H2S Detection Sensing Characteristic of CuO/SnO2 Sensor
Abstract
:Introduction
Experimental
Results and Discussion
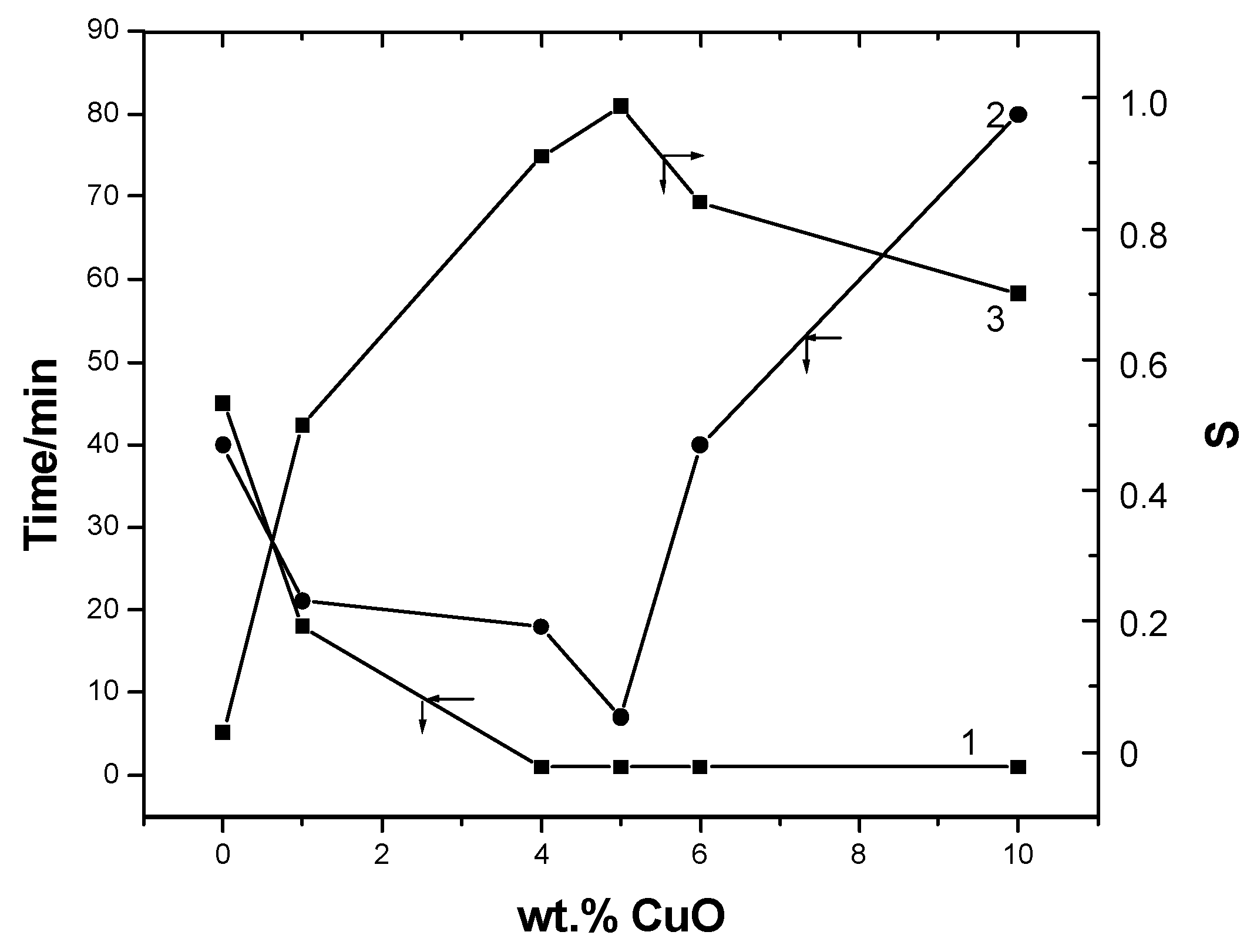
Effects of Additives on H2S Sensitivity
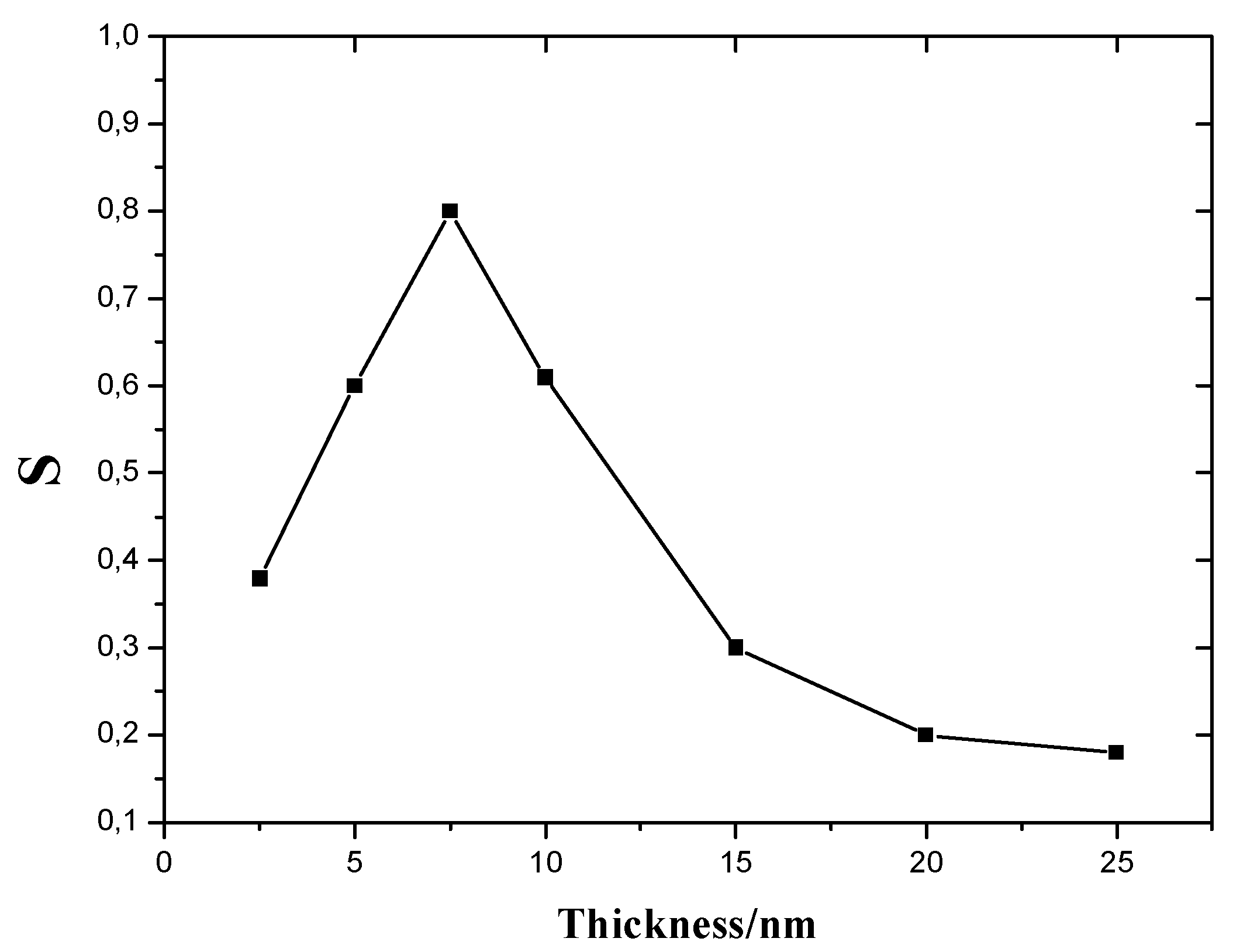
Effects of CuO Film Thickness on H2S Sensitivity
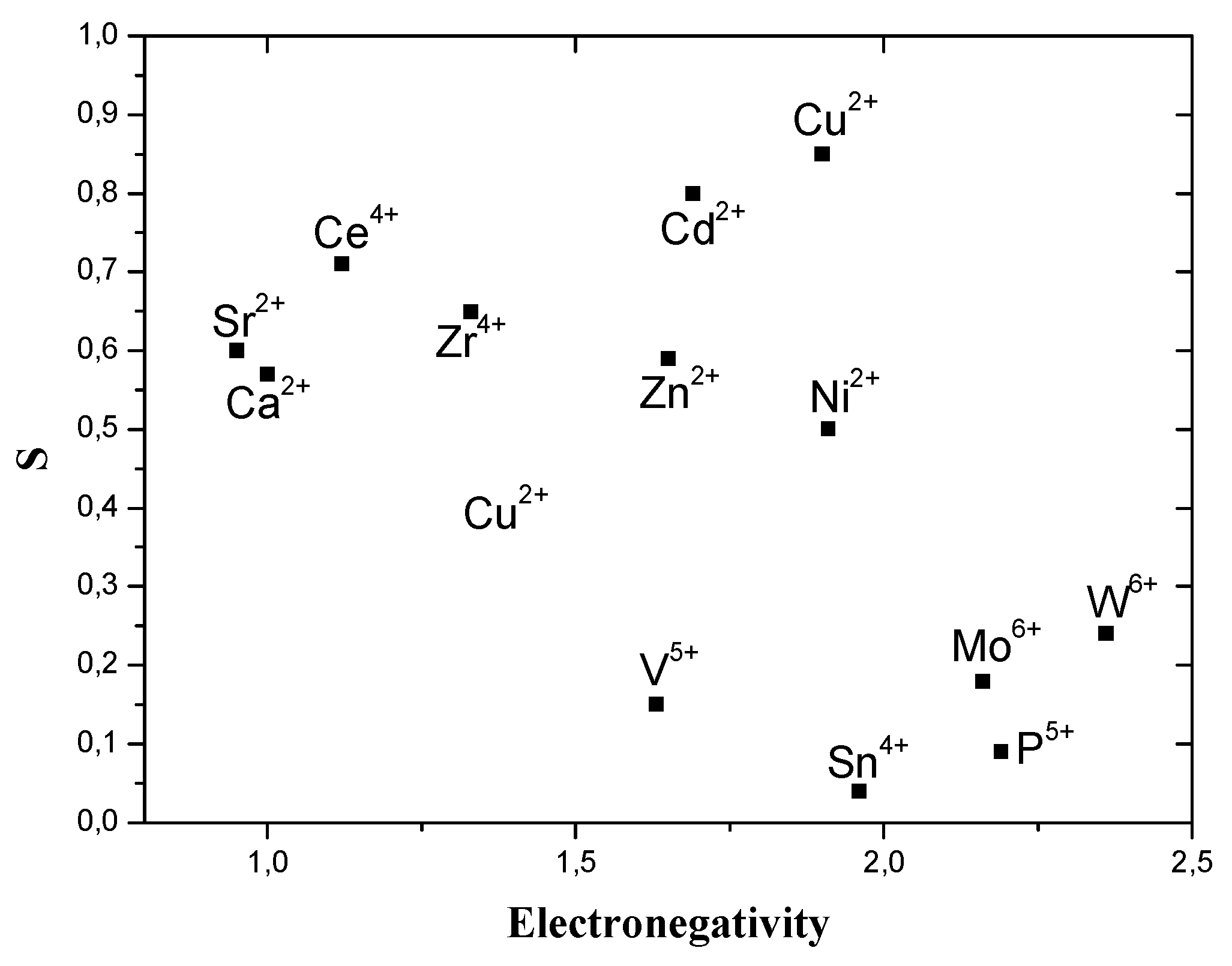
