Hardware and Software Setup for Quantitative 23Na Magnetic Resonance Imaging at 3T: A Phantom Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coil Sensitivity Measurement
2.2. MRI Acquisition Protocol and Postprocessing of the 23Na Sequence
3. Results
3.1. Coil Sensitivity Measurement
3.2. Repeatability Analysis of Sodium Measurement Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berendsen, H.J.; Edzes, H.T. The observation and general interpretation of sodium magnetic resonance in biological material. Ann. N. Y. Acad. Sci. 1973, 204, 459–485. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, J.A.; Magnuson, N.S. NMR studies of sodium and potassium in various biological tissues. Ann. N. Y. Acad. Sci. 1973, 204, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, D.A.; Crooks, L.A.; Kaufman, L.; Brant-Zawadzki, M.; Posin, J.P.; Arakawa, M.; Watts, J.C.; Hoenninger, J. Magnetic resonance imaging performance: A comparison of sodium and hydrogen. Radiology 1985, 156, 133–138. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Hilal, S.K. Biological aspects of sodium-23 imaging. Br. Med. Bull. 1984, 40, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Moseley, M.E.; Chew, W.M.; Nishimura, M.C.; Richards, T.L.; Murphy-Boesch, J.; Young, G.B.; Marschner, T.M.; Pitts, L.H.; James, T.L. In vivo sodium-23 magnetic resonance surface coil imaging: Observing experimental cerebral ischemia in the rat. Magn. Reson. Imaging 1985, 3, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Ra, J.B.; Hilal, S.K.; Cho, Z.H. A method for in vivo MR imaging of the short T2 component of sodium-23. Magn. Reson. Med. 1986, 3, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Granot, J. Sodium imaging of human body organs and extremities in vivo. Radiology 1988, 167, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Thulborn, K.R. Quantitative sodium MR imaging: A review of its evolving role in medicine. Neuroimage 2018, 168, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Poku, L.O.; Phil, M.; Cheng, Y.; Wang, K.; Sun, X. 23Na -MRI as a Noninvasive Biomarker for Cancer Diagnosis and Prognosis. J. Magn. Reson. Imaging 2021, 53, 995–1014. [Google Scholar] [CrossRef]
