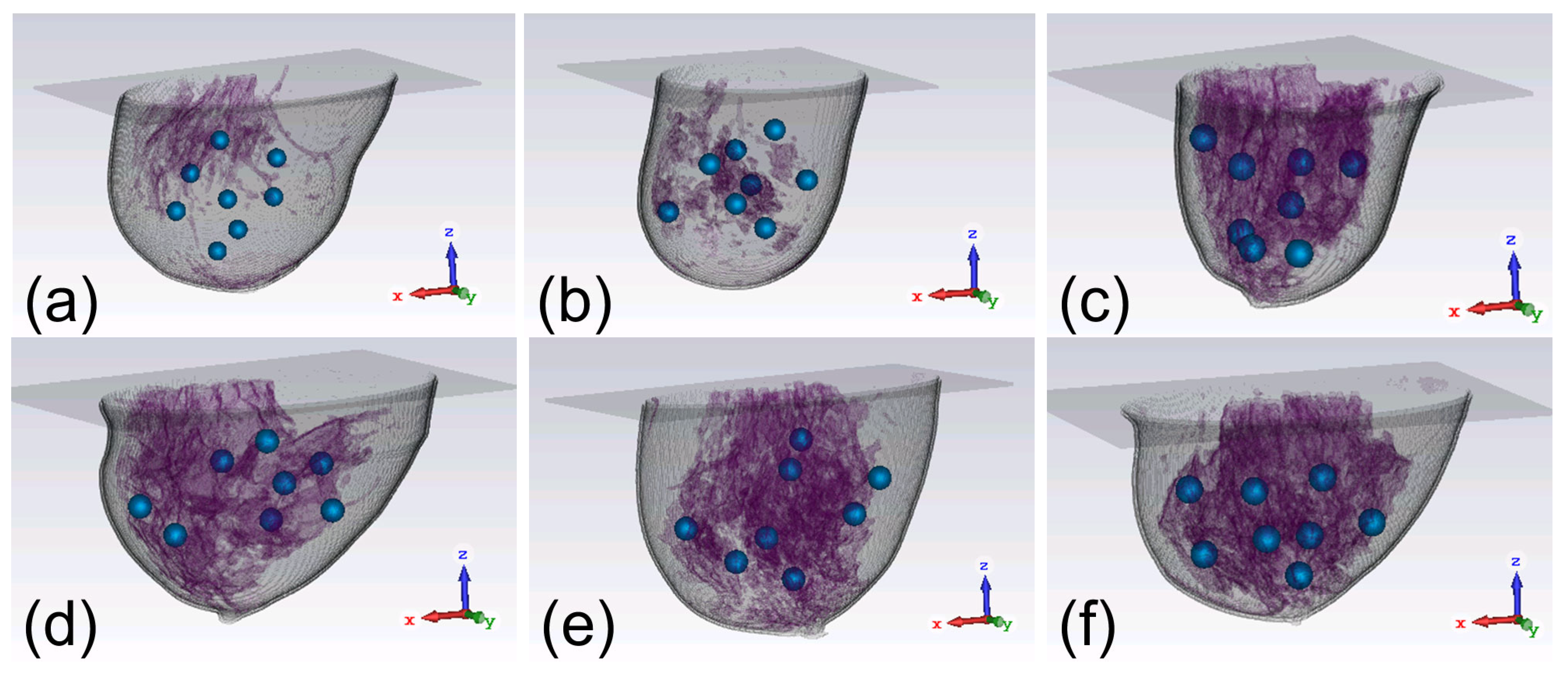
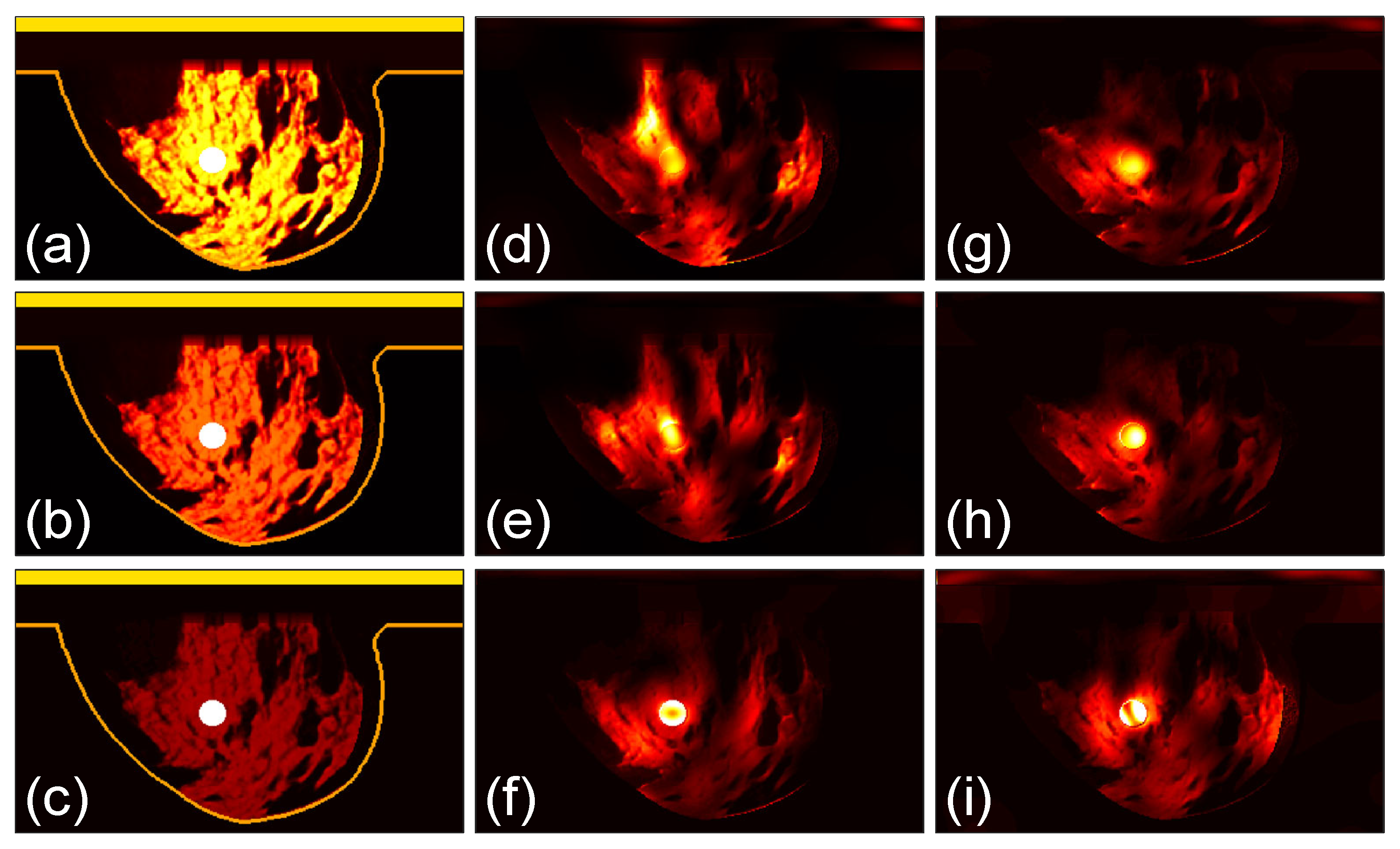
3.1. Focusing Results Using Original Breast Phantoms
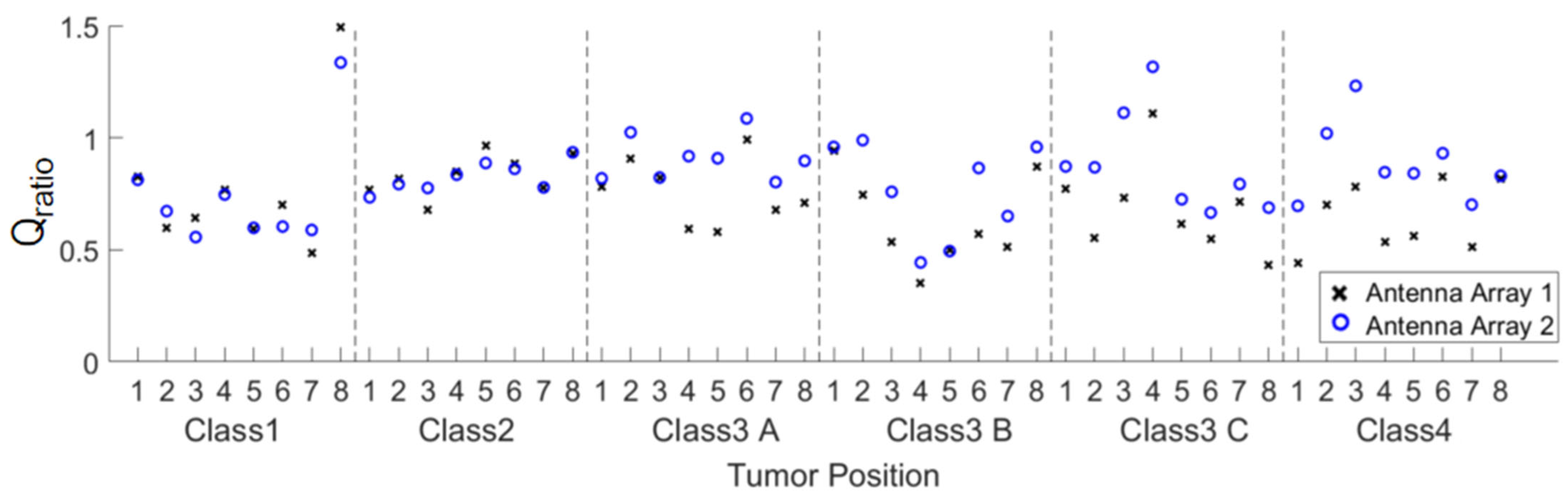
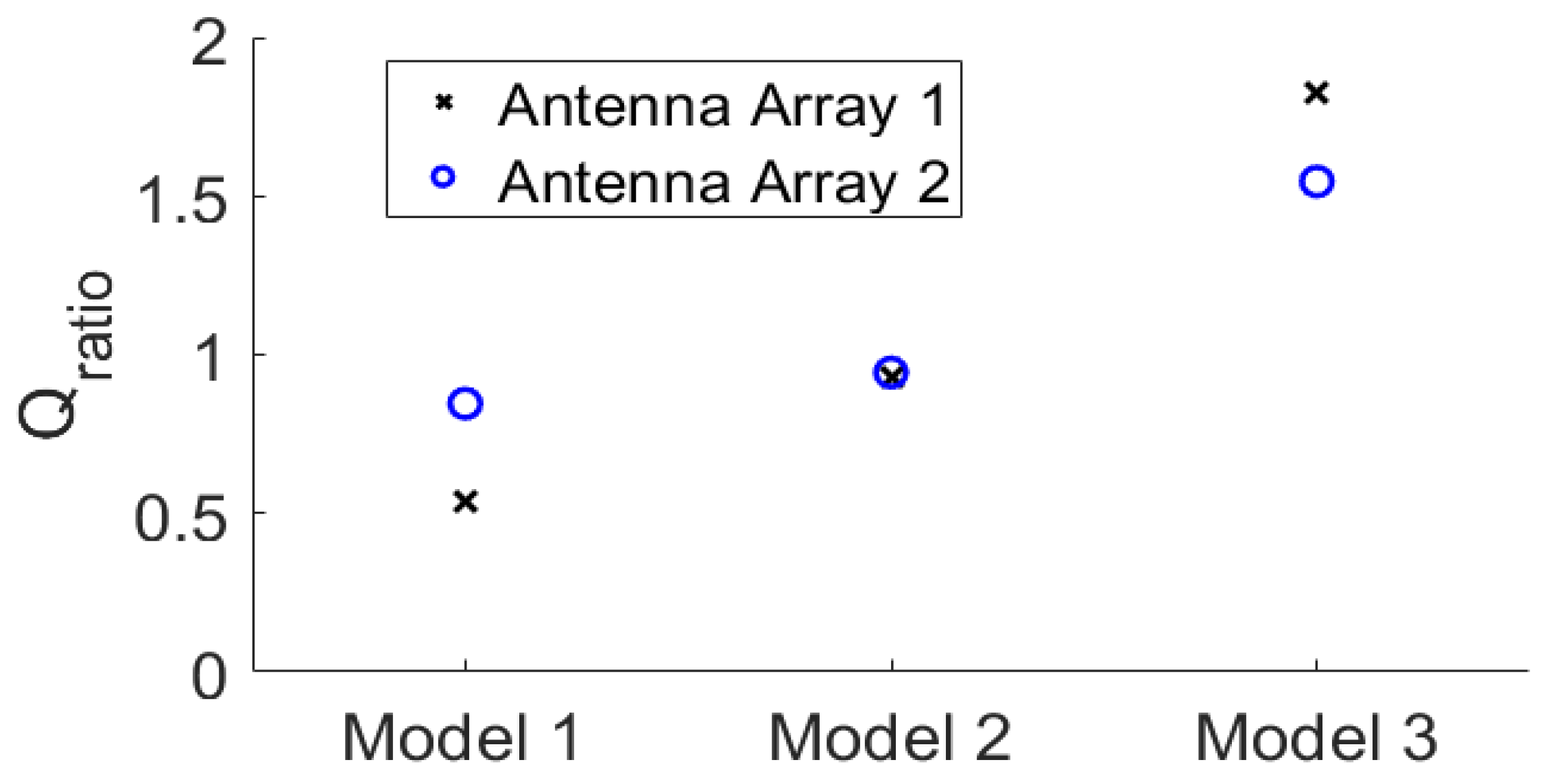
3.2. Quantitative Comparison
3.3. Focusing Results Using Modified Breast Models
4. Discussions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, F.; Liu, P.; Xu, L.X. Recent advances in thermal treatment techniques and thermally induced immune responses against cancer. IEEE Trans. Biomed. Eng. 2014, 61, 1497–1505. [Google Scholar] [CrossRef]
- Van der Zee, J. Heating the patient: A promising approach. Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef]
- Mendecki, J.; Friedenthal, E.; Botstein, C.; Sterzer, F.; Paglione, R.; Nowogrodzki, M.; Beck, E. Microwave-induced hyperthermia in cancer treatment: Apparatus and preliminary results. Int. J. Radiat. Oncol. Biol. Phys. 1978, 4, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Saniei, N. Hyperthermia and cancer treatment. Heat Transf. Eng. 2009, 30, 915–917. [Google Scholar] [CrossRef]
- Kim, K.; Seo, T.; Sim, K.; Kwon, Y. Magnetic nanoparticle-assisted microwave hyperthermia using an active integrated heat applicator. IEEE Trans. Microw. Theory Tech. 2016, 64, 2184–2197. [Google Scholar] [CrossRef]
- Falk, M.H.; Issels, R.D. Hyperthermia in oncology. Int. J. Hyperth. 2001, 17, 1–18. [Google Scholar] [CrossRef]