Effects of H2S Gas Concentration
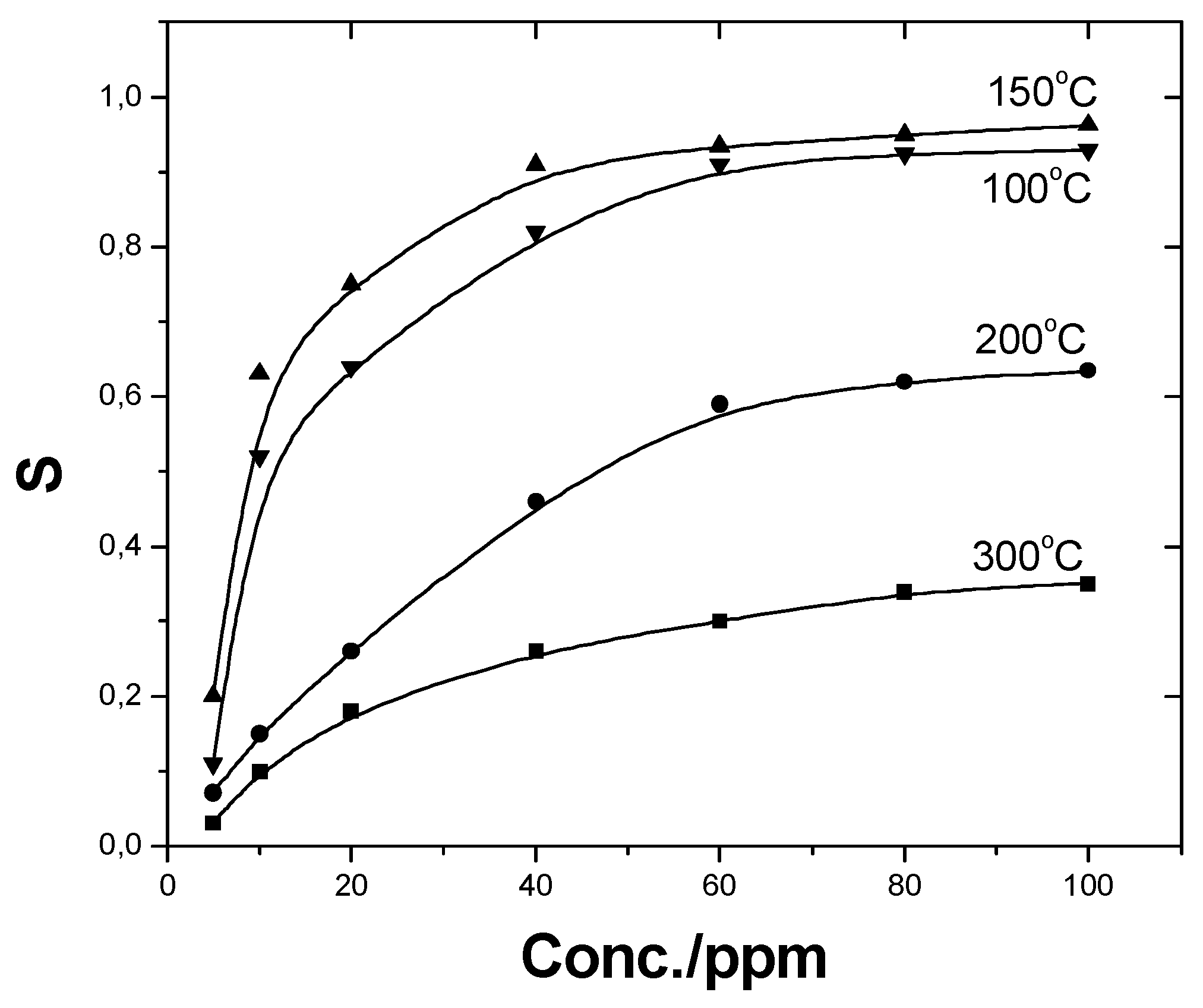
Effects of Cuo Loadings and Operation Temperature

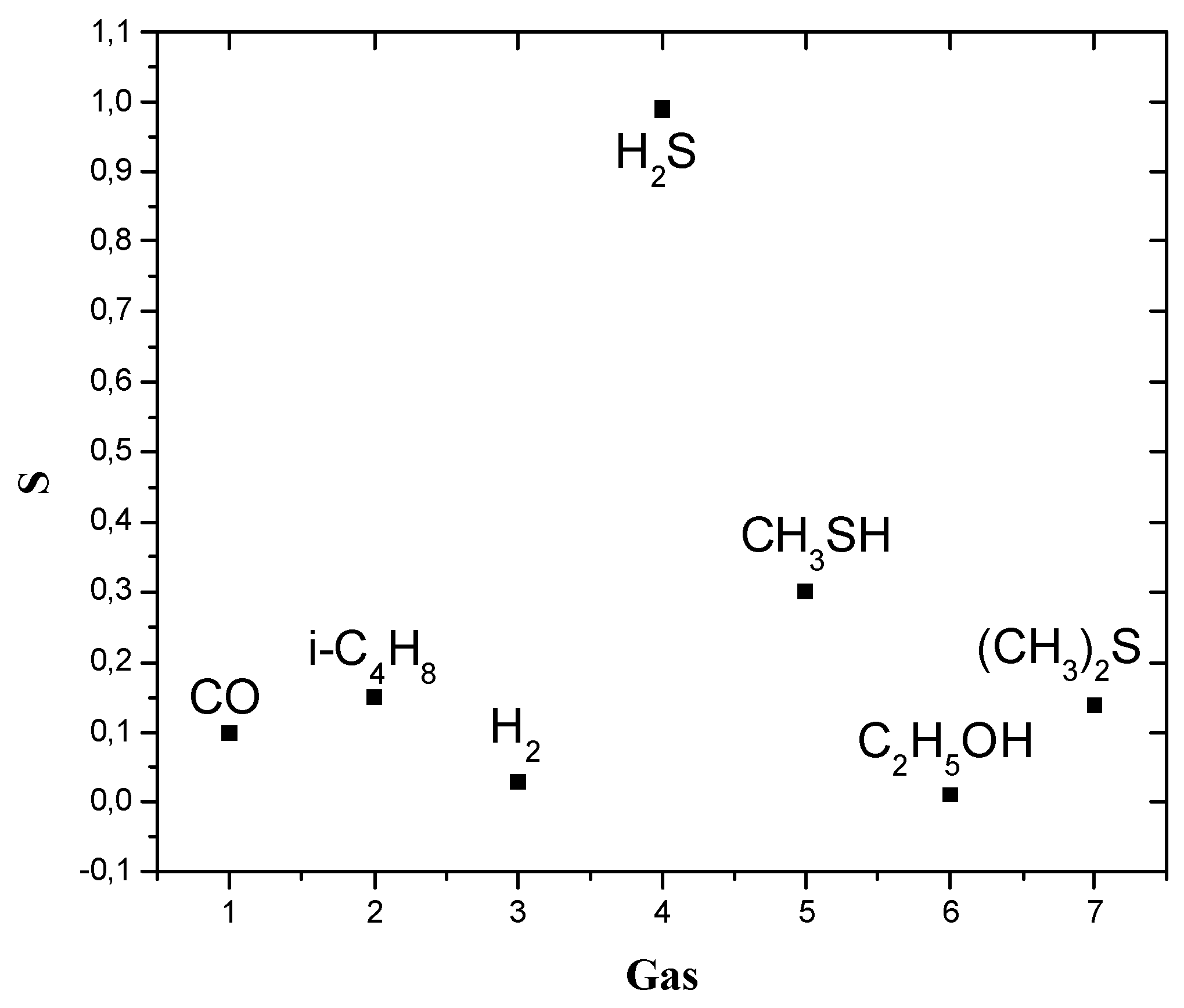
The Selectivity of CuO/SnO2 Element
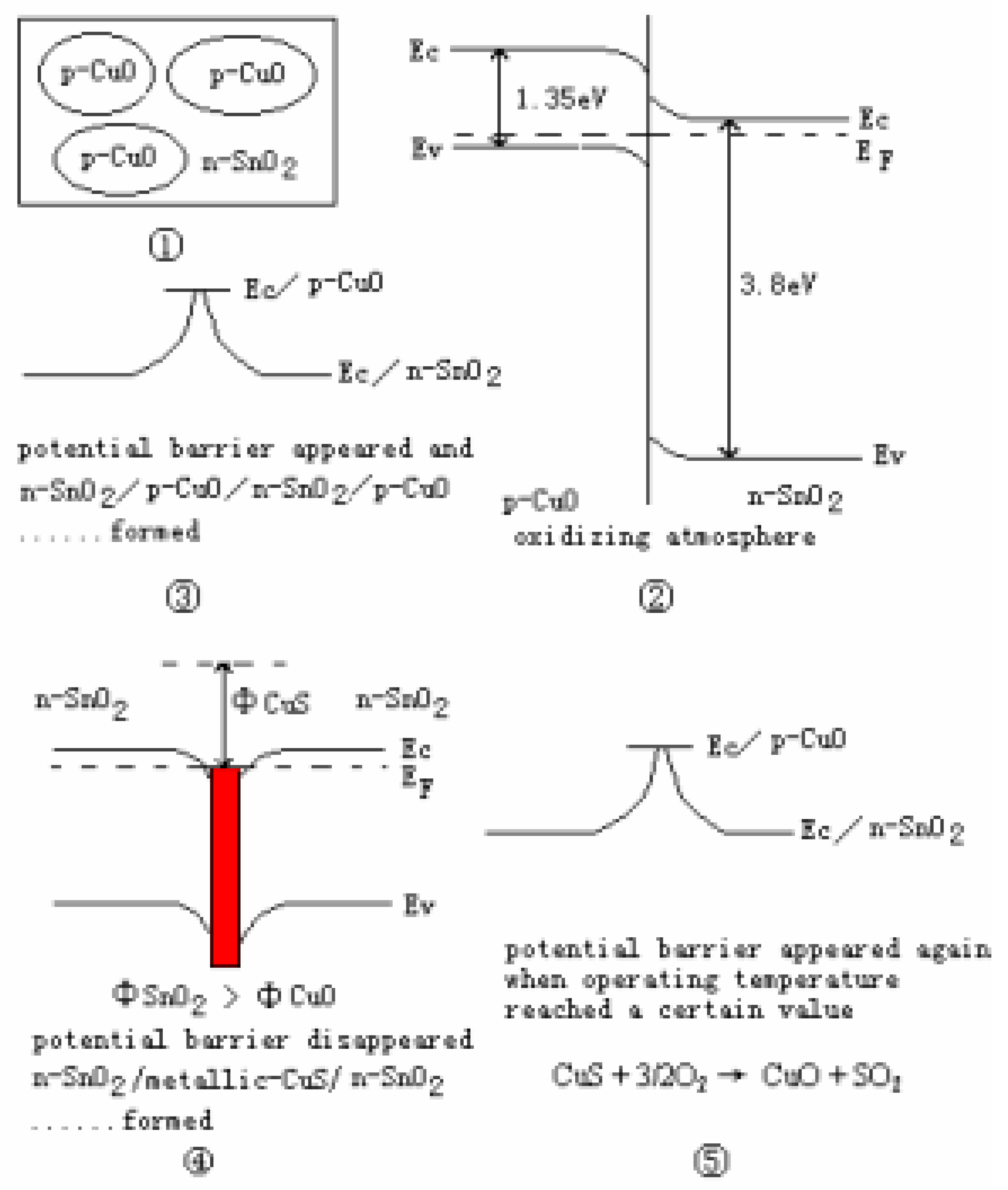
CuO/SnO2 Sensing Mechanism
Conclusion
Acknowledge
References
- Lantto, V.; Romppainen, P. J. Electrochem. Soc. 1988, 135, 2550–2556.
