PVC Based Selective Sensors for Ni2+ Ions Using Carboxylated and Methylated Porphine
Abstract
:Introduction
Experimental
Reagents

Preparation of membranes
Potential measurements
 Saturated calomel electrodes (SCE) were used as reference electrodes. The response time of the membrane sensors is the time in which stable and constant potentials are obtained.
Saturated calomel electrodes (SCE) were used as reference electrodes. The response time of the membrane sensors is the time in which stable and constant potentials are obtained.| Electrode/ Membrane No. | %(w/w) of various components | Working concn. range, M | Slope, mV/decade of activity | Response time, sec | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TBAP | DBP | DOP | DBBP | CN | NaTPB | PVC | ||||
| 1 | 10 | - | - | - | - | 5 | 85 | 5.5 x 10-5 – 1.0 x 10-1 | 26.0 | 60 |
| 2 | 10 | 30 | - | - | - | 5 | 55 | 2.0 x 10-5 – 1.0 x 10-1 | 28.5 | 15 |
| 3 | 10 | - | 30 | - | - | 5 | 55 | 5.5 x 10-5 – 1.0 x 10-1 | 34.0 | 20 |
| 4 | 10 | - | - | 30 | - | 5 | 55 | 2.0 x 10-6 – 1.0 x 10-1 | 29.6 | 15 |
| 5 | 10 | - | - | - | 30 | 5 | 55 | 5.6 x 10-5 – 1.0 x 10-1 | 35.3 | 10 |
| Electrode/ Membrane No. | %(w/w) of various components | Working concen. Range, M | Slope, mV/decade of activity | Response time, sec | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OMP | DBP | DOP | DBBP | CN | NaTPB | PVC | ||||