- Perez, C.A.; Gillespie, B.; Pajak, T.; Hornback, N.B.; Emami, D.; Rubin, P. Quality assurance problems in clinical hyperthermia and their impact on therapeutic outcome: A report by the Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Frew, D.G.; Dobson, J.M.; Stenning, S.P.; Bleehen, N.M. Response of 145 spontaneous canine head and neck tumours to radiation versus radiation plus microwave hyperthermia: Results of a randomized phase III clinical study. Int. J. Hyperth. 1995, 11, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef]
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Torres-Reveron, J.; Tomasiewicz, H.C.; Shetty, A.; Amankulor, N.M.; Chiang, V.L. Stereotactic laser induced thermotherapy (LITT): A novel treatment for brain lesions regrowing after radiosurgery. J. Neurooncol. 2013, 113, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Eckert, R.; Naguib, N.N.; Beeres, M.; Gruber-Rouh, T.; Nour-Eldin, N.E.A. Thermal ablation of colorectal lung metastases: Retrospective comparison among laser-induced thermotherapy, radiofrequency ablation, and microwave ablation. AJR. Am. J. Roentgenol. 2016, 207, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.C.; Johannessen, A.; Raatikainen, P.; Hindricks, G.; Walfridsson, H.; Kongstad, O.; Pehrson, S.; Englund, A.; Hartikainen, J.; Mortensen, L.S.; et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N. Engl. J. Med. 2012, 367, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Stang, J.; Haynes, M.; Carson, P.; Moghaddam, M. A preclinical system prototype for focused microwave thermal therapy of the breast. IEEE Trans. Biomed. Eng. 2012, 59, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Abbosh, A.; Crozier, S. Microwave hyperthermia for breast cancer treatment using electromagnetic and thermal focusing tested on realistic breast models and antenna arrays. IEEE Trans. Antennas Propag. 