Pulsed Amperometry for Anti-fouling of Boron-doped Diamond in Electroanalysis of β-Agonists: Application to Flow Injection for Pharmaceutical Analysis
Abstract
:1. Introduction
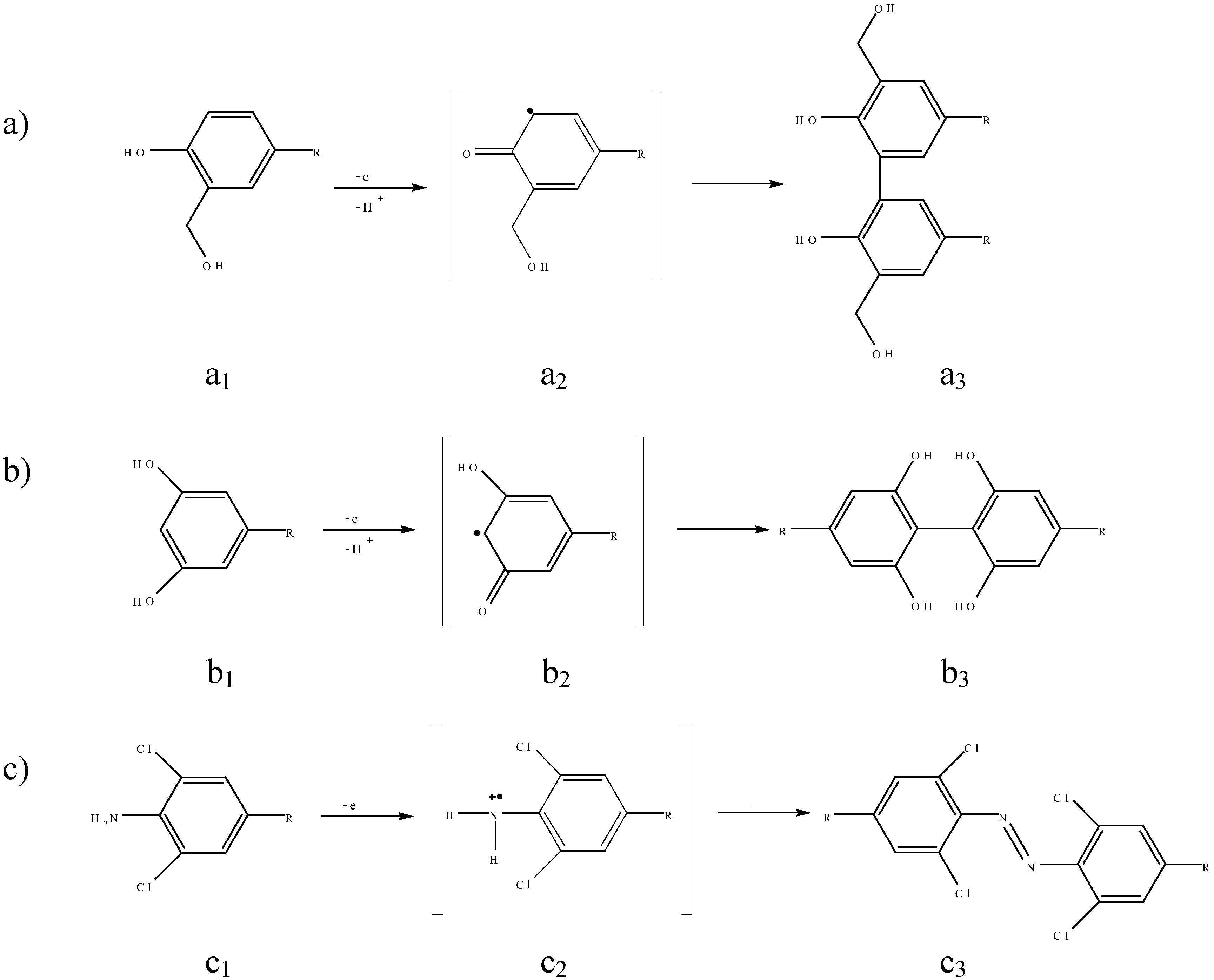
2. Experimental
2.1 The BDD Electrode
2.2 Cyclic voltammetry
2.3 Amperometric detection by flow injection
2.4 Chemicals
2.5 Sample preparation
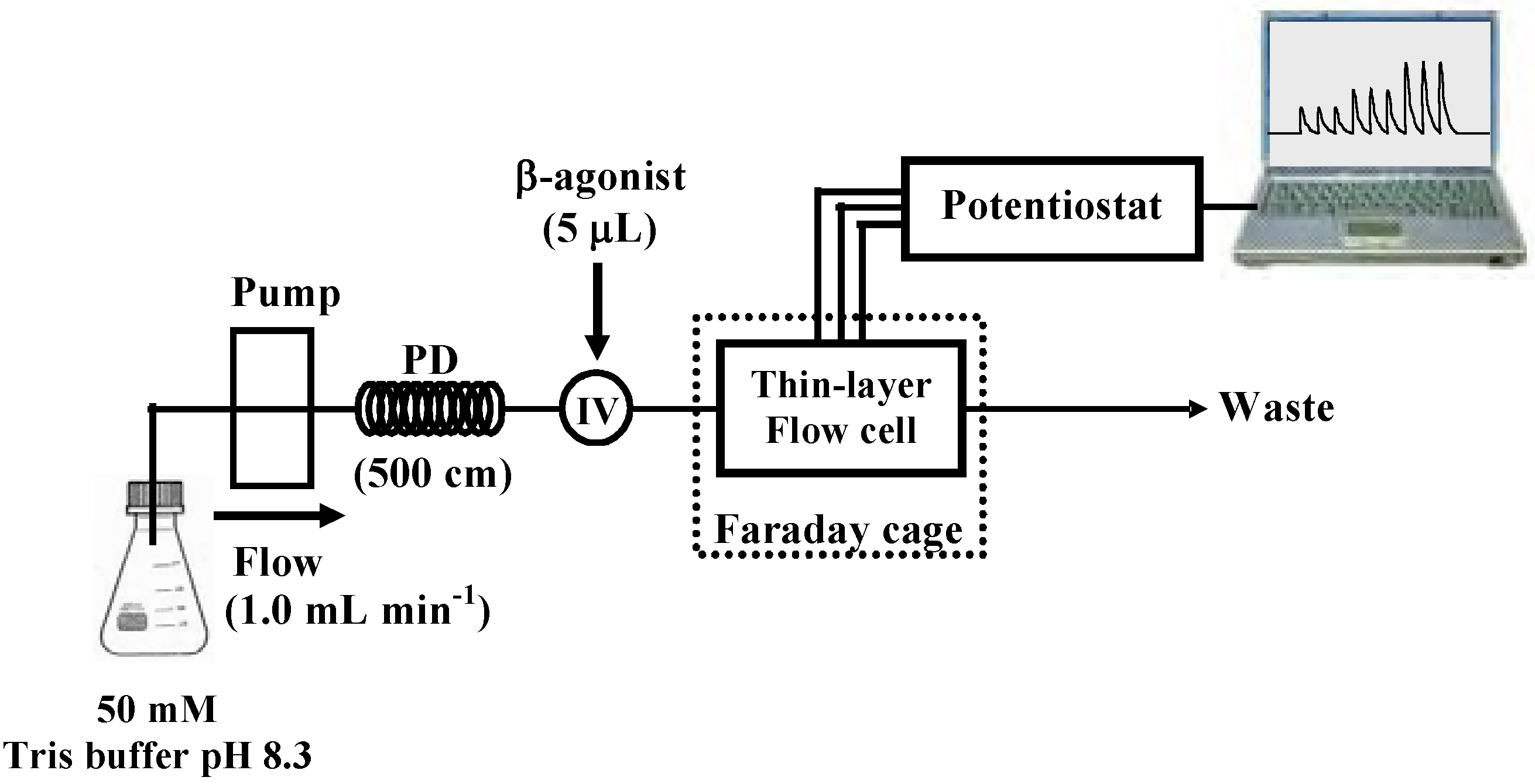
2.6 Analysis of samples by capillary electrophoresis
3. Results and Discussion
3.1 Cyclic voltammetry
3.1.1 Performance of BDD compared to GC
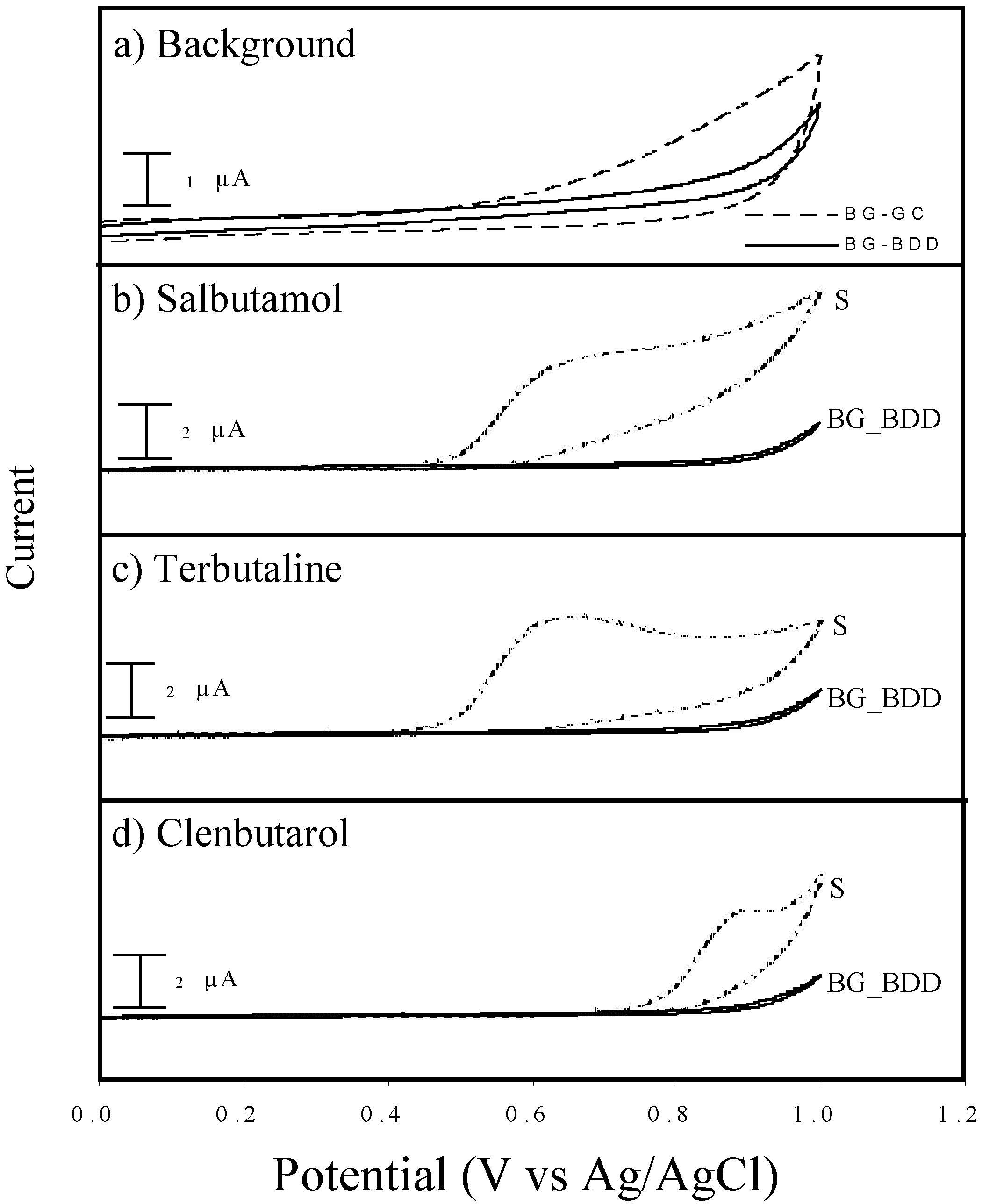
3.1.2 Selection of buffer
3.2 Amperometric flow injection experiments with BDD
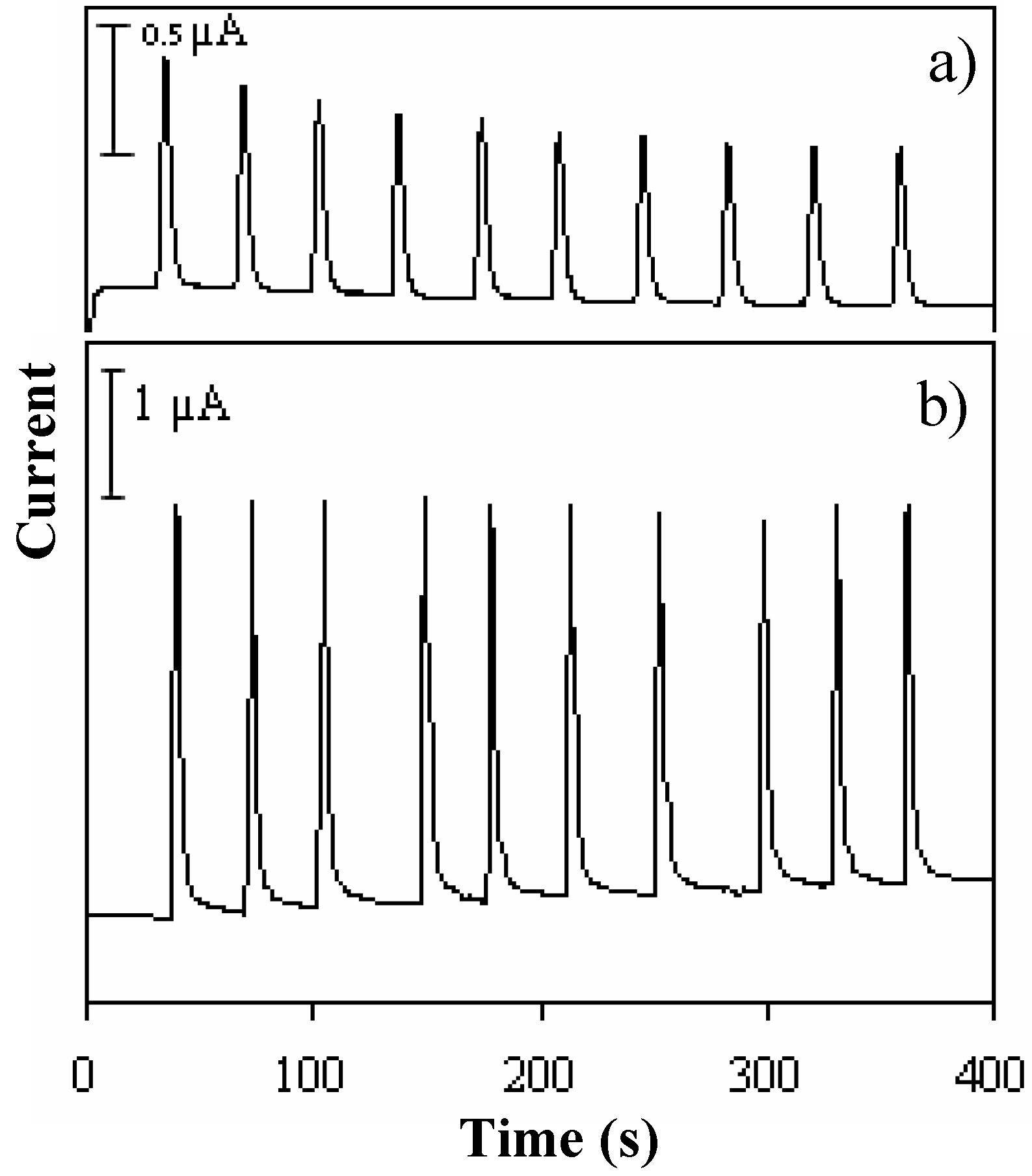
3.2.1 Fouling of BDD
| Wave form | Potential | Time (s) | ||||
|---|---|---|---|---|---|---|
| Studied range | Optimum | Studied range | Optimum | |||
 | Edet | 0.5-1.0 | 0.6s,t, 0.9c | tdel | 0.03-0.6 | 0.03 |
| tint | 0.03-0.2 | 0.03 | ||||