| 6 | 10 | - | - | - | - | 5 | 85 | 1.0 x 10-4 – 1.0 x 10-1 | 32.4 | 60 |
| 7 | 10 | 30 | - | - | - | 5 | 55 | 5.0 x 10-5 – 1.0 x 10-1 | 37.7 | 20 |
| 8 | 10 | - | 30 | - | - | 5 | 55 | 3.6 x 10-5 – 1.0 x 10-1 | 38.8 | 15 |
| 9 | 10 | - | - | 30 | - | 5 | 55 | 5.6 x 10-5 – 1.0 x 10-1 | 30.1 | 15 |
| 10 | 10 | - | - | - | 30 | 5 | 55 | 1.0 x 10-5 – 1.0 x 10-1 | 29.0 | 10 |
Results and Discussion
Working concentration range
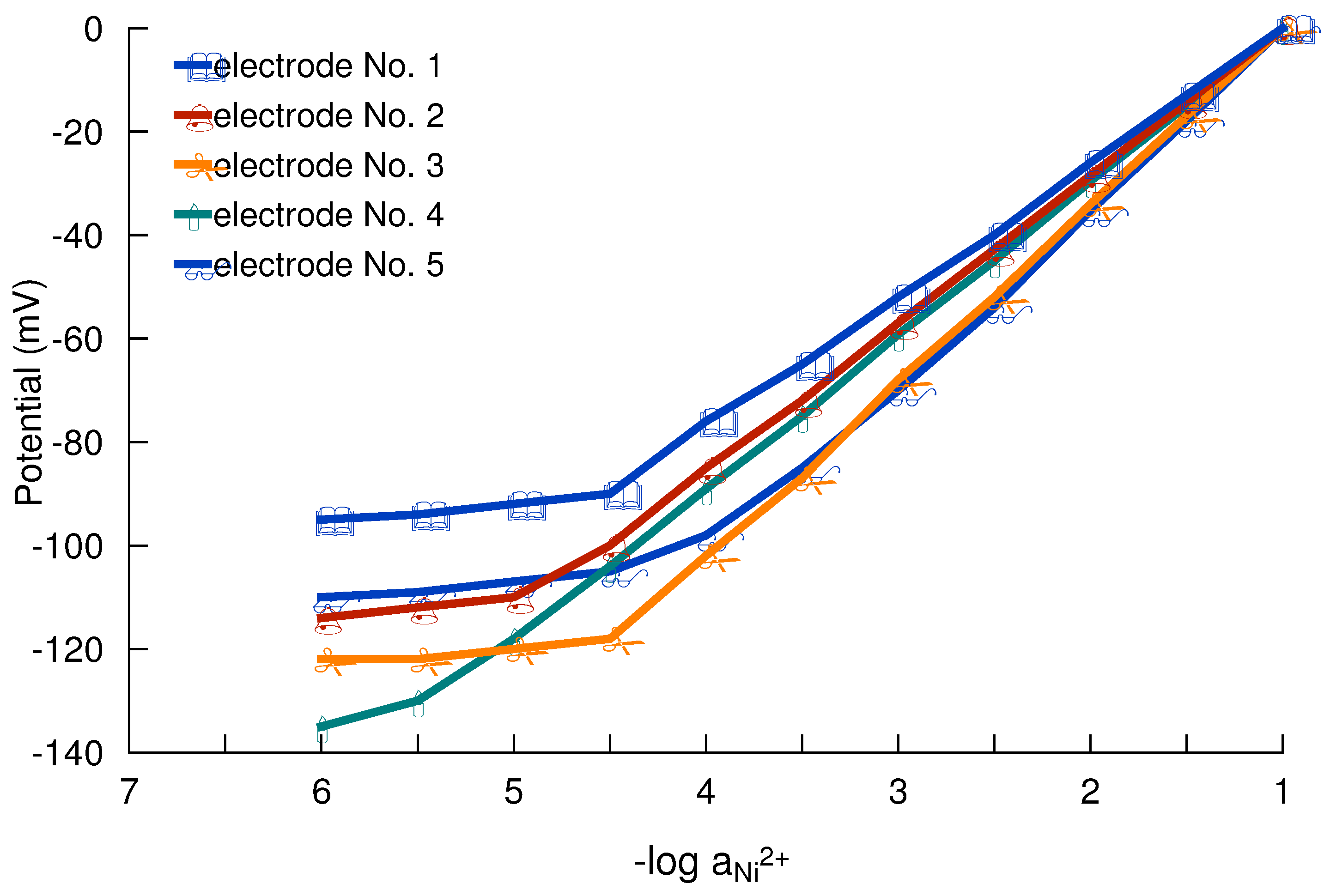
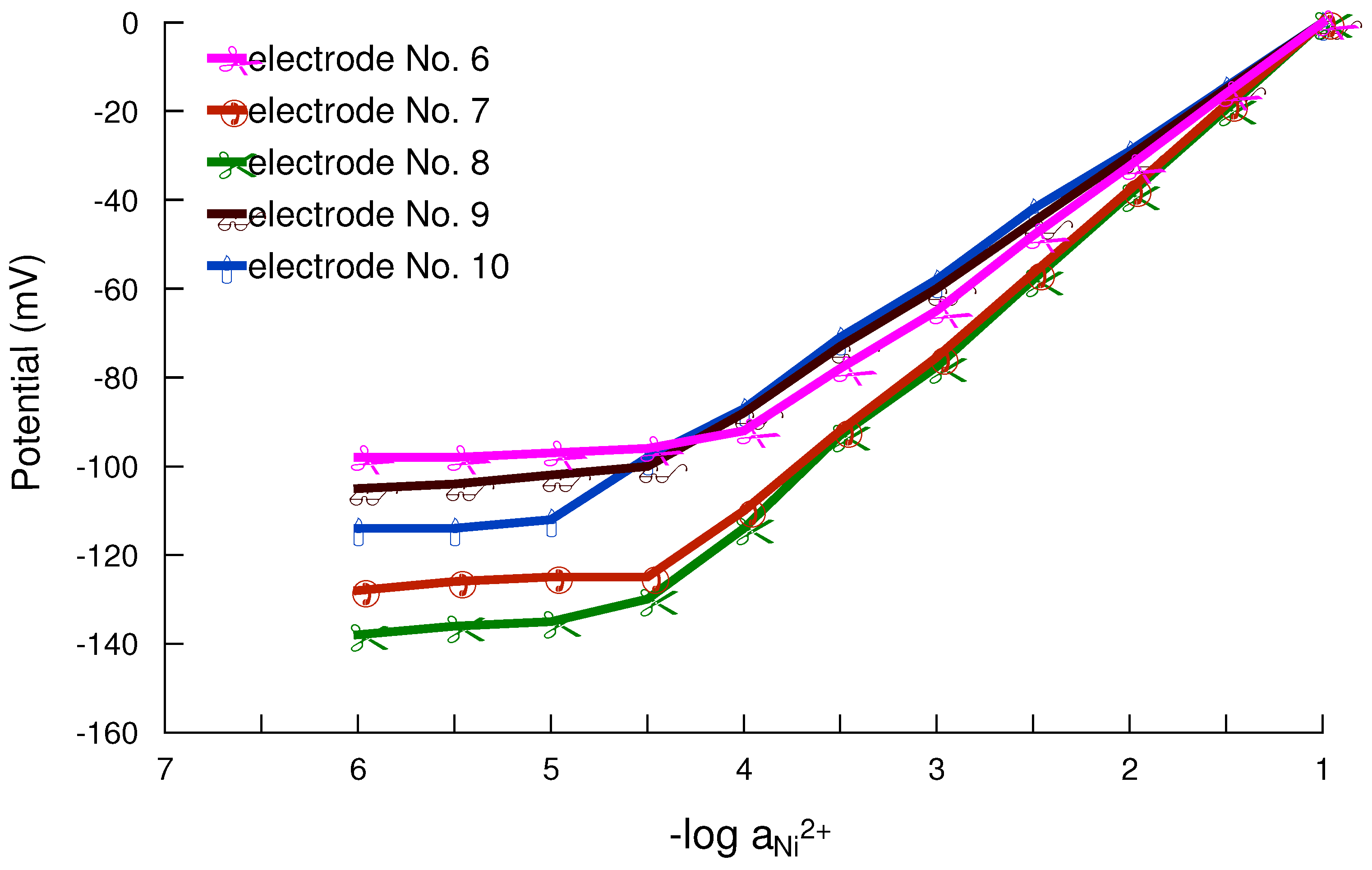
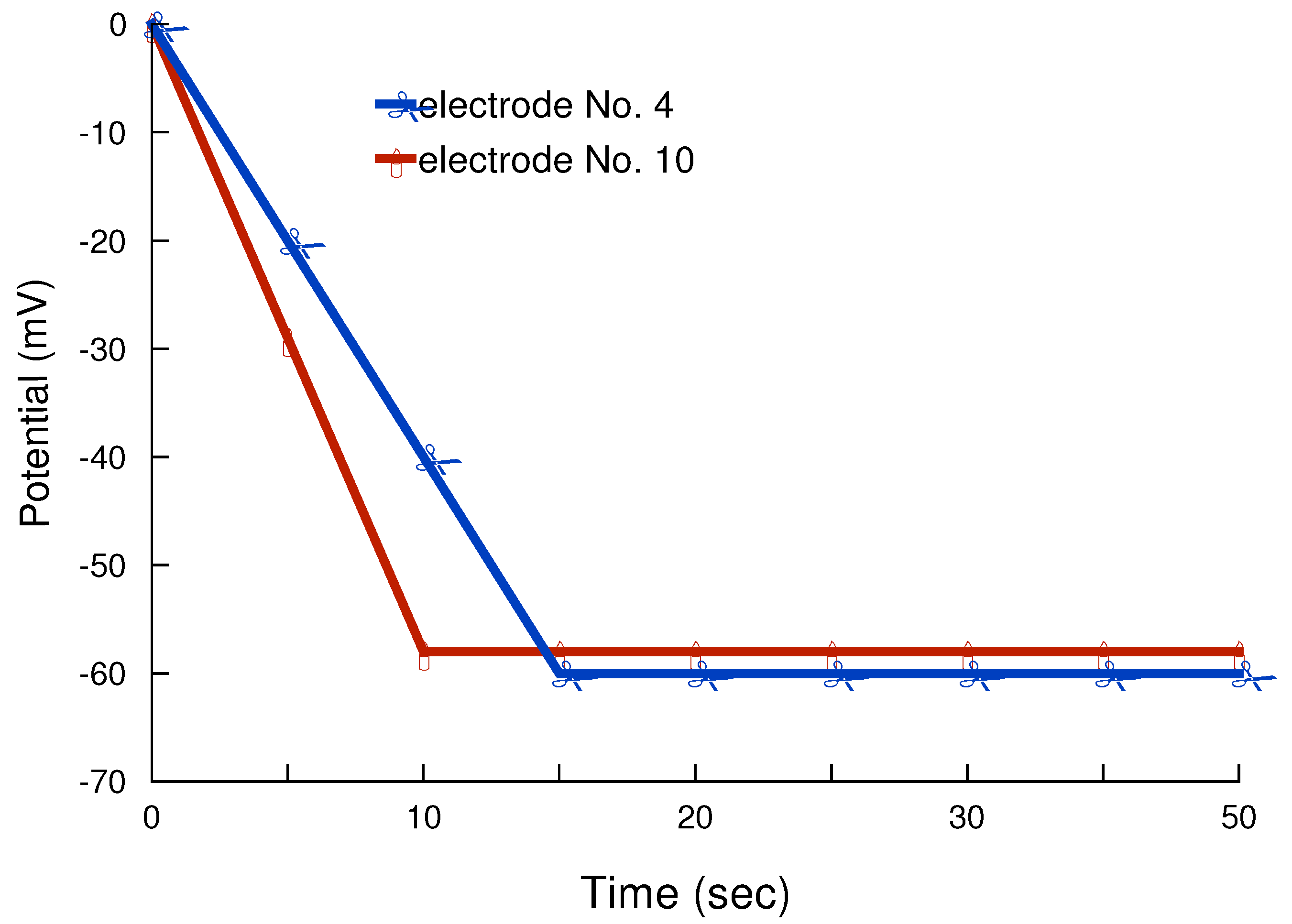
Response and lifetime
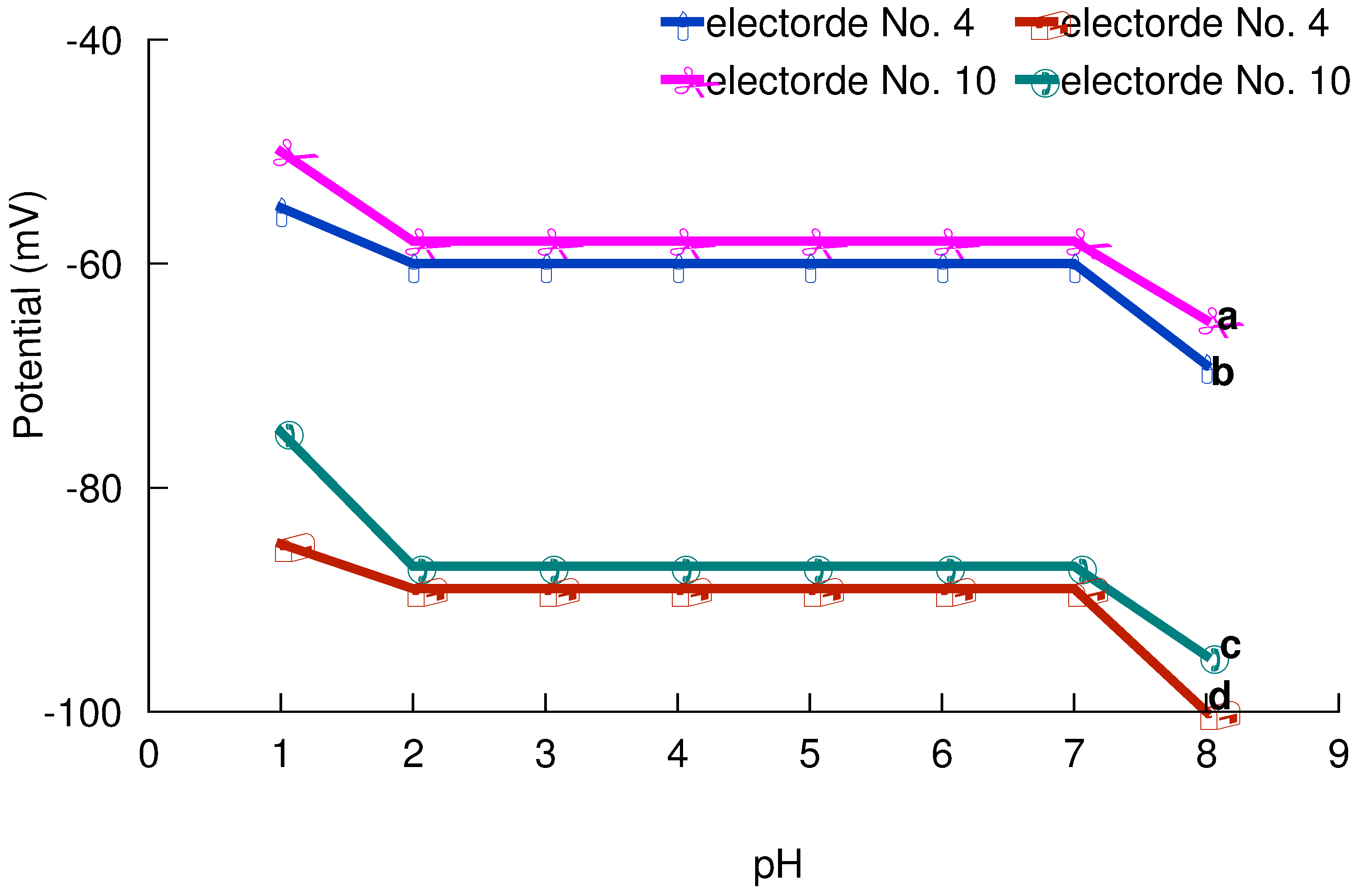
pH and non-aqueous effect
Potentiometric selectivity
 for Ni2+ -selective sensors (electrode nos. 4 and 10) as calculated by Match Potential Method
