Thermal Stability of Aqueous Polyurethanes Depending on the Applied Catalysts
Abstract
:Introduction
Experimental
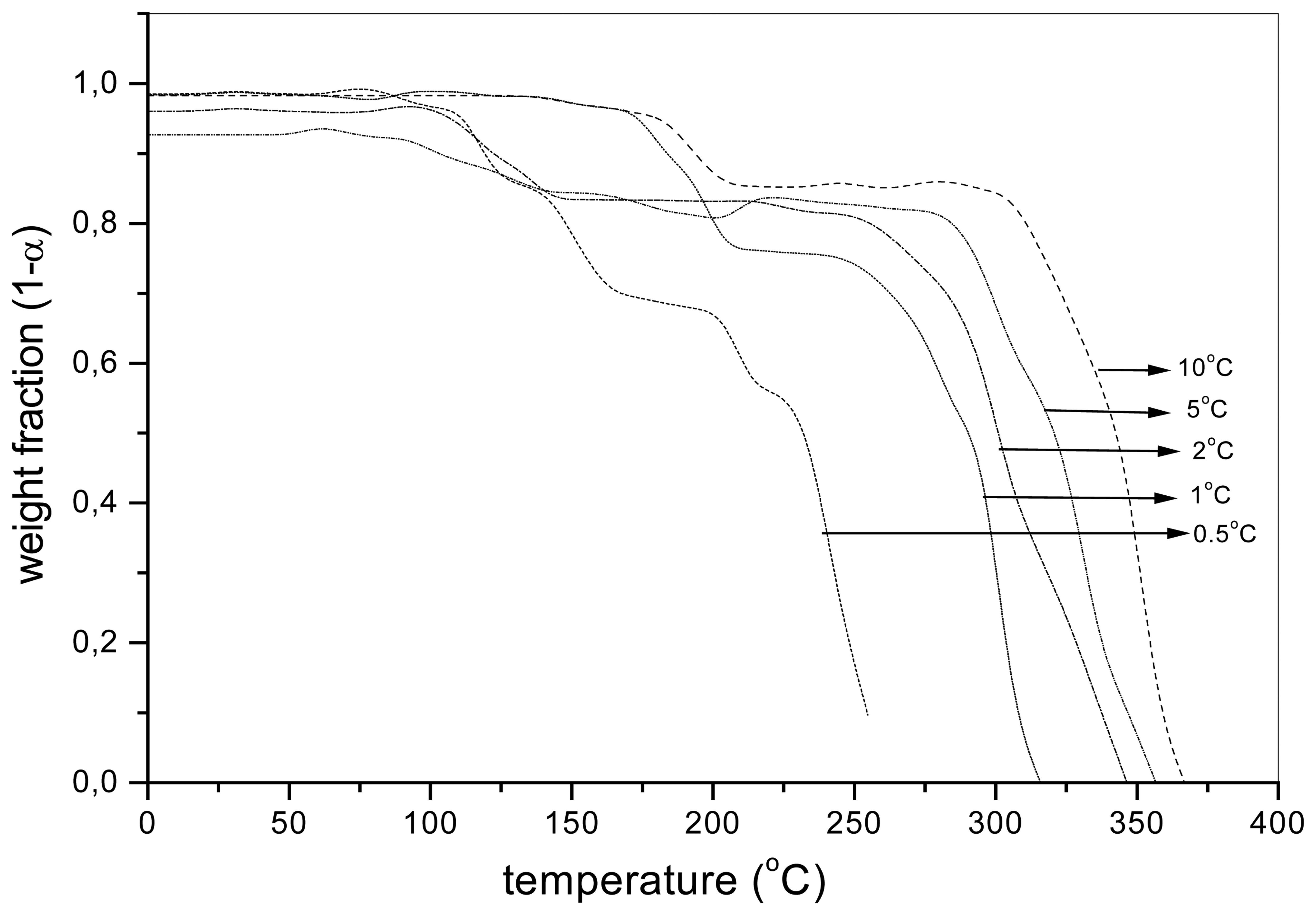
Results and Discussion
Conclusion
Acknowledgments
References
- Brinkman, E.; Vandevoorde, P. Waterborne two-pack isocyanate-free systems for industrial coatings. Progr. Org. Coatings 1997, 34, 21–25. [Google Scholar]
- Billiani, J.; Wilfinger, W. New low VOC acrylic polyol dispersions for two-component polyurethane coatings. 79th Annual meeting of the FSCT, Atalanta, 5-7.november, 2001.