- Boada, F.E.; Qian, Y.; Nemoto, E.; Jovin, T.; Jungreis, C.; Jones, S.C.; Weimer, J.; Lee, V. Sodium MRI and the assessment of irreversible tissue damage during hyper-acute stroke. Transl. Stroke Res. 2012, 3, 236–245. [Google Scholar] [CrossRef]
- Weber, C.E.; Nagel, K.; Ebert, A.; Roßmanith, C.; Paschke, N.; Adlung, A.; Platten, M.; Schad, L.R.; Gass, A.; Eisele, P. Diffusely appearing white matter in multiple sclerosis: Insights from sodium (23Na) MRI. Mult. Scler. Relat. Disord. 2021, 49, 102752. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Herrmann, K.; Adlung, A.; Paschke, N.; Hausner, L.; FrÖlich, L.; Schad, L.; Groden, C.; Kerl, H.U. Evaluation of Sodium (23Na) MR-imaging as a Biomarker and Predictor for Neurodegenerative Changes in Patients With Alzheimer’s Disease. In Vivo 2021, 35, 429–435. [Google Scholar] [CrossRef]
- Coppini, R.; Ferrantini, C.; Mazzoni, L.; Sartiani, L.; Olivotto, I.; Poggesi, C.; Cerbai, E.; Mugelli, A. Regulation of intracellular Na+ in health and disease: Pathophysiological mechanisms and implications for treatment. Glob. Cardiol. Sci. Pract. 2013, 2013, 222–242. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.; Stobbe, R.W.; Bhagat, Y.A.; Emery, D.; Butcher, K.S.; Manawadu, V.; Rizvi, N.; Maheshwari, P.; Scozzafava, J.; Shuaib, A.; et al. Sodium imaging intensity increases with time after human ischemic stroke. Ann. Neurol. 2009, 66, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sandstede, J.J.W.; Hillenbrand, H.; Beer, M.; Pabst, T.; Butter, F.; Machann, W.; Bauer, W.; Hahn, D.; Neubauer, S. Time course of 23Na signal intensity after myocardial infarction in humans. Magn. Reson. Med. 2004, 52, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, R.; Bottomley, P.A.; Solaiyappan, M.; Spooner, A.E.; Tomaselli, G.F.; Wu, K.C.; Weiss, R.G. Tissue sodium concentration in myocardial infarction in humans: A quantitative 23Na MR imaging study. Radiology 2008, 248, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.; Weidensteiner, C.; Scheffer, H.; Meininger, M.; de Groot, M.; Remkes, H.; Dienesch, C.; Przyklenk, K.; von Kienlin, M.; Neubauer, S. Detection of myocardial viability based on measurement of sodium content: A (23)Na-NMR study. Magn. Reson. Med. 2001, 45, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Dyke, J.P.; Meyring-Wösten, A.; Zhao, Y.; Linz, P.; Thijssen, S.; Kotanko, P. Reliability and agreement of sodium (23Na) MRI in calf muscle and skin of healthy subjects from the US. Clin. Imaging 2018, 52, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Thulborn, K.R.; Lu, A.; Atkinson, I.C.; Damen, F.; Villano, J.L. Quantitative Sodium MR Imaging and Sodium Bioscales for the Management of Brain Tumors. Neuroimaging Clin. N. Am. 2009, 19, 615–624. [Google Scholar] [CrossRef]
- Zaric, O.; Juras, V.; Szomolanyi, P.; Schreiner, M.; Raudner, M.; Giraudo, C.; Trattnig, S. Frontiers of Sodium MRI Revisited: From Cartilage to Brain Imaging. J. Magn. Reson. Imaging 2021, 54, 58–75. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Adlung, A.; Ruder, A.M.; Hoesl, M.A.U.; Schad, L.; Groden, C.; Giordano, F.A.; Neumaier-Probst, E. MRI Detection of Changes in Tissue Sodium Concentration in Brain Metastases after Stereotactic Radiosurgery: A Feasibility Study. J. Neuroimaging 2020, 31, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Mispelter, J.; Lupu, M.; Briguet, A. Nmr Probeheads for Biophysical and Biomedical Experiments: Theoretical Principles and Practical Guidelines; Imperial College Press: London, UK, 2006. [Google Scholar]
- Hartwig, V.; Giovannetti, G.; Vanello, N.; Landini, L.; Santarelli, M.F. Numerical Calculation of Peak-to-Average Specific Absorption Rate on Different Human Thorax Models for Magnetic Resonance Safety Considerations. Appl. Magn. Reson. 2010, 38, 337–348. [Google Scholar] [CrossRef]