for Ni2+ -selective sensors (electrode nos. 4 and 10) as calculated by Match Potential Method
| Interfering ion, B | Selectivity coefficients,  | |
|---|---|---|
| Electrode No. 4 | Electrode No. 10 | |
| NH4+ | 1.4 x 10-2 | 2.0 x 10-2 |
| Na+ | 9.0 x 10-2 | 8.2 x 10-2 |
| K+ | 5.3 x 10-2 | 2.0 x 10-2 |
| Tl+ | 3.2 x 10-2 | 1.9 x 10-2 |
| Cs+ | 4.7 x 10-2 | 1.4 x 10-2 |
| Cu2+ | 1.2 x 10-3 | 1.8 x 10-3 |
| Ca2+ | 1.4 x 10-3 | 1.2 x 10-3 |
| Sr2+ | 1.9 x 10-3 | 2.5 x 10-3 |
| Ba2+ | 2.0 x 10-3 | 2.0 x 10-3 |
| Mg2+ | 1.4 x 10-3 | 2.8 x 10-3 |
| Co2+ | 2.3 x 10-1 | 4.8 x 10-1 |
| Cd2+ | 2.6 x 10-3 | 2.4 x 10-3 |
| Zn2+ | 2.2 x 10-3 | 2.6 x 10-3 |
| Hg2+ | 1.4 x 10-3 | 1.4 x 10-3 |
| Pb2+ | 1.4 x 10-3 | 1.6 x 10-3 |
| Fe3+ | 1.8 x 10-3 | 1.4 x 10-3 |
| Al3+ | 2.0 x 10-3 | 1.9 x 10-3 |
| Ce3+ | 2.8 x 10-3 | 1.8 x 10-3 |
Analytical applications
Estimation of Ni2+ in effluents

| Samples | PH | Ni2+ as determined byAAS, mg l-1 | Ni2+ as determined by sensor 4, mg l-1 | |
|---|---|---|---|---|
| Found | after Adjustment | |||
| 1 | 6.80 | 3.70 | 44.0 | 47.0 |
| 2 | 6.65. | 3.80 | 43.5 | 47.0 |
| 3 | 6.70 | 3.65 | 43.0 | 46.0 |
| 4 | 6.90 | 3.75 | 42.0 | 45.0 |
Potentiometric titration

Conclusion
Acknowledgements
References
- Pungor, E.; Toth, K.; Havas, J. Acta. Chim. Acad. Sci. Hung. 1966, 48, 17.
- Buchanan, E.B.; Scago, J.L. Acta. Chim. 1968, 40, 517.