2015, 63, 4426–4434. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Abbosh, A.M.; Crozier, S. 3-D focused microwave hyperthermia for breast cancer treatment with experimental validation. IEEE Trans. Antennas Propag. 2017, 65, 3489–3500. [Google Scholar] [CrossRef]
- Bellizzi, G.G.; Crocco, L.; Battaglia, G.M.; Isernia, T. Multi-frequency constrained SAR focusing for patient specific hyperthermia treatment. IEEE J. Electromagn. RF Microw. Med. Biol. 2017, 1, 74–80. [Google Scholar] [CrossRef]
- Takook, P.; Persson, M.; Trefná, H.D. Performance evaluation of hyperthermia applicators to heat deep-seated brain tumors. IEEE J. Electromagn. RF Microw. Med. Biol. 2018, 2, 18–24. [Google Scholar] [CrossRef]
- Mahmoud, K.R.; Montaser, A.M. Design of multiresonance flexible antenna array applicator for breast cancer hyperthermia treatment. IEEE Access 2022, 10, 93338–93352. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Abbosh, A.; Crozier, S. Three-dimensional microwave hyperthermia for breast cancer treatment in a realistic environment using particle swarm optimization. IEEE Trans. Biomed. Eng. 2017, 64, 1335–1344. [Google Scholar] [CrossRef]
- He, X.; Geyi, W.; Wang, S. A Hexagonal focused array for microwave hyperthermia: Optimal design and experiment. IEEE Trans. Antennas Propag. 2016, 15, 56–59. [Google Scholar] [CrossRef]
- Iero, D.A.M.; Crocco, L.; Isernia, T. Thermal and microwave constrained focusing for patient-specific breast cancer hyperthermia: A robustness assessment. IEEE Trans. Antennas Propag. 2014, 62, 814–821. [Google Scholar] [CrossRef]