- Jin, J. Electromagnetic Analysis and Design; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Bottomley, P.A. Sodium MRI in human heart: A review. NMR Biomed. 2015, 29, 187–196_2015. [Google Scholar] [CrossRef] [PubMed]
- Madelin, G.; Regatte, R.R. Biomedical applications of sodium MRI in vivo. J. Magn. Reson. Imaging 2013, 38, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Niesporek, S.C.; Hoffmann, S.H.; Berger, M.C.; Benkhedah, N.; Kujawa, A.; Bachert, P.; Nagel, A.M. Partial volume correction for in vivo 23Na -MRI data of the human brain. NeuroImage 2015, 112, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, G.; Valvano, G.; Virgili, G.; Giannoni, M.; Flori, A.; Frijia, F.; De Marchi, D.; Hartwig, V.; Landini, L.; Aquaro, G.D.; et al. Design and simulation of a dual-tuned 1H/23Na birdcage coil for MRS studies in human calf. Appl. Magn. Reson. 2015, 46, 1221–1238. [Google Scholar] [CrossRef]
- Riemer, F.; McHugh, D.; Zaccagna, F.; Lewis, D.; McLean, M.A.; Graves, M.J.; Gilbert, F.J.; Parker, G.J.M.; Gallagher, F.A. Measuring Tissue Sodium Concentration: Cross-Vendor Repeatability and Reproducibility of 23Na -MRI Across Two Sites. J. Magn. Reson. Imaging 2019, 50, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Freed, J.H.; Leniart, D. Theory of saturation and double resonance effects in ESR spectra: RF coherence and line shapes. J. Chem. Phys. 1967, 47, 2762–2773. [Google Scholar] [CrossRef]
- Giovannetti, G.; Frijia, F.; Menichetti, L.; Ardenkjaer-Larsen, J.H.; Hartwig, V.; De Marchi, D.; Positano, V.; Landini, L.; Lombardi, M.; Santarelli, M.F. Coil sensitivity estimation with perturbing sphere method: Application to 13C birdcages. App. Magn. Res. 2012, 42, 511–518. [Google Scholar] [CrossRef]
- Darrasse, L.; Kassab, G. Quick measurement of NMR-coil sensitivity with a dual-loop probe. Rev. Sci. Instrum. 1993, 64, 1841–1844. [Google Scholar] [CrossRef]