- Billiani, J.; Wilfinger, W. Waterborne acrylic polyols for 2k waterborne polyuethane coatings. European coatings show, Nurnberg, 2-5. april, 2001.
- Blank, W.J.; He, Z.A.; Hessell, E.T. Catalysis of the isocyanate-hydroxyl reaction by non-tin catalysts. Progr. Org. Coatings 1999, 35, 19–29. [Google Scholar]
- He, Z.A.; Blank, W.J.; Picci, M.E. A selective catalyst for two-component waterborne polyurethane coatings. J. Coatings Tech. 2002, 74, 31–36. [Google Scholar]
- Stamenković, J.; Cakić, S.; Nikolić, G. Study of the catalytic selectivity of an aqueous two-component polyurethane system by FTIR spectroscopy. Chem. Industry 2003, 57, 559–569. [Google Scholar]
- Stamenković, J.; Cakić, S.; Konstantinović, S.; Stoilković, S. Catalysis of the isocyanate-hydroxyl reaction by non-tin catalysts in waterborne two-component polyurethane coatings. Facta Univesitatis 2004, 2, 243–250. [Google Scholar]
- Petrović, Z.S.; Zavargo, Z. Reliability of methods for determination of kinetic parameters from thermogravimetry and DSC measurements. J. Appl. Polym. Sci. 1986, 32, 4353–4368. [Google Scholar]
- Jimenez, A.; Berenguer, V.; Lopez, J.; Sanchez, A. Thermal degradation study of poly(vinyl chloride). Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1993, 50, 1565–1573. [Google Scholar]
- Petrović, Z.S.; Zavargo, Z.; Flynn, J.H.; Macknight, W. Thermal degradation of segmented polyurethanes. J. Appl. Polym. Sci. 1994, 51, 1087–1095. [Google Scholar]
- Denq, B.L.; Chiu, W.Y.; Lin, K.F. Kinetic model of thermal degradation of polymers for nonisothermal process. J. Appl. Polym. Sci. 1997, 66, 1855–1868. [Google Scholar]
- Pegoretti, A.; Penati, A.; Kolarik, J. Effect of hydrolysis on molar mass and thermal properties of poly(ester urethanes). J. Therm. Anal. 1944, 41, 1441–1452. [Google Scholar]
- Fambri, L.; Pegoretti, A.; Kolarik, J.; Gavazza, C.; Penati, A. Thermal stabilities of different polyurethanes after hydrolytic treatment. J. Therm. Anal. 1998, 52, 789–797. [Google Scholar]
- Park, J.W.; Oh, S.C.; Lee, H.P.; Hee, T.K.; Yoo, K.O. A kinetic analysis of thermal degradation of polymers using a dynamic method. Polym. Degrad. Stab. 2000, 67, 535–540. [Google Scholar]
- Cakic, S.; Lacnjevac, C.; Rajkovic, M.B.; Raskovic, Lj.; Stamenkovic, J. Reticulation of Agueous Polyurethane Systems Controlled by DSC Method. Sensors 2006, 6, 536–545. [Google Scholar]
- Agic, A.; Bajsic, E.G.; Rek, V. Kinetic parameters estimation for thermal degradation of polyurethane elastomers. J. Elast. Plast. 2006, 38(2), 105–118. [Google Scholar]
- Coutinho, F.M.B.; Delpech, M.C. Degradation profile of films cast from aqueous polyurethane dispersions. Polym. Degrad.Stab. 2000, 70, 49–57. [Google Scholar]
- Chattopadhyay, D.K.; Sreedhar, B.; Raju, K.V.S.N. Thermal stability of chemically crosslinked moisture-cured polyurethane coatings. J. Appl. Polym. Sci. 2005, 96(6), 1509–1518. [Google Scholar]
- Fambri, L.; Pegoretti, A.; Gavazza, C.; Penati, A. Thermooxidative stability of different polyurethanes evaluated by isothermal and dynamic methods. J. Appl. Polym. Sci. 2001, 81, 1216–1225. [Google Scholar]
- Flynn, J.H. Temperature dependence of the rate of reaction in thermal analysis. The Arrhenius equation in condensed phase kinetics. J.Therm. Anal. 1990, 36(4), 1579–1593. [Google Scholar]
- Flynn, J.H. General differential technique for the determination of parameters for d(α)/dt = f(α)A exp (-E/RT). Energy of activation, preexponential factor and order of reaction (when applicable). J. Therm. Anal. 1991, 37, 293–305. [Google Scholar]
- K-KAT®XC-6212 a product of King Industries Inc. US patent 5.846.897.
