Development of a Rotation-Robust PPG Sensor for a Smart Ring
Abstract
1. Introduction
2. Methods
2.1. Monte Carlo Simulation
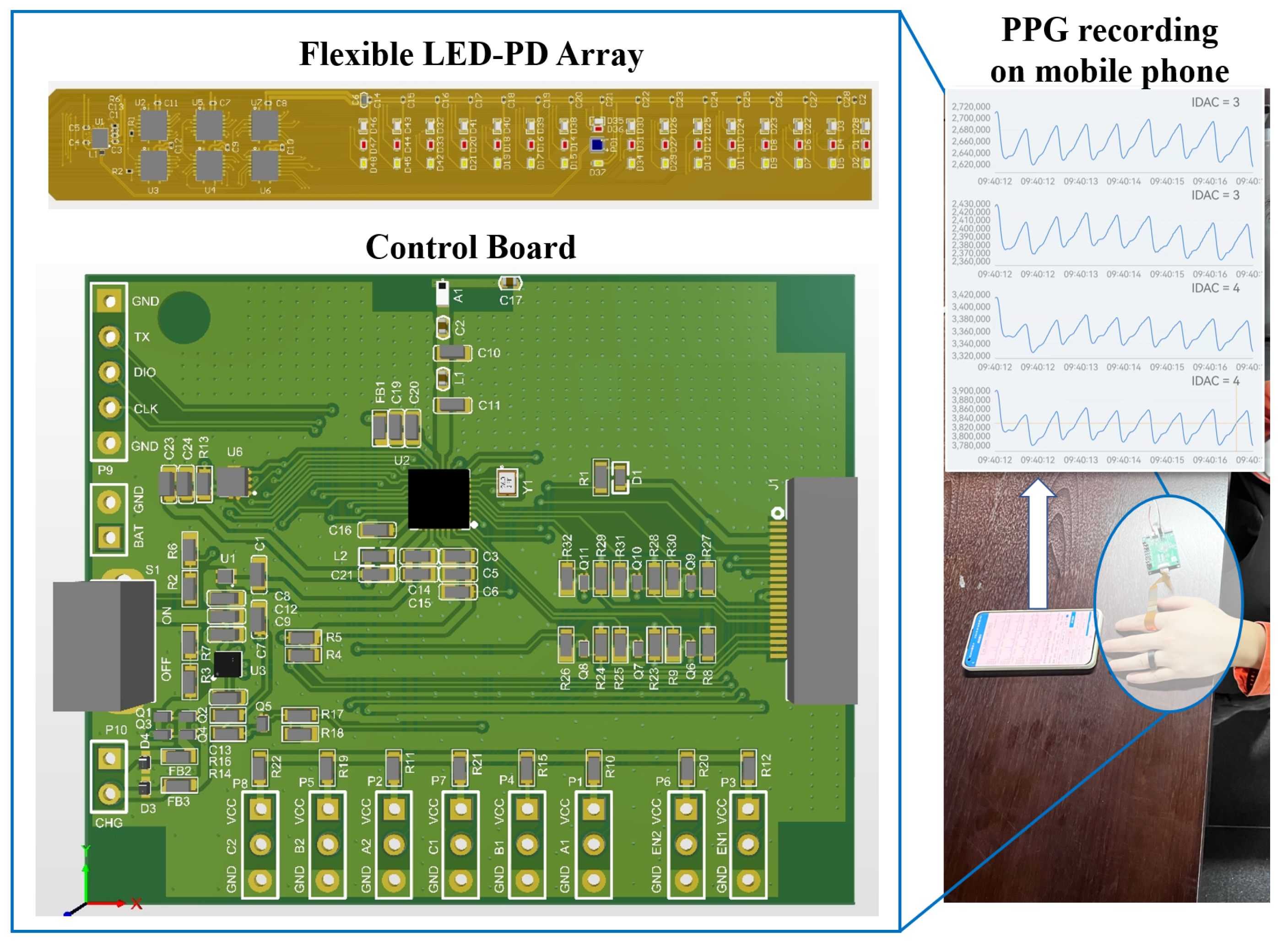
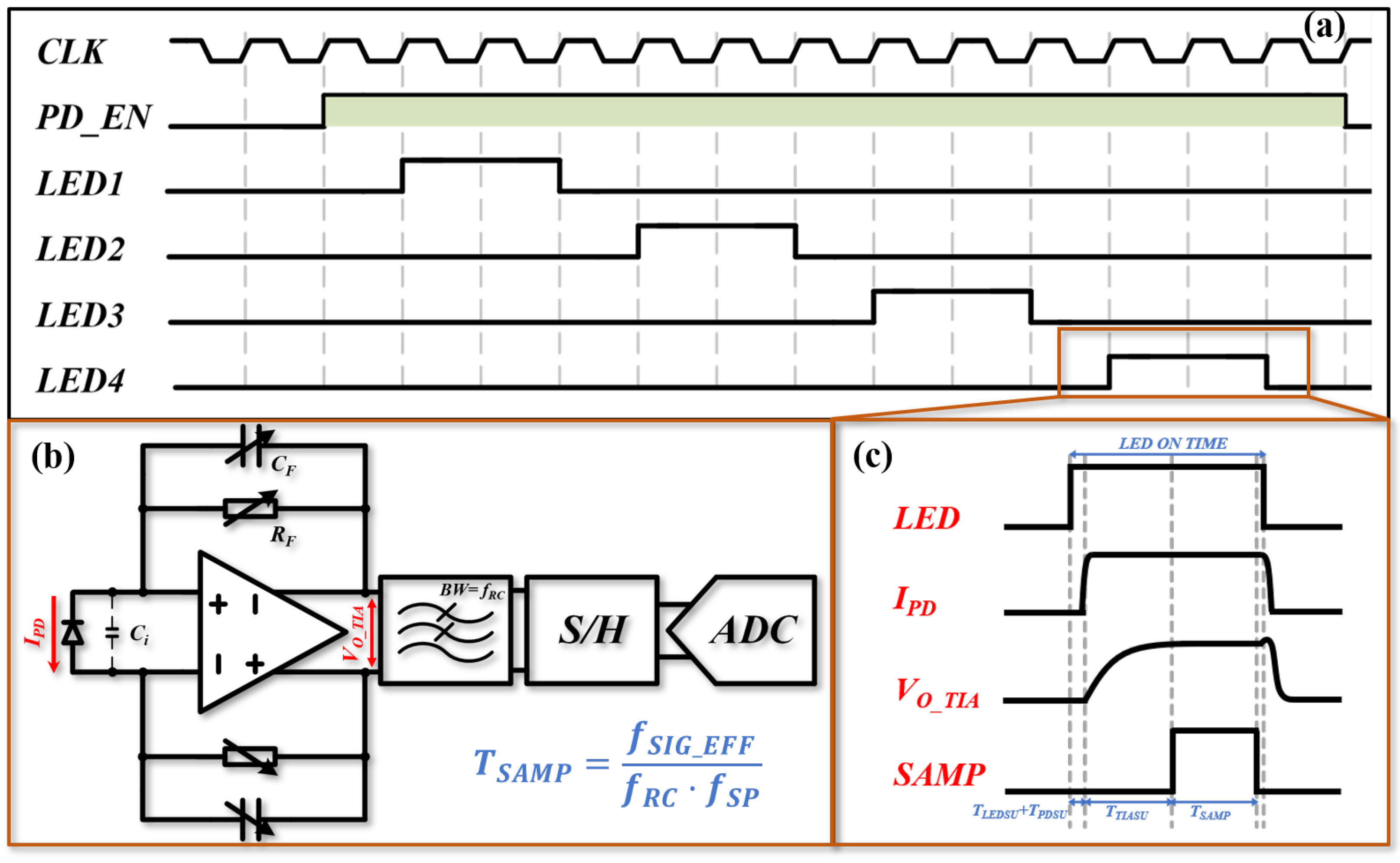
2.2. Test Platform
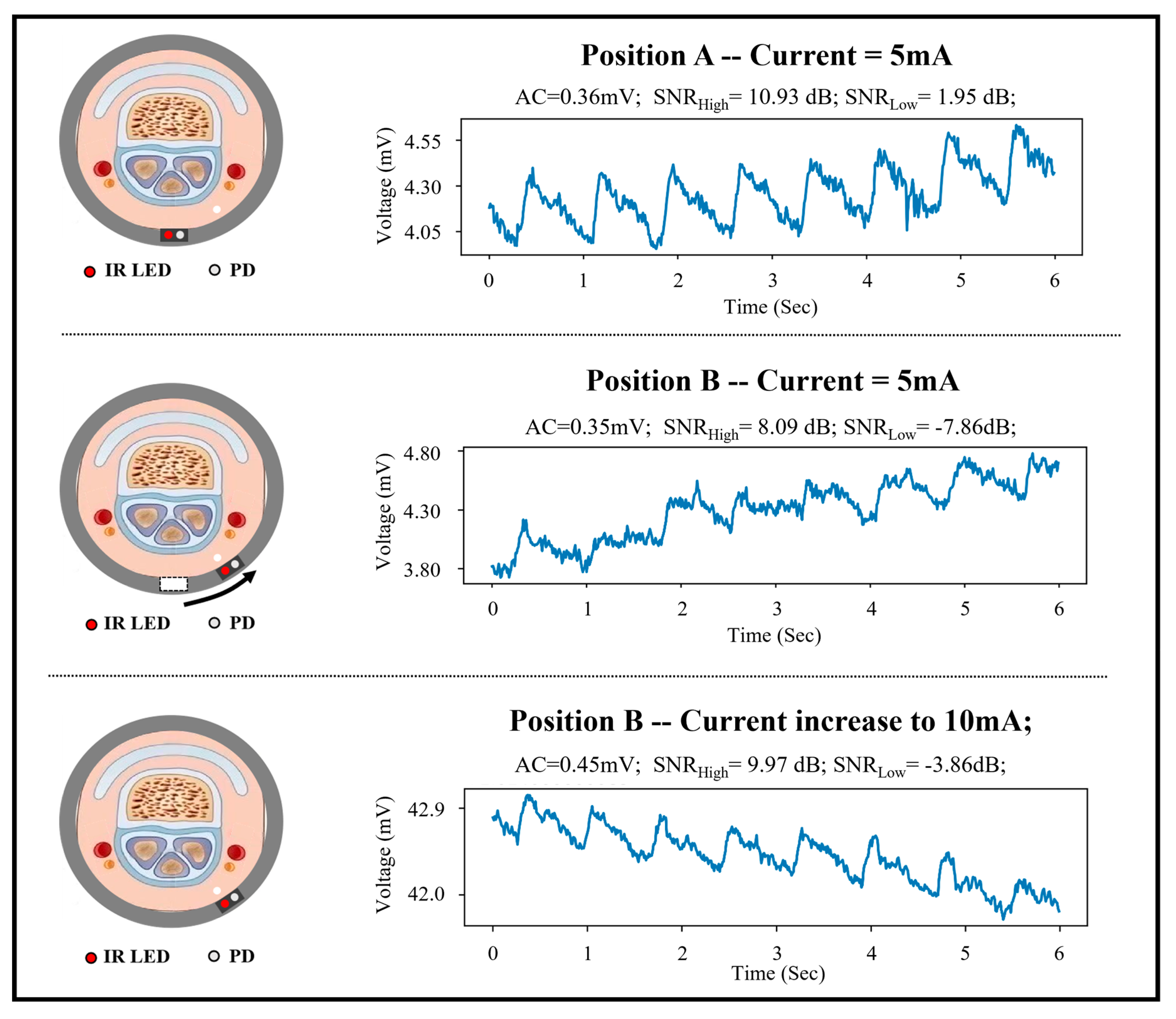
2.3. Signal Quality Indices
2.4. Signal Processing
3. Results
3.1. Finger Anatomy Analysis and Assumption
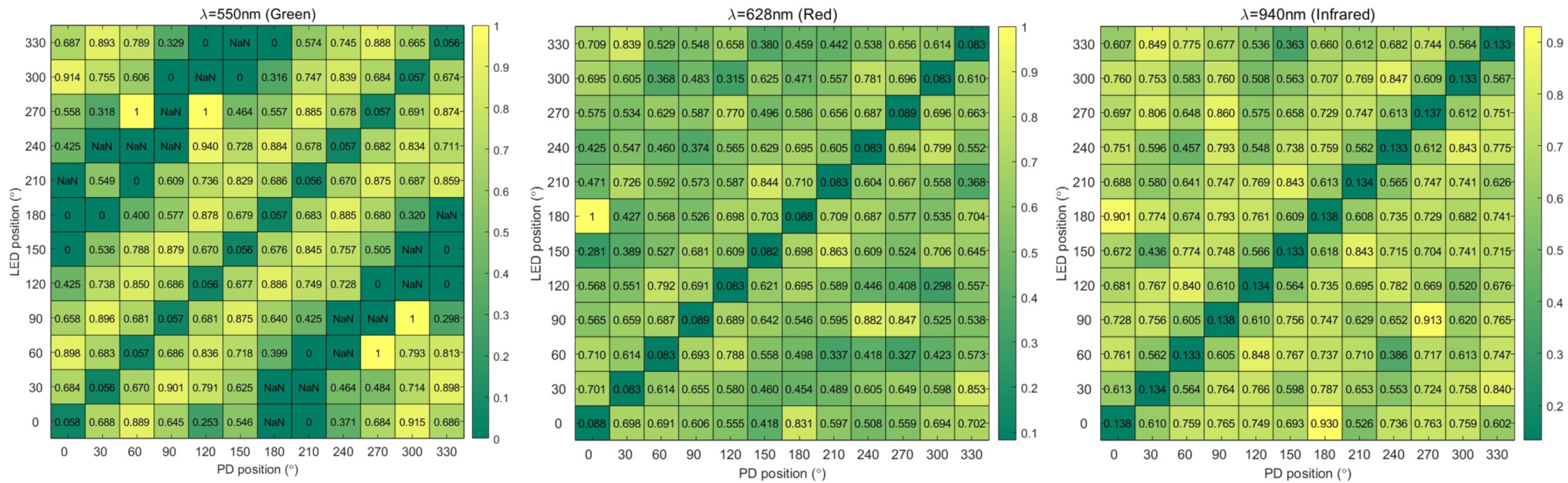
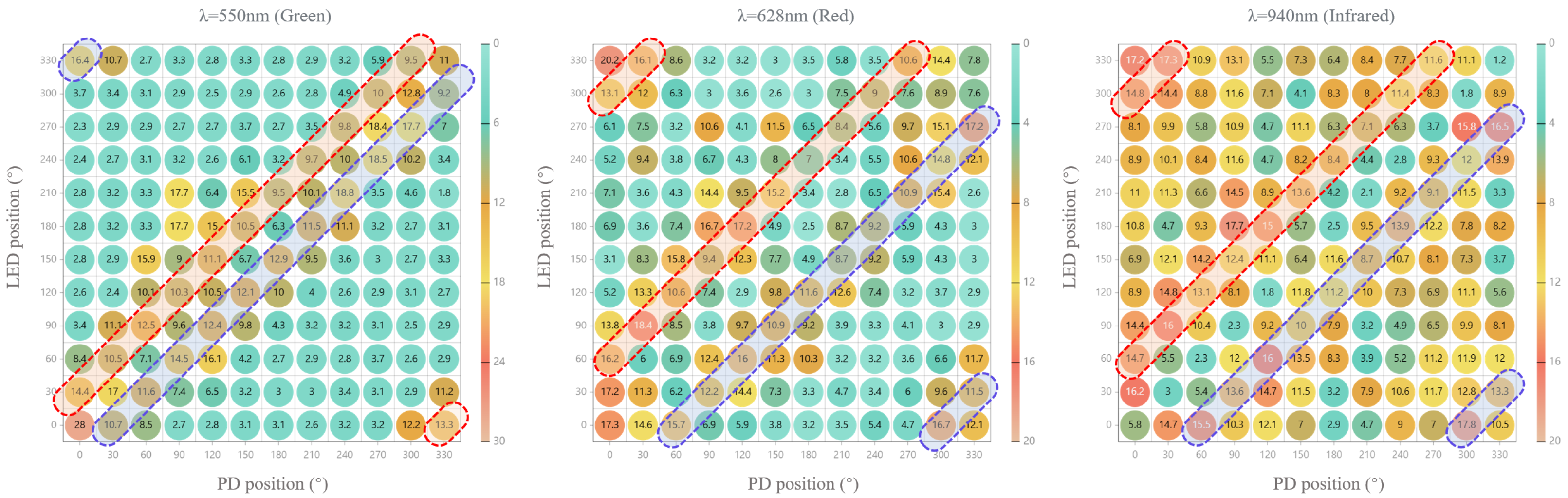
3.2. FoM Results