- Hoult, D.I. The principle of reciprocity in signal strength calculations—A mathematical guide. Concepts Magn. Reson. 2000, 12, 173–187. [Google Scholar] [CrossRef]
- Giovannetti, G.; Viti, V.; Liu, Y.; Yu, W.; Mittra, R.; Landini, L.; Benassi, A. An accurate simulator for magnetic resonance coil sensitivity estimation. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2008, 33, 209–215. [Google Scholar] [CrossRef]
- Hartwig, V.; Vanello, N.; Giovannetti, G.; De Marchi, D.; Lombardi, M.; Landini, L.; Santarelli, M.F. B1+/actual flip angle and reception sensitivity mapping methods: Simulation and comparison. Magn. Reson. Imaging 2011, 29, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Schulte, R.F.; Sacolick, L.; Deppe, M.H.; Janich, M.A.; Schwaiger, M.; Wild, J.M.; Wiesinger, F. Transmit gain calibration for non proton MR using the Bloch–Siegert shift. NMR Biomed. 2011, 24, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Gerhalter, T.; Gast, L.V.; Marty, B.; Uder, M.; Carlier, P.G.; Nagel, A.M. Assessing the variability of 23Na MRI in skeletal muscle tissue: Reproducibility and repeatability of tissue sodium concentration measurements in the lower leg at 3 T. NMR Biomed. 2020, 33, e4279. [Google Scholar] [CrossRef] [PubMed]
- Costea, A.; Boumezbeura, F.; Vignauda, A.; Madelinb, G.; Reetzc, K.; Le Bihana, D.; Rabrait-Lermana, C.; Romanzetti, S. Tissue sodium concentration and sodium T1 mapping of the human brain at 3 T using a Variable Flip Angle method. Magn. Reson. Imaging 2019, 58, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.J.; Nishimura, D.G.; Pauly, J.M. Rapid gridding reconstruction with a minimal oversampling ratio. IEEE Trans. Med. Imaging 2005, 24, 799–808. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Madelina, G.; Babbb, J.S.; Xiaa, D.; Changa, G.; Jerschowc, A.; Regattea, R.R. Reproducibility and Repeatability of Quantitative Sodium MRI In Vivo in Articular Cartilage at 3T and 7T. Magn. Reson. Med. 2012, 68, 841–849. [Google Scholar] [CrossRef]