- Brizi, D.; Fontana, N.; Giovannetti, G.; Menichetti, L.; Cappiello, L.; Doumett, S.; Ravagli, C.; Baldi, G.; Monorchio, A. A Radiating system for low-frequency highly focused hyperthermia with magnetic nanoparticles. IEEE J. Electromagn. RF Microw. Med. Biol. 2020, 4, 109–116. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; Haar, G.T.; Wust, P. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X. Comparison of two optimization algorithms for focused microwave breast cancer hyperthermia. In Proceedings of the 2018 International Applied Computational Electromagnetics Society (ACES) Symposium, Beijing, China, 29 July–1 August 2018. [Google Scholar]
- Xu, L.; Wang, X. Focused microwave breast hyperthermia monitored by thermoacoustic imaging: A computational feasibility study applying realistic breast phantoms. IEEE J. Electromagn. RF Microw. Med. Biol. 2020, 4, 81–88. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; Zhang, D.; Li, C.; Zhu, Y.; Zou, Y.; Chen, B.; Wu, T.; Wang, X. A preclinical system prototype for focused microwave breast hyperthermia guided by compressive thermoacoustic tomography. IEEE Trans. Biomed. Eng. 2021, 68, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Androulakis, I.; Sumser, K.; Machielse, M.N.D.; Koppert, L.; Jager, A.; Nout, R.; Franckena, M.; van Rhoon, G.C.; Curto, S. Patient-derived breast model repository, a tool for hyperthermia treatment planning and applicator design. Int. J. Hyperth. 