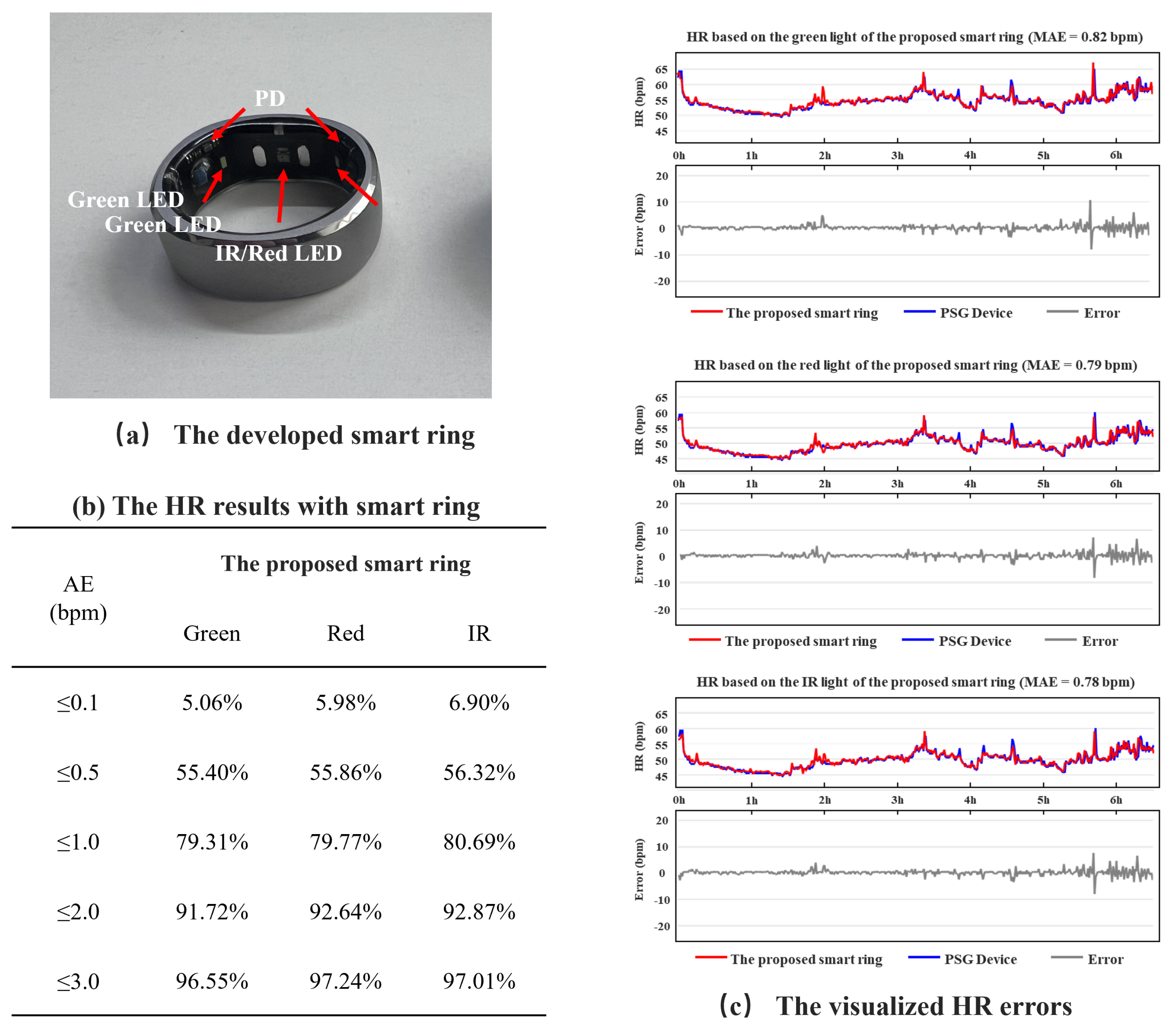
3.3. Performance Evaluation
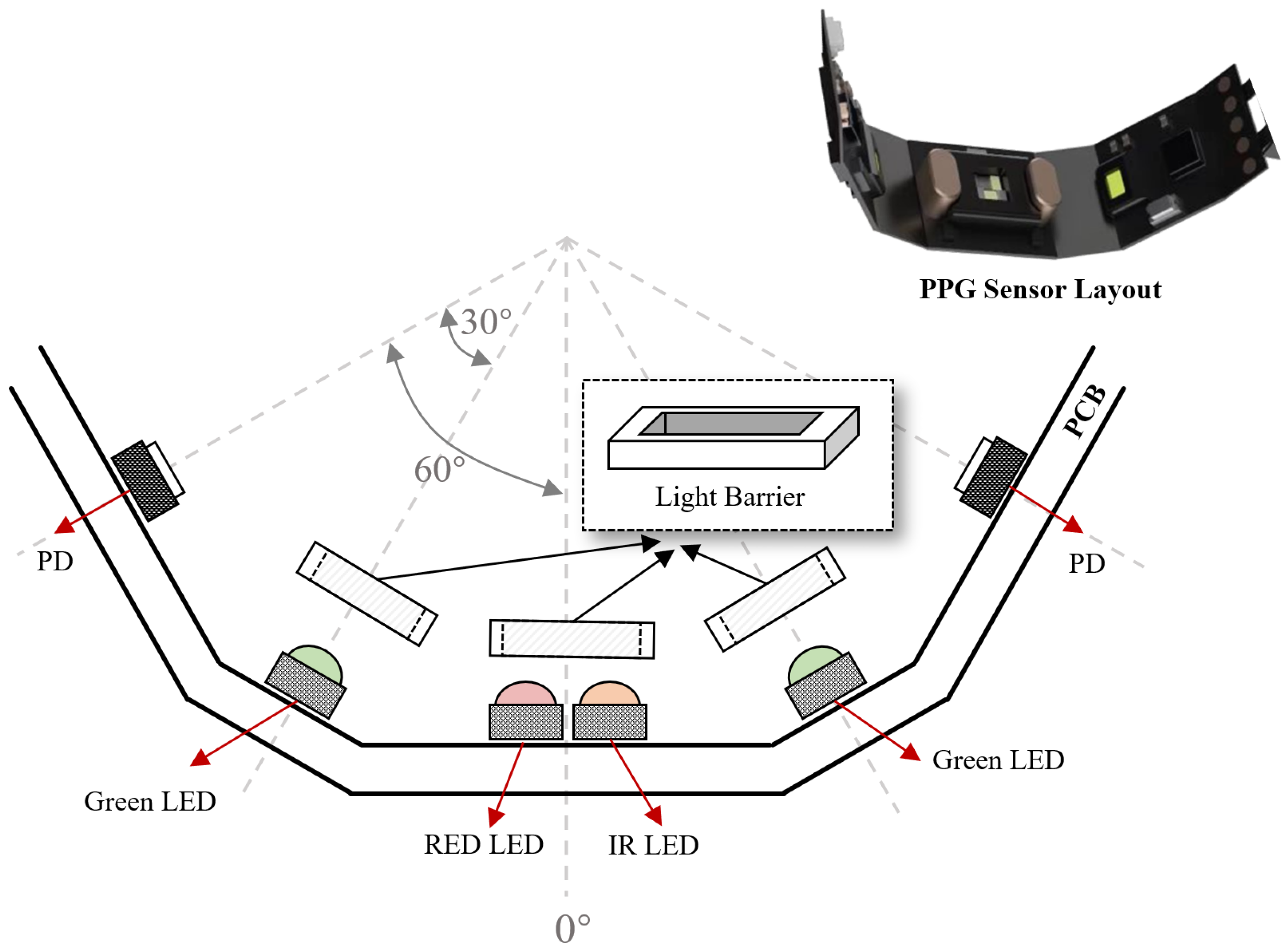
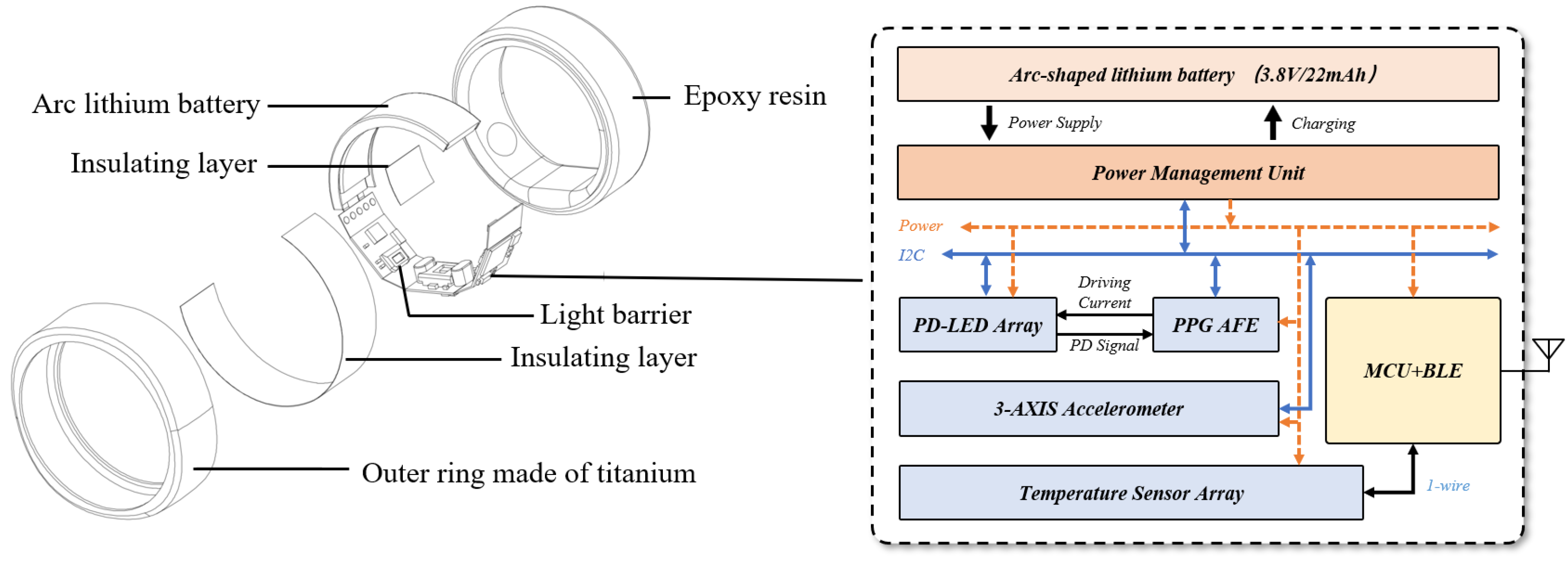
3.4. Design of the Smart Ring
3.5. Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deepu, C.J.; Zhang, X.; Heng, C.H.; Lian, Y. A 3-Lead ECG-on-Chip with QRS Detection and Lossless Compression for Wireless Sensors. IEEE Trans. Circuits Syst. II Express Briefs 2016, 63, 1151–1155. [Google Scholar] [CrossRef]