- Barendregt, R.B.; Berg, P. The degradation of polyurethane. Thermochim. Acta 1980, 38, 181–195. [Google Scholar]



| Component A, weight % | Control | ZrCAT | MnCAT |
|---|---|---|---|
| Polyol VSM 6299 | 44.1 | 44.1 | 44.1 |
| Water | 41.2 | 41.2 | 41.2 |
| Component B, weight % | |||
| Bayhydur VP LS 2319 | 5.88 | 5.88 | 5.88 |
| Dezmodur N 3600 | 5.88 | 5.88 | 5.88 |
| Methoxypropyl acetate | 2.94 | 2.94 | 2.94 |
| Zr catalyst, relative to 2 % (4%) resin solids | no catalyst | 0.65 (1.30) | - |
| Mn catalyst, relative to 2 % (4%) resin solids | no catalyst | - | 0.65 (1.30) |
| Total components mass | 100.00 | 100.6 | 100.6 |
| Component A, weight % | Control | ZrCAT | MnCAT |
|---|---|---|---|
| Polyol VSM 2521 | 56.2 | 56.2 | 56.2 |
| Water | 22.6 | 22.6 | 22.6 |
| Component B, weight % | |||
| Bayhydur VP LS 2336 | 9.8 | 9.8 | 9.8 |
| Bayhydur VP LS 2150 BA | 9.8 | 9.8 | 9.8 |
| Methoxypropyl acetate | 1.1 | 1.1 | 1.1 |
| Zr catalyst, relative to 2 % (4%) resin solids | no catalyst | 0.8 (1.60) | - |
| Mn catalyst, relative to 2 % (4%) resin solids | no catalyst | - | 0.8 (1.60) |
| Total | 99.5 | 100.3 | 100.3 |
| β Heating rate, (°C min-1) | α Degradation | aqPUR1-control (°C) | aqPUR1-ZrCAT 2%(°C) | aqPUR1-MnCAT 2% (°C) | aqPUR1-ZrCAT 4% (°C) | aqPUR1-MnCAT 4% (°C) |
|---|---|---|---|---|---|---|
| 0.5 | 0.025 | 127 | 139 | 153 | 150 | 164 |
| 0.050 | 163 | 173 | 188 | 185 | 200 | |
| 0.100 | 181 | 190 | 205 | 202 | 217 | |
| 1 | 0.025 | 131 | 144 | 157 | 155 | 168 |
| 0.050 | 175 | 186 | 199 | 198 | 211 | |
| 0.100 | 181 | 192 | 205 | 204 | 217 | |
| 2 | 0.025 | 143 | 154 | 169 | 166 | 180 |
| 0.050 | 178 | 188 | 203 | 199 | 214 | |
| 0.100 | 192 | 201 | 216 | 213 | 228 | |
| 5 | 0.025 | 174 | 184 | 199 | 195 | 210 |
| 0.050 | 221 | 228 | 245 | 240 | 257 | |
| 0.100 | 248 | 254 | 271 | 266 | 283 | |
| 10 | 0.025 | 181 | 191 | 205 | 202 | 217 |
| 0.050 | 265 | 271 | 287 | 283 | 300 | |
| 0.100 | 277 | 282 | 299 | 294 | 312 |
| β Heating rate, (°C min-1) | α Degradation | aqPUR2-control (°C) | aqPUR2-ZrCAT 2%(°C) | aqPUR2-MnCAT 2% (°C) | aqPUR2-ZrCAT 4% (°C) | aqPUR2-MnCAT 4% (°C) |
|---|---|---|---|---|---|---|
| 0.5 | 0.025 | 141 | 153 | 176 | 164 | 178 |
| 0.050 | 178 | 188 | 203 | 199 | 214 | |
| 0.100 | 190 | 199 | 214 | 211 | 226 | |
| 1 | 0.025 | 150 | 161 | 179 | 173 | 190 |
| 0.050 | 181 | 190 | 210 | 202 | 222 | |
| 0.100 | 203 | 211 | 232 | 223 | 244 | |
| 2 | 0.025 | 165 | 176 | 190 | 187 | 201 |