2022, 39, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xiong, N.; Sun, Y.; Zhang, L.; Li, C.; Li, J.; Wang, Z.; Chen, Z.; Zhang, Y.; Wang, X. Microwave-induced thermoacoustic imaging of small animals applying scanning orthogonal polarization excitation. IEEE J. Electromagn. RF Microw. Med. Biol. 2022, 6, 123–130. [Google Scholar] [CrossRef]
- He, Y.; Liu, C.; Lin, L.; Wang, L.V. Comparative effects of linearly and circularly polarized illumination on microwave-induced thermoacoustic tomography. IEEE Antennas Wirel. Propag. Lett. 2017, 16, 1593–1596. [Google Scholar] [CrossRef]
- He, Y.; Shen, Y.; Feng, X.; Liu, C.; Wang, L.V. Homogenizing microwave illumination in thermoacoustic tomography by a linear-to-circular polarizer based on frequency selective surfaces. Appl. Phys. Lett. 2017, 111, 063703. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Wang, X. A computational study on number of elements in antenna array for focused microwave breast hyperthermia. In Proceedings of the 2019 IEEE MTTS International Microwave Biomedical Conference (IMBioC), Nanjing, China, 6–8 May 2019; pp. 1–3. [Google Scholar]
- Nemez, K.; Baran, A.; Asefi, M.; LoVetri, J. Modeling error and calibration techniques for a faceted metallic chamber for magnetic field microwave imaging. IEEE Trans. Microw. Theory Techn. 2017, 65, 4347–4356. [Google Scholar] [CrossRef]
- Wu, D.; Huang, L.; Jiang, M.; Jiang, H. Contrast agents for photoacoustic and thermoacoustic imaging: A review. Int. J. Mol. Sci. 2014, 15, 23616–23639. [Google Scholar] [CrossRef] [PubMed]