- Panula, T.; Sirkiä, J.P.; Kaisti, M. Continuous Blood Pressure Monitoring Using Nonpulsatile Photoplethysmographic Components for Low-Frequency Vascular Unloading. IEEE Trans. Instrum. Meas. 2023, 72, 1–10. [Google Scholar] [CrossRef]
- Cowie, M.; Linz, D.; Redline, S.; Somers, V.K.; Simonds, A.K. Sleep Disordered Breathing and Cardiovascular Disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 78, 608–624. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, Y.; Liang, Y.; Zhan, Z.; Jiang, M.; Zhang, X.; da Silva, D.S.; Wu, W.; de Albuquerque, V.H.C. Edge2Analysis: A novel AIoT platform for atrial fibrillation recognition and detection. IEEE J. Biomed. Health Inform. 2022, 26, 5772–5782. [Google Scholar] [CrossRef]
- Ren, W.; Liu, Z.; Wu, Y.; Zhang, Z.; Hong, S.; Liu, H.; on behalf of the Missing Data in Electronic health Records (MINDER) Group. Moving beyond medical statistics: A systematic review on missing data handling in electronic health records. Health Data Sci. 2024, 4, 0176. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, V.; Liu, H.; Chen, F.; Qiu, Q.; Hughes, S.; Zheng, D. Quantitative Comparison of Photoplethysmographic Waveform Characteristics: Effect of Measurement Site. Front. Physiol. 2019, 10, 198. [Google Scholar] [CrossRef]
- Magno, M.; Salvatore, G.A.; Jokic, P.; Benini, L. Self-Sustainable Smart Ring for Long-Term Monitoring of Blood Oxygenation. IEEE Access 2019, 7, 115400–115408. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, H.; Zhang, Y.; Ye, X. Study of a Ring-Type Surgical Pleth Index Monitoring System Based on Flexible PPG Sensor. IEEE Sens. J. 2021, 21, 14360–14368. [Google Scholar] [CrossRef]
- Boukhayma, A.; Barison, A.; Haddad, S.; Caizzone, A. Ring-Embedded Micro-Power mm-Sized Optical Sensor for Accurate Heart Beat Monitoring. IEEE Access 2021, 9, 127217–127225. [Google Scholar] [CrossRef]
- Smart Ring News. Latest Updates on Smart Ring Technology. 2025. Available online: https://smartringnews.com/ (accessed on 20 January 2025).