| Eoxd | 1.0-1.4 | 1.3 | toxd | 0.03-1.0 | 1.0 | |
| Ered | -0.2-0.3 | -0.1 | tred | 0.03-0.8 | 0.08 | |
 | ||||||
3.2.2 Electropolishing by PAD and waveform optimization
3.2.3 Optimization of injection volume
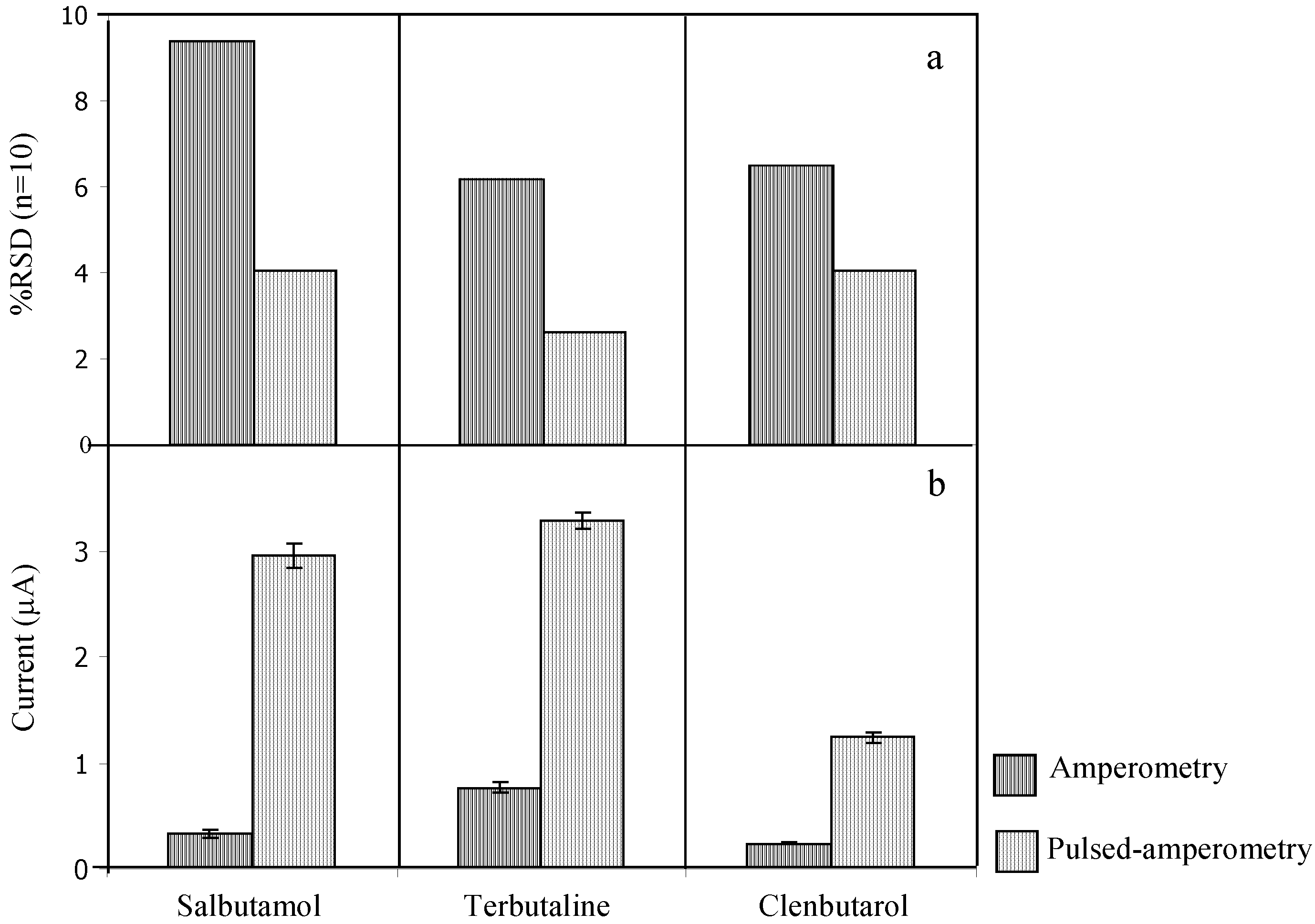
3.3 Analytical features
| Parameters | Salbutamol | Terbutaline | Clenbuterol |
|---|---|---|---|
| 1. Linear range (μM) | 0.5-100 | 1.0-100 | 0.5-50 |
| 2. Regression equation (slope x 10-3) | y = (6.2±0.3)x + (15.4±11.8) | y = (6.4±0.4)x + (20.2±23.0) | y = (6.0±0.3)x + (59.0±5.3) |
| 3. Correlation coefficient (r2) | 0.999 | 0.999 | 0.999 |
| 4. Detection limit (μM: 3S/N) | 0.1 | 0.5 | 0.3 |
| 5. Repeatability (%RSD, n=10) | 4.1 | 2.6 | 4.0 |
3.4 Performance for pharmaceutical applications
| Trade name | β-agonist | Type | Unit | Content of agonist | ||
|---|---|---|---|---|---|---|
| Label | CE-UVa | PAD-BDDb (this method) | ||||
| 1. Sabumol | Salbutamol | syrup | mg/L | 400 | 411 ± 19 | 408 ± 9 |