| 0.050 | 215 | 223 | 239 | 235 | 251 | |
| 0.100 | 220 | 228 | 244 | 240 | 256 | |
| 5 | 0.025 | 169 | 180 | 193 | 191 | 204 |
| 0.050 | 224 | 232 | 246 | 244 | 258 | |
| 0.100 | 251 | 258 | 272 | 270 | 285 | |
| 10 | 0.025 | 192 | 199 | 215 | 211 | 227 |
| 0.050 | 273 | 276 | 294 | 288 | 307 | |
| 0.100 | 290 | 292 | 311 | 304 | 323 |
| Method | α Degradation | aqPUR1-control (min) | aqPUR1-ZrCAT 2% (min) | aqPUR1-MnCAT 2% (min) | aqPUR1-ZrCAT 4% (min) | aqPUR1-MnCAT 4% (min) |
|---|---|---|---|---|---|---|
| Dynamic method, from 30 °C | 0.025 | 395.4 | 440.9 | 493.8 | 483.2 | 535.7 |
| 0.050 | 546.7 | 584.7 | 640.2 | 629.8 | 685.4 | |
| 0.100 | 602.3 | 637.5 | 693.1 | 683.1 | 738.8 | |
| Dynamic method, from 100 °C | 0.025 | 129.4 | 174.9 | 227.8 | 217.2 | 269.7 |
| 0.050 | 280.7 | 318.7 | 374.2 | 363.8 | 419.4 | |
| 0.100 | 336.3 | 371.5 | 427.1 | 417.1 | 472.8 |
| Method | α Degradation | aqPUR2-control (min) | aqPUR2-ZrCAT 2% (min) | aqPUR2-MnCAT 2% (min) | aqPUR2-ZrCAT 4% (min) | aqPUR2-MnCAT 4% (min) |
|---|---|---|---|---|---|---|
| Dynamic method, from 30 °C | 0.025 | 453.5 | 496.9 | 572.1 | 539.8 | 596.0 |
| 0.050 | 602.6 | 637.5 | 700.1 | 681.1 | 743.8 | |
| 0.100 | 658.2 | 689.8 | 753.5 | 735.4 | 799.3 | |
| Dynamic method, from 100 °C | 0.025 | 187.5 | 230.9 | 306.1 | 273.8 | 330.0 |
| 0.050 | 336.6 | 371.5 | 434.1 | 415.1 | 477.8 | |
| 0.100 | 392.2 | 423.8 | 487.5 | 469.4 | 533.3 |
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Cakic, S.; Nikolic, G.; Lacnjevac, C.; Gligoric, M.; Stamenkovic, J.; Rajkovic, M.B.; Barac, M. Thermal Stability of Aqueous Polyurethanes Depending on the Applied Catalysts. Sensors 2006, 6, 1697-1707. https://doi.org/10.3390/s6111697
Cakic S, Nikolic G, Lacnjevac C, Gligoric M, Stamenkovic J, Rajkovic MB, Barac M. Thermal Stability of Aqueous Polyurethanes Depending on the Applied Catalysts. Sensors. 2006; 6(11):1697-1707. https://doi.org/10.3390/s6111697
Chicago/Turabian StyleCakic, Suzana, Goran Nikolic, Caslav Lacnjevac, Miladin Gligoric, Jakov Stamenkovic, Milos B. Rajkovic, and Miroljub Barac. 2006. "Thermal Stability of Aqueous Polyurethanes Depending on the Applied Catalysts" Sensors 6, no. 11: 1697-1707. https://doi.org/10.3390/s6111697
APA StyleCakic, S., Nikolic, G., Lacnjevac, C., Gligoric, M., Stamenkovic, J., Rajkovic, M. B., & Barac, M. (2006). Thermal Stability of Aqueous Polyurethanes Depending on the Applied Catalysts. Sensors, 6(11), 1697-1707. https://doi.org/10.3390/s6111697