- Zhang, J.; Li, J.; Huang, Z.; Huang, D.; Yu, H.; Li, Z. Recent progress in wearable brain–computer interface (BCI) devices based on electroencephalogram (EEG) for medical applications: A review. Health Data Sci. 2023, 3, 0096. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, C.; Wu, H.; Zhang, J.; Zhou, S.; Wang, G. Will Smart Ring be Next Wave of Wearables? In Proceedings of the 2024 IEEE Biomedical Circuits and Systems Conference (BioCAS), Xi’an, China, 24–26 October 2024; pp. 1–5. [Google Scholar]
- Mehrgardt, P.; Khushi, M.; Poon, S.; Withana, A. Deep Learning Fused Wearable Pressure and PPG Data for Accurate Heart Rate Monitoring. IEEE Sens. J. 2021, 21, 27106–27115. [Google Scholar] [CrossRef]
- Berwal, D.; Kuruba, A.; Shaikh, A.M.; Udupa, A.; Baghini, M.S. SpO2 Measurement: Non-Idealities and Ways to Improve Estimation Accuracy in Wearable Pulse Oximeters. IEEE Sens. J. 2022, 22, 11653–11664. [Google Scholar] [CrossRef]
- Mohammed, H.; Chen, H.B.; Li, Y.; Sabor, N.; Wang, J.G.; Wang, G. Meta-Analysis of Pulse Transition Features in Non-Invasive Blood Pressure Estimation Systems: Bridging Physiology and Engineering Perspectives. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 1257–1281. [Google Scholar] [CrossRef]
- Guo, H.; Wu, H.; Xia, J.; Cheng, Y.; Guo, Q.; Chen, Y.; Xu, T.; Wang, J.; Wang, G. OSAHS Detection Capabilities of RingConn Smart Ring: A Feasibility Study. In Proceedings of the 2024 IEEE 6th International Conference on AI Circuits and Systems (AICAS), Abu Dhabi, United Arab Emirates, 22–25 April 2024; pp. 597–601. [Google Scholar]
- Kao, Y.H.; Chao, P.C.P.; Wey, C.L. Design and Validation of a New PPG Module to Acquire High-Quality Physiological Signals for High-Accuracy Biomedical Sensing. IEEE J. Sel. Top. Quantum Electron. 2018, 25, 69000210. [Google Scholar] [CrossRef]
- Kao, Y.H.; Chao, P.C.P.; Hung, Y.; Wey, C.L. A New Reflective PPG LED-PD Sensor Module for Cuffless Blood Pressure Measurement at Wrist Artery. In Proceedings of the IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Lin, Y.; Chen, C.; Ma, Z.; Sabor, N.; Wei, Y.; Zhang, T.; Sawan, M.; Wang, G.; Zhao, J. Emulation of brain metabolic activities based on a dynamically controllable optical phantom. Cyborg Bionic Syst. 2023, 4, 0047. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, V.; Pramanik, M. Advances in Monte Carlo simulation for light propagation in tissue. IEEE Rev. Biomed. Eng. 2017, 10, 122–135. [Google Scholar] [CrossRef]
- McEwen, M.P.; Bull, G.P.; Reynolds, K.J. Vessel calibre and haemoglobin effects on pulse oximetry. Physiol. Meas. 2009, 30, 869. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M. Optimal Signal Quality Index for Photoplethysmogram Signals. Bioengineering 2016, 3, 21. [Google Scholar] [CrossRef]
- Reddy, G.N.K.; Manikandan, M.S.; Murty, N.V.L.N. On-Device Integrated PPG Quality Assessment and Sensor Disconnection/Saturation Detection System for IoT Health Monitoring. IEEE Trans. Instrum. Meas. 2020, 69, 6351–6361. [Google Scholar] [CrossRef]
- Krishnan, R.; Natarajan, B.; Warren, S. Two-stage approach for detection and reduction of motion artifacts in photoplethysmographic data. IEEE Trans. Biomed. Eng. 2010, 57, 1867–1876. [Google Scholar] [CrossRef]
- Orphanidou, C. Signal Quality Assessment in Physiological Monitoring, 1st ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Vadrevu, S.; Manikandan, M.S. Real-Time PPG Signal Quality Assessment System for Improving Battery Life and False Alarms. IEEE Trans. Circuits Syst. II Express Briefs 2019, 66, 1910–1914. [Google Scholar] [CrossRef]
- Kamshilin, A.A.; Nippolainen, E.; Sidorov, I.S.; Vasilev, P.V.; Erofeev, N.P.; Podolian, N.P.; Romashko, R.V. A new look at the essence of the imaging photoplethysmography. Sci. Rep. 2015, 5, 10494. [Google Scholar] [CrossRef]
- Näslund, J.; Pettersson, J.; Lundeberg, T.; Linnarsson, D.; Lindberg, L.G. Non-invasive continuous estimation of blood flow changes in human patellar bone. Med. Biol. Eng. Comput. 2006, 44, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, P.A.; Allen, J. (Eds.) Photoplethysmography: Technology, Signal Analysis and Applications; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Volkov, M.V.; Margaryants, N.B.; Potemkin, A.V.; Volynsky, M.A.; Gurov, I.P.; Mamontov, O.V.; Kamshilin, A.A. Video capillaroscopy clarifies mechanism of the photoplethysmographic waveform appearance. Sci. Rep. 2017, 7, 13298. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.; Zhang, Q.; Wang, G. Removal of Motion Artifacts in Photoplethysmograph Sensors During Intensive Exercise for Accurate Heart Rate Calculation Based on Frequency Estimation and Notch Filtering. Sensors 2019, 19, 3312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lu, T.; Liu, Y.; Zhang, S.; Liu, R.; Gowda, M. One Ring to Rule Them All: An Open Source Smartring Platform for Finger Motion Analytics and Healthcare Applications. In Proceedings of the 8th ACM/IEEE Conference on Internet of Things Design and Implementation, New York, NY, USA, 9–12 May 2023; IoTDI ’23. pp. 27–38. [Google Scholar] [CrossRef]