| 2. Ventolin | Salbutamol | tablet | mg/tablet | 2 | 2.03 ± 0.10 | 1.91 ± 0.03 |
| 3. Bricanyl | Terbutaline | syrup | mg/L | 300 | 287 ± 2.9 | 306 ± 3.3 |
| 4. Fasma | Terbutaline | tablet | mg/tablet | 2.5 | 2.55 ± 0.04 | 2.40 ± 0.07 |
4. Conclusion
Acknowledgements
References
- Fujishima, A.; Einaga, Y.; Rao, T. N.; Tryk, D. A. Diamond Electrochemistry; BKC INC; Tokyo and Elsevier B. V.: Amsterdam, 2005; pp. 1–25. [Google Scholar]
- Hupert, M.; Muck, A.; Wang, J.; Stotter, J.; Cvackova, Z.; Haymond, S.; Show, Y.; Swain, G. M. Conductive Diamond Thin-Films in Electrochemistry. Diam. Relat Mater. 2003, 12, 1940–1949. [Google Scholar] [CrossRef]
- Rao, T. N.; Fujishima , A. Recent Advances in Electrochemistry of Diamond. Diam. Relat Mater. 2000, 9, 384–389. [Google Scholar] [CrossRef]
- Compton, R. G.; Foord, J. S.; Marken, F. Electroanalysis at diamond-like and doped-diamond electrodes. Electroanalysis 2003, 15, 1349–1363. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Chatrathi, M. P.; Fujishima, A.; Tryk, D. A. Microchip Capillary Electrophoresis Coupled with a Boron-Doped Diamond Electrode-Based Electrochemical Detector. Anal. Chem. 2003, 75, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, G.; Muck, A.; Shin, D.; Fujishima, A. Microchip Capillary Electrophoresis with a Boron-doped Diamond Electrode for Rapid Separation and Detection of Purines. J. Chromatogr. A. 2004, 1022, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Q.; Swain, G. M. Anthraquinonedisulfonate Electrochemistry: A Comparison of Glassy Carbon, Hydrogenated Glassy Carbon, Highly Oriented Pyrolytic Graphite, and Diamond Electrodes. Anal. Chem. 1998, 70, 3146–3154. [Google Scholar] [CrossRef] [PubMed]
- Rao, T. N.; Yagi, I.; Miwa, T.; Tryk, D. A.; Fujishima, A. Electrochemical Oxidation of NADH at Highly Boron-Doped Diamond Electrodes. Anal. Chem. 1999, 71, 2506–2511. [Google Scholar] [CrossRef] [PubMed]