- Bialkowski, M.E. Ultra wideband microwave system with novel image reconstruction strategies for breast cancer detection. In Proceedings of the 40th European Microwave Conference, Paris, France, 28–30 September 2010; pp. 537–540. [Google Scholar]
- Razzicchia, E.; Lu, P.; Guo, W.; Karadima, O.; Sotiriou, I.; Ghavami, N.; Kallos, E.; Palikaras, G.; Kosmas, P. Metasurface-enhanced antennas for microwave brain imaging. Diagnostics 2021, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Wang, X.; Ye, K.; Wang, X. Performance evaluation of focused microwave brain hyperthermia guided by microwave-induced thermoacoustic tomography. IEEE J. Electromagn. RF Microw. Med. Biol. 2023, 7, 383–391. [Google Scholar] [CrossRef]
- Kim, I.; Kim, E. Quad-Band Uniformly Spaced Array Antenna Using Diverse Patch and Fractal Antennas. Appl. Sci. 2023, 13, 3675. [Google Scholar] [CrossRef]
- Meerabeab, S.; Wounchoum, P.; Jantarachote, V. A suspended conformal patch antenna for a constant-gain beam-switching phased antenna array. AEU—Int. J. Electron. C. 2023, 161, 154538. [Google Scholar] [CrossRef]
- Wang, X.; Qin, T.; Witte, R.S.; Xin, H. Computational feasibility study of contrast-enhanced thermoacoustic imaging for breast cancer detection using realistic numerical breast phantoms. IEEE Trans. Microw. Theory Techn. 2015, 63, 1489–1501. [Google Scholar] [CrossRef]
- Zastrow, E.; Davis, S.K.; Lazebnik, M.; Kelcz, F.; Van Veem, B.D.; Hagness, S.C. Database of 3D Grid-Based Numerical Breast Phantoms for Use in Computational Electromagnetics Simulations; University of Wisconsin–Madison: Madison, WI, USA, 2007; Available online: http://uwcem.ece.wisc.edu/MRIdatabase/ (accessed on 1 November 2023).