- Yu, T.C.; Hu, G.; Zhang, R.; Lim, H.; Mahmud, S.; Lee, C.J.; Li, K.; Agarwal, D.; Nie, S.; Oh, J.; et al. Ring-a-Pose: A Ring for Continuous Hand Pose Tracking. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2024, 8, 1–30. [Google Scholar] [CrossRef]
- Bi, R.; Du, Y.; Singh, G.; Ho, C.J.H.; Zhang, S.; Attia, A.B.E.; Li, X.; Olivo, M. Fast pulsatile blood flow measurement in deep tissue through a multimode detection fiber. J. Biomed. Opt. 2020, 25, 055003. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, R.; Meng, L.; Du, Y.; Low, J.; Qi, Y.; Rajarahm, P.; Lai, A.Y.F.; Tan, V.S.Y.; Ho, P.; et al. A portable optical pulsatile flowmetry demonstrates strong clinical relevance for diabetic foot perfusion assessment. APL Bioeng. 2024, 8, 016109. [Google Scholar] [CrossRef]
- Zhao, Q.; Geng, S.; Wang, B.; Sun, Y.; Nie, W.; Bai, B.; Yu, C.; Zhang, F.; Tang, G.; Zhang, D.; et al. Deep learning in heart sound analysis: From techniques to clinical applications. Health Data Sci. 2024, 4, 0182. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, X.; Yu, Q.; Zhang, Y.; Li, Y.; Zhao, J.; Wang, G. Lungbrn: A smart digital stethoscope for detecting respiratory disease using bi-resnet deep learning algorithm. In Proceedings of the 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–4. [Google Scholar]

| Δ | 0° | 30° | 60° | 90° | 120° | 150° | 180° | 210° | 240° | 270° | 300° | 330° |
| λ = 550 nm | 0.057 | 0.680 | 0.878 | 0.707 | 0.517 | NaN | NaN | NaN | 0.483 | 0.707 | 0.876 | 0.680 |
| λ = 628 nm | 0.085 | 0.668 | 0.727 | 0.566 | 0.516 | 0.512 | 0.583 | 0.558 | 0.488 | 0.574 | 0.724 | 0.670 |
| λ = 940 nm | 0.135 | 0.594 | 0.784 | 0.751 | 0.658 | 0.648 | 0.649 | 0.685 | 0.694 | 0.744 | 0.782 | 0.595 |
| Δ | 0° | 30° | 60° | 90° | 120° | 150° |
| λ = 550 nm | 12.29 ± 6.2 | 13.86 ± 3.25 | 8.96 ± 3.39 | 3.74 ± 1.12 | 2.81 ± 0.42 | 2.92 ± 0.16 |
| λ = 628 nm | 7.26 ± 4.3 | 10.53 ± 4.41 | 13.03 ± 2.88 | 10.31 ± 3.15 | 5.82 ± 2.16 | 4.17 ± 2.00 |
| λ = 940 nm | 2.98 ± 1.60 | 11.22 ± 3.36 | 13.22 ± 2.97 | 11.51 ± 2.30 | 8.53 ± 3.05 | 7.12 ± 2.7 |
| Δ | 180° | 210° | 240° | 270° | 300° | 330° |
| λ = 550 nm | 3.00 ± 0.25 | 2.98 ± 0.37 | 4.18 ± 4.27 | 5.8 ± 5.31 | 9.17 ± 4.15 | 10.93 ± 1.61 |
| λ = 628 nm | 4.45 ± 2.21 | 3.69 ± 1.06 | 6.73 ± 3.36 | 10.05 ± 4.26 | 12.52 ± 3.96 | 8.57 ± 4.47 |
| λ = 940 nm | 7.76 ± 2.89 | 7.25 ± 2.8 | 9.83 ± 2.56 | 11.00 ± 3.76 | 12.87 ± 3.02 | 8.48 ± 3.54 |
| Module | Consumption |
| Wireless Transmission | 201 μA |
| 4-channel PPG readout | 157 μA (@100 Hz sampling rate) |
| LEDs driving current | 179 μA (2 mA for green LEDs and 8 mA for Red/IR LEDs) |
| 3-axis accelerometer | 21 μA (@25 Hz sampling rate) |
| 4-channel temperature | 1.5 μA |
| Others | 35 μA |
| Total | 594.5 μA |
| Parameter | Device | |||||
|---|---|---|---|---|---|---|
| This Work | [8] | [9] | [10] | [33] | [34] | |
| Width | 7.8 mm | 22 mm | 10 mm | 7.9 mm | N.A. | 11 mm |
| Thickness | 2.6 mm | N.A. | 2 mm | 3.8 mm | 1.6 mm | 3.58 mm |
| Weight | 3–5 g | N.A. | 11 g | N.A. | 2.5–2.8 g | 4.3 g |
| Avg. Power | 0.59 mA | 3.76 mA | 3 mA | 2.5 mA | N.A. | 40 mA |
| PPG Sensors | 4 | 2 | 1 | 2 | 1 | 0 |
| Accelerometer | 3 | 3 | 0 | 0 | 9 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Shi, W.; Zhang, J.; Chen, J.; Lin, Q.; Chen, C.; Wang, G. Development of a Rotation-Robust PPG Sensor for a Smart Ring. Sensors 2025, 25, 6326. https://doi.org/10.3390/s25206326
Wang M, Shi W, Zhang J, Chen J, Lin Q, Chen C, Wang G. Development of a Rotation-Robust PPG Sensor for a Smart Ring. Sensors. 2025; 25(20):6326. https://doi.org/10.3390/s25206326
Chicago/Turabian StyleWang, Min, Wenqi Shi, Jianyu Zhang, Jiarong Chen, Qingliang Lin, Cheng Chen, and Guoxing Wang. 2025. "Development of a Rotation-Robust PPG Sensor for a Smart Ring" Sensors 25, no. 20: 6326. https://doi.org/10.3390/s25206326
APA StyleWang, M., Shi, W., Zhang, J., Chen, J., Lin, Q., Chen, C., & Wang, G. (2025). Development of a Rotation-Robust PPG Sensor for a Smart Ring. Sensors, 25(20), 6326. https://doi.org/10.3390/s25206326