- Wang, Y.; Tadimalla, S.; Rai, R.; Goodwin, J.; Foster, S.; Liney, G.; Holloway, L.; Haworth, A. Quantitative MRI: Defining repeatability, reproducibility and accuracy for prostate cancer imaging biomarker development. Magn. Reson. Imaging 2021, 77, 169–179. [Google Scholar] [CrossRef]
- Raunig, D.L.; McShane, L.M.; Pennello, G.; Gatsonis, C.; Carson, P.L.; Voyvodic, J.T.; Wahl, R.L.; Kurland, B.F.; Schwarz, A.J.; Gönen, M.; et al. Quantitative imaging biomarkers: A review of statistical methods for technical performance assessment. Stat. Methods Med. Res. 2015, 24, 27–67. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.M.; Haneder, S.; Konstandin, S.; Budjan, J.; Morelli, J.N.; Schad, L.R.; Kerl, H.U.; Schoenberg, S.O.; Kabbasch, C. Repeatability and reproducibility of cerebral 23Na imaging in healthy subjects. BMC Med. Imaging 2019, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Mirkes, C.C.; Hoffmann, J.; Shajan, G.; Pohmann, R.; Scheffler, K. High-resolution quantitative sodium imaging at 9.4 Tesla. Magn. Reson. Med. 2015, 73, 342–351. [Google Scholar] [CrossRef]
- Constantinides, C.D.; Gillen, J.S.; Boada, F.E.; Pomper, M.G.; Bottomley, P.A. Human skeletal muscle: Sodium MR imaging and quantification-potential applications in exercise and disease. Radiology 2000, 216, 559–568. [Google Scholar] [CrossRef] [PubMed]


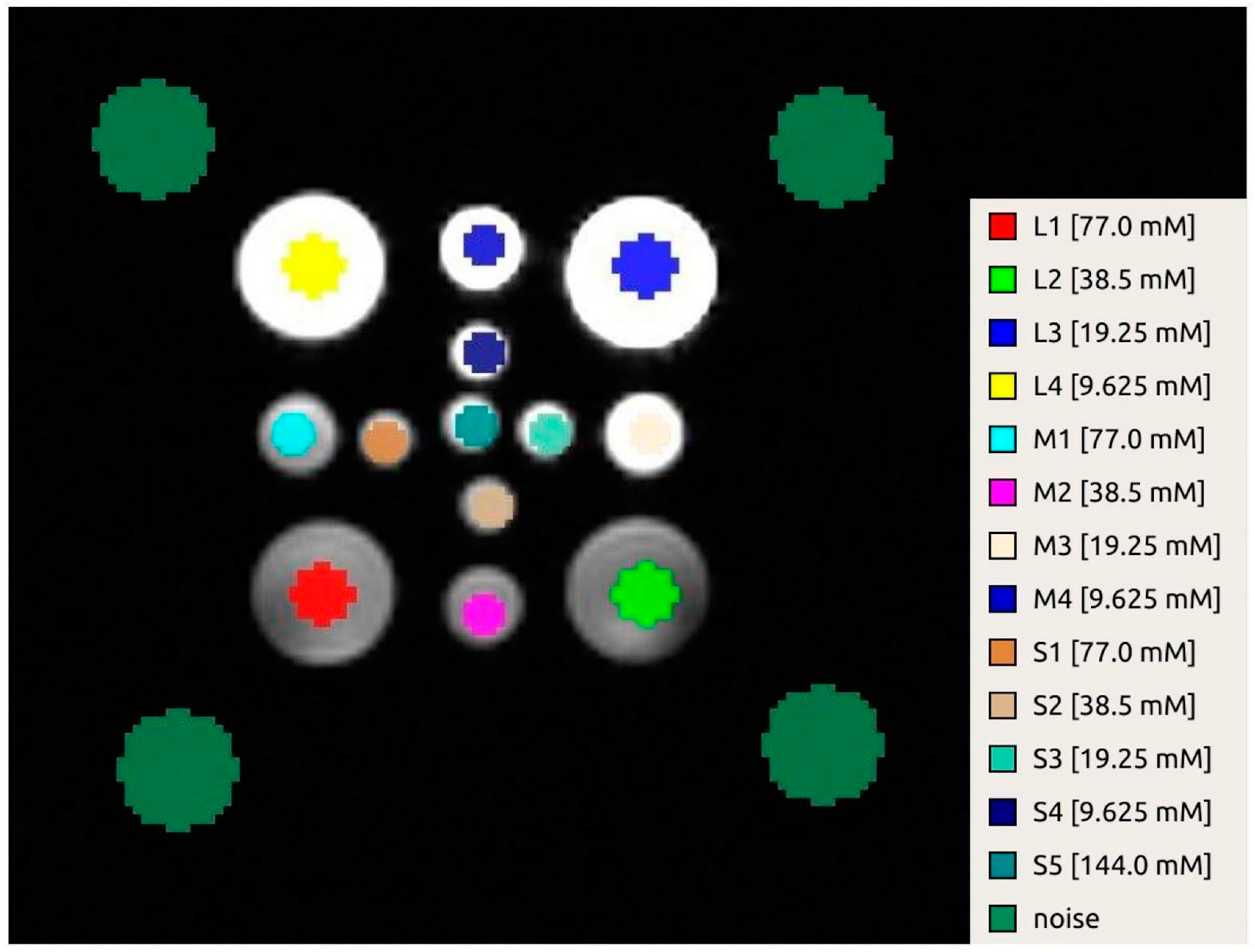
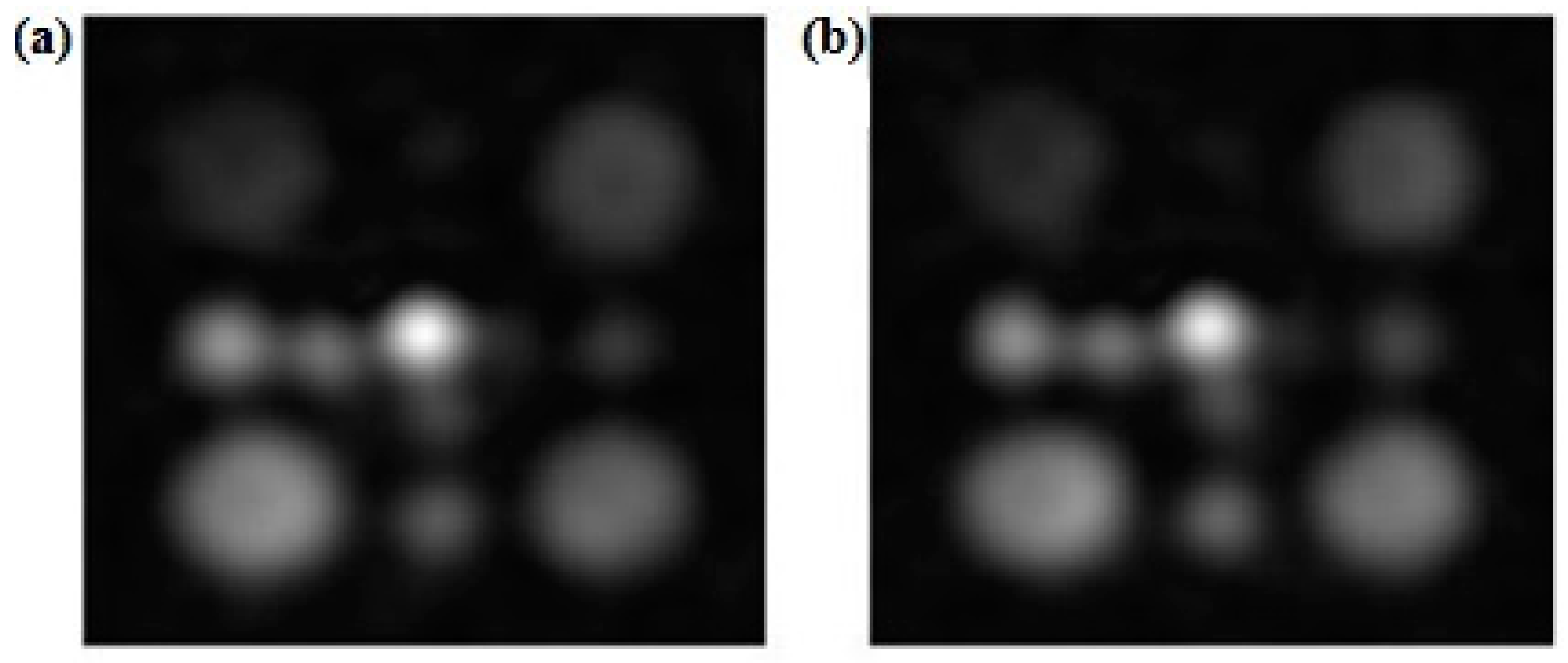
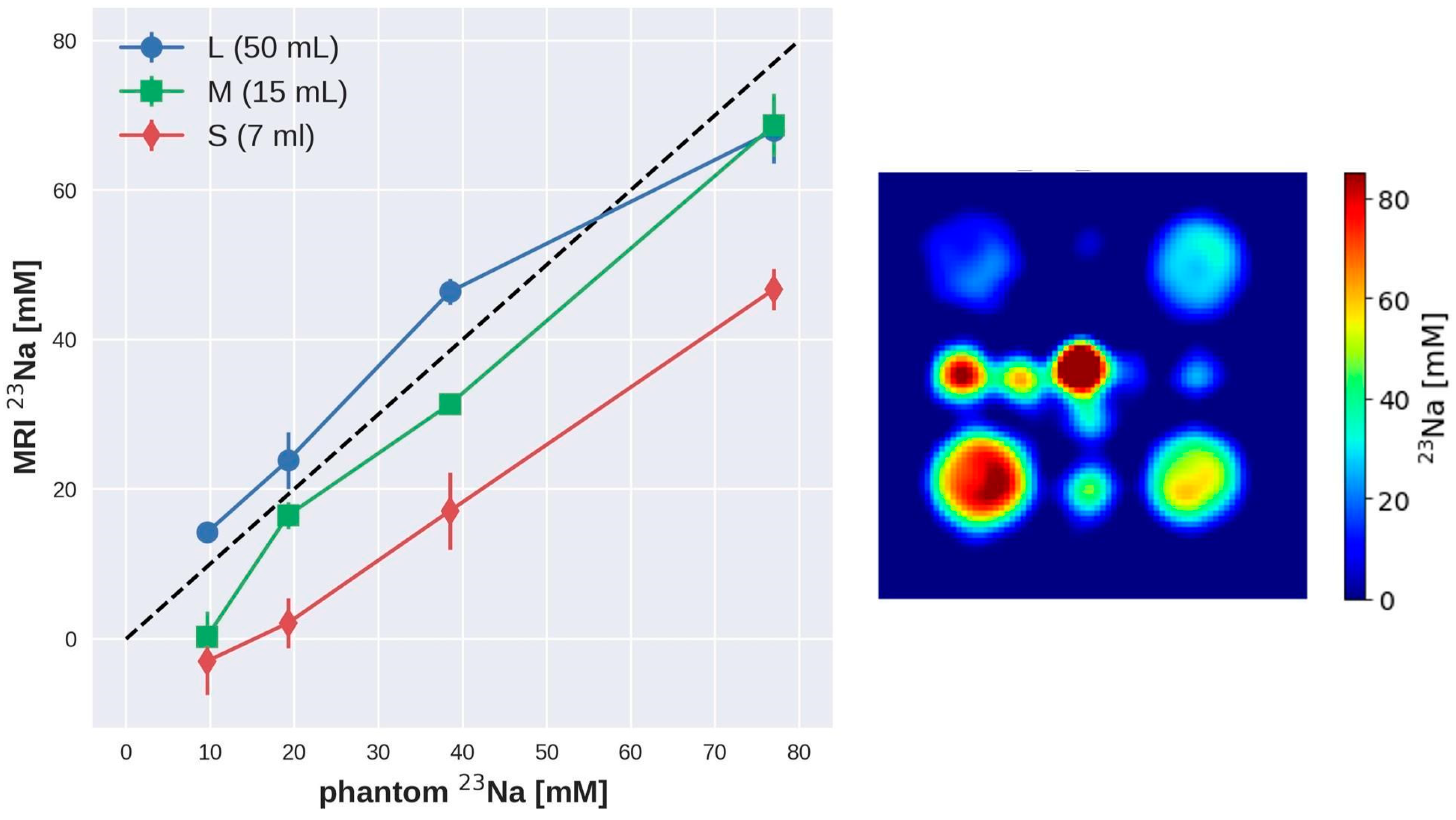
| SNR 1 Day | SNR 2 Day | SNR 3 Day | |
|---|---|---|---|
| FA = 15° | 70 | 61 | 60 |
| FA = 30° | 79 | 65 | 64 |
| Vial Size | Nominal Concentration [mM] | Estimated Concentration [mM] FA 15° | Estimated Concentration [mM] FA 30° |
|---|---|---|---|
| Large | 77 | 61.5 ± 2.4 | 68.4 ± 2.1 |
| Large | 38.5 | 52.5 ± 0.6 | 46.4 ± 1.0 |
| Large | 19.25 | 25.6 ± 2.2 | 23.6 ± 1.7 |