- Yoo, D.-S. The dielectric properties of cancerous tissues in a nude mouse xenograft model. Bioelectromagnetics 2004, 25, 492–497. [Google Scholar] [CrossRef]
- Sinkus, R.; Tanter, M.; Bercoff, J.; Siegmann, K.; Pernot, M.; Athanasiou, A.; Fink, M. Potential of MRI and ultrasound radiation force in elastography: Applications to diagnosis and therapy. Proc. IEEE 2008, 96, 490–499. [Google Scholar] [CrossRef]
- Pernot, M.; Tanter, M.; Bercoff, J.; Waters, K.R.; Fink, M. Temperature estimation using ultrasonic spatial compound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, Y.; Wang, Z.; Wang, X. Three-dimensional microwave-induced thermoacoustic imaging based on compressive sensing using an analytically constructed dictionary. IEEE Trans. Microw. Theory Techn. 2020, 68, 377–386. [Google Scholar] [CrossRef]
- Price, K.; Storn, R. Differential evolution—A simple and efficient heuristic for global optimization over continuous spaces. J. Glob. Optim. 1997, 11, 341–359. [Google Scholar]
- Cappiello, G.; McGinley, B.; Elahi, M.A.; Drizdal, T.; Paulides, M.M.; Glavin, M.; O’Halloran, M.; Jones, E. Differential evolution optimization of the SAR distribution. IEEE Trans. Biomed. Eng. 2017, 64, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- CST GmbH. CST Users’ Manuals. Available online: www.cst.com (accessed on 1 November 2023).
- Xu, K.; Qian, Z.; Zhong, Y.; Su, J.; Gao, H.; Li, W. Learning-assisted inversion for solving nonlinear inverse scattering problem. IEEE Trans. Microw. Theory Techn. 2023, 71, 2384–2395. [Google Scholar] [CrossRef]
- Ostadrahimi, M.; Zakaria, A.; LoVetri, J.; Shafai, L. A Near-field dual polarized (TE–TM) microwave imaging system. IEEE Trans. Microw. Theory Techn. 2013, 61, 1376–1384. [Google Scholar] [CrossRef]
- Xi, Z.; Feng, J.; Ye, K.; Liu, D.; Wang, X.; Wang, X.; Wang, X. A Preclinical system prototype and experimental validation of focused microwave brain hyperthermia. IEEE Trans. Microw. Theory Techn. 2024, in press. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xi, Z.; Ye, K.; Gong, Z.; Chen, Y.; Wang, X. Improvement of Phased Antenna Array Applied in Focused Microwave Breast Hyperthermia. Sensors 2024, 24, 2682. https://doi.org/10.3390/s24092682
Wang X, Xi Z, Ye K, Gong Z, Chen Y, Wang X. Improvement of Phased Antenna Array Applied in Focused Microwave Breast Hyperthermia. Sensors. 2024; 24(9):2682. https://doi.org/10.3390/s24092682
Chicago/Turabian StyleWang, Xuanyu, Zijun Xi, Ke Ye, Zheng Gong, Yifan Chen, and Xiong Wang. 2024. "Improvement of Phased Antenna Array Applied in Focused Microwave Breast Hyperthermia" Sensors 24, no. 9: 2682. https://doi.org/10.3390/s24092682