- Kanefusa, S.; Nitta, M.; Haradome, M. J. Electrochem. Soc. 1985, 132, 1770–1773.
- Kandfusa, S.; Nitta, M.; Haradome, M. Tech. Digest, 7th chemical Sensor Symp., Saitama, Japan, Sept. 1988, 25–26, 145–148. [Google Scholar]
- Fang, G.; Liu, Z.; Liu, C.; Yao, K-L. Sensors and Actuators B 2000, 66, 46–48.
- Nakahara, T.; Takahata, K.; Matsuura, S. Proc.Sym.Chem.,Sensors,Honolulu,HI,USA,Oct. 1987, 18–23, 55–64. [Google Scholar] [PubMed]
- Mizei, J.; Lanto, V. Sensors and Actuators B 1991, 4, 163–168.
- Mizei, J. Sensors and Actuators B 1993, 15-16, 328–333.
- Sample Availability: Available from the authors.
© 2003 by MDPI (http://www.mdpi.net). Reproductio is permitted for noncommercial purposes.
Share and Cite
Liu, J.; Huang, X.; Ye, G.; Liu, W.; Jiao, Z.; Chao, W.; Zhou, Z.; Yu, Z. H2S Detection Sensing Characteristic of CuO/SnO2 Sensor. Sensors 2003, 3, 110-118. https://doi.org/10.3390/s30500110
Liu J, Huang X, Ye G, Liu W, Jiao Z, Chao W, Zhou Z, Yu Z. H2S Detection Sensing Characteristic of CuO/SnO2 Sensor. Sensors. 2003; 3(5):110-118. https://doi.org/10.3390/s30500110
Chicago/Turabian StyleLiu, Jinhuai, Xingjiu Huang, Gang Ye, Wei Liu, Zheng Jiao, Wnaglian Chao, Zhongbai Zhou, and Zenglian Yu. 2003. "H2S Detection Sensing Characteristic of CuO/SnO2 Sensor" Sensors 3, no. 5: 110-118. https://doi.org/10.3390/s30500110