- Chailapakul, O.; Amatatongchai, M.; Wilairat, P.; Grudpan, K.; Nacapricha, D. Flow-injection Determination of Iodide Ion in Nuclear Emergency Tablets, Using Boron-Doped Diamond Thin Film Electrode. Talanta 2004, 64, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Siangproh, W.; Wangfuengkanagul, N.; Chailapakul, O. Electrochemical Oxidation of Tiopronin at Diamond Film Electrodes and Its Determination by Amperometric Flow Injection Analysis. Anal. Chim. Acta. 2003, 499, 183–189. [Google Scholar] [CrossRef]
- Preechaworapan, A.; Chuanuwatanakul, S.; Einaga, Y.; Grudpan, K.; Motomizu, S.; Chailapakul, O. Electroanlysis of Sulfonamides by Flow Injection System/High-Performance Liquid Chromatography Coupled with Amperometric Detection Using Boron-Doped Diamond Electrode. Talanta 2006, 68, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Boonsong, K.; Chuanuwatanakul, S.; Wangfuengkanagul, N.; Chailapakul, O. Electroanalysis of Lincomycin Using Boron-Doped Diamond Thin-Film Electrode Applied to Flow Injection System. Sensor Actuat. B 2005, 108, 627–632. [Google Scholar] [CrossRef]
- Witek, M. A.; Swain, G.M. Aliphatic Polyamine Oxidation Response Variability and Stability at Boron-Doped Diamond Thin-Film Electrodes as Studied by Flow-Injection Analysis. Anal. Chim. Acta. 2001, 440, 119–129. [Google Scholar] [CrossRef]
- Granger, M. G.; Xu, J.; Strojek, J. W.; Swain, G. M. Polycrystalline Diamond Electrodes: Basic Propoties and Applications as Amerometric Detectors in Flow Injection and Liquid Chromatography. Anal. Chim. Acta. 1999, 397, 145–161. [Google Scholar] [CrossRef]
- Chevolleau, S.; Tulliez, J. Optimization of the Separatioon of β-agonists by Capillary electrophoresis on untreated and C18 Bonded Silica Cpillaries. J. Chromatrogr. A 1995, 715, 345–354. [Google Scholar] [CrossRef]
- http://www.wada-ama.org.