- Awasthi, S.P.; Kulkarni, V.T.; Sundaresan, M. J. Electrochem. Soc. India 1988, 37, 309.
- Lal, U.S.; Chattopadhyaya, M.C.; Dey, A.K. J. Ind. Chem. Soc. 1982, 49, 493.
- Materova, E.A.; Muchovikov, V.V.; Grigorieva, M.G. Anal. Lett. 1975, 8, 167.
- Luca, C.; Maria, P.; Nicolae, M. Rev. Chim. 1976, 27, 1088.
- Smirnova, E.V.; Petrukhin, O.M.; Rogatinskaya, S.L. Zh. Anal. Khim. 1982, 37, 2137.
- Hampton, M.D.; Peters, C.A.; Wellington, L.A. Acta. Chim. Acta. 1987, 194, 171. [CrossRef]
- Lebedera, O.A.; Jansons, E. Latv. PSR Zinat. Akad. Vestis. Kim. Ser. 1986, 4, 423.
- Rao, G.N.; Srivastava, S.K.; Singh, M. Talanta 1996, 43, 1821.
- Chaniotakis, N.A.; Chasser, A.M.; Meyerhoff, M.E. Anal. Chem. 1988, 60, 88.
- Jyo, A.; Minakami, R.; Kanda, Y.; Egawa, H. Sens. & Actuators B 1993, 13–14, 200.
- Gao, D.; Jun, G.; Yu, R.; Zheny, G. Analyst 1995, 120, 499.
- Singh, L.P.; Vardhan, H. Anal. Proc. 1995, 32, 193.
- Jain, A.K.; Gupta, V.K.; Singh, R.D.; Khurana, U.; Singh, L.P. Sens & Actuators B 1997, 40, 15.
- Gupta, V.K.; Jain, A.K.; Singh, L.P.; Khurana, U. Anal. Chim. Acta 1997, 335, 33. [CrossRef]
- Craggs, A; Moody, G.J.; Thomas, J.D.R. J. Chem. Edu. 1974, 51, 541.
- Assubai, F.N.; Moody, G.J.; Thomas, J.D.R. Analyst 1988, 113, 61.
- Chan, W.H.; Shiu, K.K.; Gu, X.H. Analyst 1993, 118, 863.
- Jain, A.K.; Gupta, V.K.; Singh, L.P.; Khurana, U. Analyst 1997, 122, 583.
- Saez de Viteri, F.J.; Diamond, D. Analyst 1994, 119, 749.
- Srivastava, S.K.; Gupta, V.K.; Jain, S. Analyst 1995, 120, 495.
- Srivastava, S.K.; Gupta, V.K.; Jain, S. Anal. Chem. 1996, 68, 1272.
- Jain, A.K.; Singh, L.P.; Jain, P.K. Sens & Actuators B 1995, 25, 729.
- Jain, A.K.; Gupta, V.K.; Sahoo, B.B.; Singh, L.P. Anal. Proc. 1995, 32, 99.
- Jain, A.K.; Gupta, V.K.; Singh, L.P. Anal. Proc. 1995, 32, 263.
- Sample Availability: Available from the authors.
© 2003 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Singh, L.P.; Bhatnagar, J.M. PVC Based Selective Sensors for Ni2+ Ions Using Carboxylated and Methylated Porphine. Sensors 2003, 3, 393-403. https://doi.org/10.3390/s30900393
Singh LP, Bhatnagar JM. PVC Based Selective Sensors for Ni2+ Ions Using Carboxylated and Methylated Porphine. Sensors. 2003; 3(9):393-403. https://doi.org/10.3390/s30900393
Chicago/Turabian StyleSingh, L. P., and J. M. Bhatnagar. 2003. "PVC Based Selective Sensors for Ni2+ Ions Using Carboxylated and Methylated Porphine" Sensors 3, no. 9: 393-403. https://doi.org/10.3390/s30900393
APA StyleSingh, L. P., & Bhatnagar, J. M. (2003). PVC Based Selective Sensors for Ni2+ Ions Using Carboxylated and Methylated Porphine. Sensors, 3(9), 393-403. https://doi.org/10.3390/s30900393