| Large | 9.625 | 10.5 ± 1.4 | 13.5 ± 1.1 |
| Medium | 77 | 55.5 ± 1.4 | 67.1 ± 2.9 |
| Medium | 38.5 | 32.6 ± 3.5 | 31.0 ± 0.7 |
| Medium | 19.25 | 19.5 ± 1.4 | 15.3 ± 1.9 |
| Medium | 9.625 | 2.0 ± 2.0 | 0.8 ± 2.0 |
| Small | 77 | 39.2 ± 3.5 | 45.7 ± 2.4 |
| Small | 38.5 | 17.5 ± 2.3 | 17.4 ± 2.3 |
| Small | 19.25 | 4.6 ± 5.1 | 2.2 ± 2.0 |
| Small | 9.625 | −3.1 ± 1.2 | −3.1 ± 2.0 |
| Small (Central) | 154 | 96.9 ± 6.3 | 112.8 ± 6.6 |
| INTRA-DAY Repeatability | INTER-DAY Repeatability | |||
|---|---|---|---|---|
| Vial | CV(%) FA 15° | CV(%) FA 30° | CV(%) FA 15° | CV(%) FA 30° |
| L_77 | 1.06 | 1.69 | 4.98 | 3.96 |
| L_38.5 | 1.48 | 2.27 | 1.44 | 2.27 |
| L_19.3 | 3.60 | 4.90 | 6.34 | 9.72 |
| L_9.6 | 11.89 | 5.63 | 13.56 | 4.85 |
| M_77 | 0.88 | 2.78 | 3.30 | 3.77 |
| M_38.5 | 6.56 | 2.96 | 12.97 | 0.62 |
| M_19.3 | 7.92 | 17.57 | 2.66 | 6.68 |
| M_9.6 | >50.0 | >50.0 | >50.0 | >50.0 |
| S_77 | 6.27 | 5.70 | 4.04 | 3.61 |
| S_38.5 | 9.62 | 4.37 | 9.20 | 18.52 |
| S_19.3 | >50.0 | >50.0 | >50.0 | >50.0 |
| S_9.6 | 11.92 | >50.0 | 44.50 | >50.0 |
| Centre_154 | 0.57 | 4.76 | 9.19 | 4.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannetti, G.; Flori, A.; Martini, N.; Cademartiri, F.; Aquaro, G.D.; Pingitore, A.; Frijia, F. Hardware and Software Setup for Quantitative 23Na Magnetic Resonance Imaging at 3T: A Phantom Study. Sensors 2024, 24, 2716. https://doi.org/10.3390/s24092716
Giovannetti G, Flori A, Martini N, Cademartiri F, Aquaro GD, Pingitore A, Frijia F. Hardware and Software Setup for Quantitative 23Na Magnetic Resonance Imaging at 3T: A Phantom Study. Sensors. 2024; 24(9):2716. https://doi.org/10.3390/s24092716
Chicago/Turabian StyleGiovannetti, Giulio, Alessandra Flori, Nicola Martini, Filippo Cademartiri, Giovanni Donato Aquaro, Alessandro Pingitore, and Francesca Frijia. 2024. "Hardware and Software Setup for Quantitative 23Na Magnetic Resonance Imaging at 3T: A Phantom Study" Sensors 24, no. 9: 2716. https://doi.org/10.3390/s24092716