- Dumasia, M.C.; Houghton, E. Screening and Confirmatory Analysis of Beta-agonists, Beta-antagonists and Their Metabolites in Horse Urine by Capillary Gas Chromatography-Mass Spectrometry. J. Chromatogr. 1991, 564, 503–513. [Google Scholar] [CrossRef]
- Montrade, M.P.; Bizec, B. Le.; Monteau , F.; Siliart, B.; Andre, F. Multi-residue analysis for β-Agonistic Drugs in Urine of Meat-Producing Animals by Gas Chromatography-Mass Spectrometry. Anal. Chim. Acta 1993, 275, 253–68. [Google Scholar] [CrossRef]
- Haasnoot, W.; Ploum, M.E.; Paulussen, R.J.A.; Schilt, R.; Huf, F.A. Rapid Determination of Clenbuterol Residues in Urine by High-Performance Liquid Chromatography with On-line Automated Sample Processing using Immunoaffinity Chromatography. J. Chromatogr. 1990, 519, 323–35. [Google Scholar] [CrossRef]
- Lin, L. A.; Tomlinson, R. S.; Stazger, R. D. Detection of Clenbuterol in Bovine Retina Tissue by High Performance Liquid Chromatography with Electrochemical Detection. J. Chromatogr. A 1997, 762, 275–280. [Google Scholar] [CrossRef]
- Debrauwer, L.; Delous, G.; Bories, G. Comparison of Various Hyphenated Chromatography - Mass Spectrometry Methods for the Analysis of β-agonists. Chromatographia 1993, 36, 218–224. [Google Scholar] [CrossRef]
- Debrauwer, L.; Bories, G. Determination of Clenbuterol Residues by Liquid Chromatographiy-Electrospray Mass Spectrometry. Anal. Chim. Acta 1993, 275, 231–239. [Google Scholar] [CrossRef]
- Lamoree, M. H.; Reinhoud, N. J.; Tjaden, U. R.; Niessen, W.M.A.; van der, Greef. On-capillary Isotachophoresis for Loadability Enhancement in Capillary Zone Electrophoresis/Mass Spectrometry of Beta-agonists. J. Biol. Mass Spectrom. 1994, 23, 339–45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Hu, Q.; Yu, H.; Fang, Y. Separation Determination of β-agonists in Serum by Capillary Zone Electrophoresis with Amperometric Detection. Anal. Chim. Acta. 2001, 441, 23–28. [Google Scholar] [CrossRef]
- Quintino, M. S. M.; Angnes, L. Bia-amperometric Quantification of Salbutamol in Pharmaceutical Products. Talanta 2004, 62, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Moane, S.; Rodriguez, Jose- Ramon B. Electrochemical Behavior of Clenbuterol at Nafion-Modified Carbon-paste Electrodes. J. Pharm. Biomed. Anal. 1995, 14, 57–63. [Google Scholar] [CrossRef]
- LaCourse, W. R. Pulsed Electrochemical Detection in High-Performance Liquid Chromatography. John Wiley & Sons, Inc.: Toronto, 1997; pp. 107–121. [Google Scholar]
- Fang Zhi, J.; Bin Wang, H.; Nakashima, T.; Rao, T. N.; Fujishima, A. Electrochemical Incineration of Organic Pollutants on Boron-Doped Diamond Electrode. Evidence for Direct Electrochemical Oxidation Pathway. J. Phys. Chem. B 2003, 107, 13389–13395. [Google Scholar]
- Yilmaz, N.; Ozkan, S. A.; Senturk, B. U. Z.; Biryol, I. Voltammetric Determination of Salbutamol Based on Electrochemical Oxidation at Platinum and Glassy Carbon Electrode. J. of Chemistry 1998, 22, 175–182. [Google Scholar]
- McGrath, G. J.; O’Kane, E.; Smyth, W. F.; Tagliaro, F. Investigation of the electrochemical oxidation of clenbuterol at a porous carbon electrode, and its application to the determination of this β–agonists in bovine hair by liquid chromatography with coulometric detection. Anal. Chim. Acta. 1996, 322, 159–166. [Google Scholar] [CrossRef]
- Siangproh, W.; Ngamukot, P.; Chailapakul, O. Electrochemical Determination of Captopril at Boron-Doped Diamond Thin Film Electrode Applied to a Flow Injection System. Sensor Actuat. B 2003, 91, 60–66. [Google Scholar] [CrossRef]
- Chailapakul, O.; Aksharanandana, P.; Frelink, T.; Einaga, Y.; Fujishima, A. The Electrooxidation of Sulfur-Containing Compounds at Boron-Doped Diamond Electrode. Sensor Actuat. B 2001, 80, 193–201. [Google Scholar] [CrossRef]
- Ho., K. K.; Hun, S.S.; Ju, K. H.; Young, J. E.; Jong-Seong, K.; Woongchon, M.; Rok, Y. J. Determination of Terbutaline Enantiomers in Human Urine by Capillary Electrophoresis Using Hydroxypropyl-beta-cyclodextrin as Chiral Selector. Archives of Pharm. Res. 2003, 26, 120–123. [Google Scholar]
- Zhou, T.; Hu, Q.; Yu, H.; Fang, Y. Separation and Determination of β-agonists in Serum by Capillary Zone Electrophoresis with Amperometric Detection. Anal. Chim. Acta. 2001, 44, 23–28. [Google Scholar] [CrossRef]
- Charoenraks, T.; Chuanuwatanakul, S.; Honda, K.; Yamaguchi, Y.; Chailapakul, O. Analysis of Tetracycline Antibiotics using HPLC With Pulsed Amperometric Detection. Anal. Sci. 2005, 21, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Charoenraks, T.; Palaharn, S.; Grudpan, K.; Siangproh, W.; Chailapakul, O. Flow Injection Analysis of Doxycycline or Chlortetracycline in Pharmaceutical Formulations With Pulsed Amperometric Detection. Talanta 2004, 64, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Agui, M. L.; Calavia, E.; Yanez-Sedeno, P.; Pingarron, J. M. Determination of the Antioxidant Irganox 1076 by Anodic Voltammetry and Flow Injection with Pulsed Amperometric Detection at a Glassy Carbon Electrode. Anal. Chim. Acta. 1995, 305, 324–331. [Google Scholar] [CrossRef]
- Miller, J. H.; Miller, J. C. Statistics and Chemometrics for Analytical Chemistry, fourth ed.Pearson Education Ltd.: Essex, 2000; pp. 48–50. [Google Scholar]
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Karuwan, C.; Mantim, T.; Chaisuwan, P.; Wilairat, P.; Grudpan, K.; Jittangprasert, P.; Einaga, Y.; Chailapakul, O.; Suntornsuk, L.; Anurukvorakun, O.; et al. Pulsed Amperometry for Anti-fouling of Boron-doped Diamond in Electroanalysis of β-Agonists: Application to Flow Injection for Pharmaceutical Analysis. Sensors 2006, 6, 1837-1850. https://doi.org/10.3390/s6121837
Karuwan C, Mantim T, Chaisuwan P, Wilairat P, Grudpan K, Jittangprasert P, Einaga Y, Chailapakul O, Suntornsuk L, Anurukvorakun O, et al. Pulsed Amperometry for Anti-fouling of Boron-doped Diamond in Electroanalysis of β-Agonists: Application to Flow Injection for Pharmaceutical Analysis. Sensors. 2006; 6(12):1837-1850. https://doi.org/10.3390/s6121837
Chicago/Turabian StyleKaruwan, Chanpen, Thitirat Mantim, Patcharin Chaisuwan, Prapin Wilairat, Kate Grudpan, Piyada Jittangprasert, Yasuaki Einaga, Orawon Chailapakul, Leena Suntornsuk, Oraphan Anurukvorakun, and et al. 2006. "Pulsed Amperometry for Anti-fouling of Boron-doped Diamond in Electroanalysis of β-Agonists: Application to Flow Injection for Pharmaceutical Analysis" Sensors 6, no. 12: 1837-1850. https://doi.org/10.3390/s6121837




