Sensing Arrays Constructed from Nanoparticle Thin Films and Interdigitated Microelectrodes
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
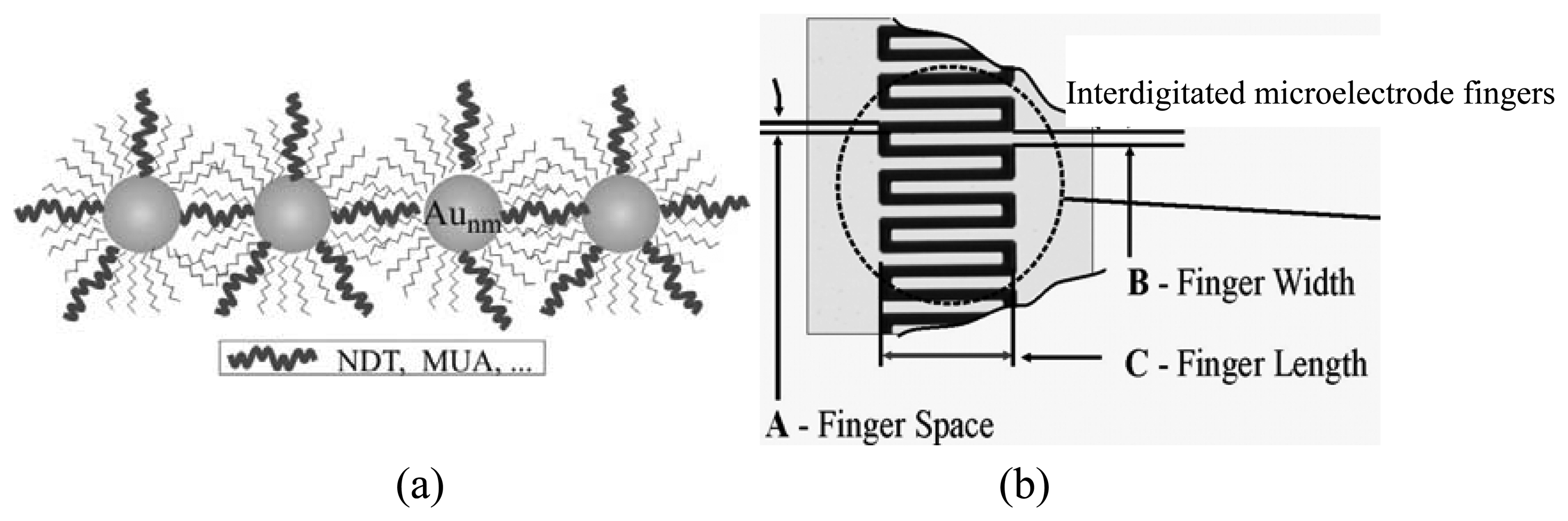
3.1. Sensor Device Characterization
3.2. Array Response Characteristics
3.3. Statistical Analysis of Sensor Array Data
4. Conclusions
Acknowledgments
References
- Wohltjen, H.; Snow, A. W. Colloidal metal-insulator-metal ensemble chemiresistor sensor. Anal. Chem. 1998, 70, 2856–2859. [Google Scholar]
- Zamborini, F. P.; Leopold, M. C.; Hicks, J. F.; Kulesza, P. J.; Malik, M. A.; Murray, R. W. Electron hopping conductivity and vapor sensing properties of flexible network polymer films of metal nanoparticles. J. Am. Chem. Soc. 2002, 124, 8958–8964. [Google Scholar]
- Grate, J. W.; Nelson, D. A.; Skaggs, R. Sorptive behavior of monolayer-protected gold nanoparticle films: Implications for chemical vapor sensing. Anal. Chem. 2003, 75, 1868–1879. [Google Scholar]
- Severin, E. J.; Lewis, N. S. Relationships among resonant frequency changes on a coated quartz crystal microbalance, thickness changes, and resistance responses of polymer-carbon black composite chemiresistors. Anal. Chem. 2000, 72, 2008–2015. [Google Scholar]Shinar, R.; Liu, G. J.; Porter, M. D. Graphite microparticles as coatings for quartz crystal microbalance-based gas sensors. Anal. Chem. 2000, 72, 5981–5987. [Google Scholar]Joseph, Y.; Besnard, I.; Rosenberger, M.; Guse, B.; Nothofer, H-G.; Wessels, J. M.; Wild, U.; Knop-Gericke, A.; Su, D.; Schlögl, R.; Yasuda, A.; Vossmeyer, T. Self-assembled gold nanoparticle/alkanedithiol films: Preparation, electron microscopy, XPS-analysis, charge transport, and vapor-sensing properties. J. Phys. Chem. B. 2003, 107, 7406. [Google Scholar]Cai, Q. Y.; Zellers, E. T. Dual-chemiresistor GC detector employing layer-protected metal nanocluster interfaces. Anal. Chem. 2002, 74, 3533. [Google Scholar]
- Han, L.; Daniel, D. R.; Maye, M. M.; Zhong, C. J. Core-shell nanostructured nanoparticle films as chemically sensitive interfaces. Anal. Chem. 2001, 73, 4441–4449. [Google Scholar]
- Han, L.; Shi, X.; Wu, W.; Kirk, F. L.; Luo, J.; Wang, L.Y.; Mott, D.; Cousineau, L.; Lim, S. I-Im; Lu, S.; Zhong, C. J. Nanoparticle-Structured Sensing Array Materials and Pattern Recognition for VOC Detection. Sens. Actuators, B. 2005, 106, 431–441. [Google Scholar]
- Shi, X.; Wang, L.Y.; Kariuki, N.; Luo, J.; Zhong, C. J.; Lu, S. A Multi-Module Artificial Neural Network Approach to Pattern Recognition with Optimized Nanostructured Sensor Array. Sens. Actuators, B 2006, in press. [Google Scholar]
- Zellers, E. T.; Pan, T. S.; Patrash, S. J.; Han, M.; Batterman, S. A. Extended disjoint principal-components regression-analysis of saw vapor sensor-array responses. Sens. Actuators B 1993, 12, 123. [Google Scholar]Bakken, G. A.; Kauffman, G. W.; Jurs, P. C.; Albert, K. J.; Stitzel, S. S. Pattern recognition analysis of optical sensor array data to detect nitroaromatic compound vapors. Sens. Actuators B 2001, 79, 1–10. [Google Scholar]Gardner, J. W. Pattern recognition part 1: Principal component and cluster analysis sensors and sctuators. Sens. Actuators B 1991, 4, 109. [Google Scholar]Corcoran, P.; Lowery, P.; Anglesea, J. Optimal configuration of a thermally cycled gas sensor array with neural network pattern recognition. Sens Actuators B 1998, 48, 448. [Google Scholar]Polikar, R.; Shinar, R.; Udpa, L.; Porter, M. D. Artificial intelligence methods for selection of an optimized sensor array for identification of volatile organic compounds. Sens Actuators B 2001, 80, 243–254. [Google Scholar]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D. J.; Whyman, R. Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system. J. Chem. Soc., Chem. Commun. 1994, 801–802. [Google Scholar]
- Hostetler, M. J.; Wingate, J. E.; Zhong, C. J.; Harris, J. E.; Vachet, R. W.; Clark, M. R.; Londono, J. D.; Green, S. J.; Stokes, J. J.; Wignall, G. D.; Glish, G. L.; Porter, M. D.; Evans, N. D.; Murray, R. W. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: core and monolayer properties as a function of core size. Langmuir 1998, 14, 17–30. [Google Scholar]
- Leibowitz, F. L.; Zheng, W. X.; Maye, M. M.; Zhong, C. J. Structures and properties of nanoparticle thin films formed via a one-step - Exchange-cross-linking - Precipitation route. Anal. Chem. 1999, 71, 5076–5083. [Google Scholar]Zheng, W. X.; Maye, M. M.; Leibowitz, F. L.; Zhong, C. J. Imparting biomimetic ion-gating recognition properties to electrodes with a hydrogen-bonding structured core-shell nanoparticle network. Anal. Chem. 2000, 72, 2190. [Google Scholar]Han, L.; Maye, M. M.; Leibowitz, F. L.; Ly, N. K.; Zhong, C. J. Quartz-crystal microbalance and spectrophotometric assessments of inter-core and inter-shell reactivities in nanoparticle thin film formation and growth. J. Mater. Chem. 2001, 11, 1259–1264. [Google Scholar]
- Maye, M. M.; Zheng, W. X.; Leibowitz, F. L.; Ly, N. K.; Zhong, C. J. Heating-induced evolution of thiolate-encapsulated gold nanoparticles: a strategy for size and shape manipulations. Langmuir 2000, 16, 490–497. [Google Scholar]Maye, M. M.; Zhong, C. J. Manipulating core-shell reactivities for processing nanoparticle sizes and shapes. J. Mater. Chem. 2000, 10, 1895–1901. [Google Scholar]
- Sotzing, G. A.; Briglin, S. M.; Grubbs, R. H.; Lewis, N. S. Preparation and properties of vapor detector arrays formed from poly(3,4 ethylenedioxy)thiophene-poly(styrene sulfonate)/insulating polymer composites. Anal. Chem. 2000, 72, 3181–3190. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 5th edition; John Wiley & Sons: New York, 2001. [Google Scholar]
- Montgomery, D. C.; Runger, G. C. Applied Statistics and Probability of Engineers; John Wiley & Sons: New York, 1999. [Google Scholar]



| Design # | FS (μm) | FW (μm) | FL (μm) |
|---|---|---|---|
| 1 | 10 (1) | 10 (1) | 100 (-1) |
| 2 | 10 (1) | 5 (-1) | 200 (1) |
| 3 | 5 (-1) | 10 (1) | 200 (1) |
| 4 | 5 (-1) | 5 (-1) | 100 (-1) |
| Film | #1 | #2 | #3 | #4 | (A)* | (B)* |
|---|---|---|---|---|---|---|
| MUA-Aunm | 54.92 | 27.01 | 11.73 | 12.92 | 3.8 | 11.7 |
| NDT-Aunm | 0.373 | 0.174 | 0.081 | 0.092 | 0.556 | 0.342 |
| RS** | NDT-Aunm/IME | MUA-Aunm/IME | (A)* | (B)* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #1 | #2 | #3 | #4 | NDT | MUA | NDT | MUA | |
| Hx | 4.72 | 5.34 | 6.98 | 6.64 | 16.4 | 18.1 | 16.3 | 21.3 | 5.49 | 18.7 | 3.1 | 19 |
| Tl | 8.84 | 3.48 | 20.1 | 19.4 | 94.8 | 98.2 | 89.8 | 102 | 31.4 | 82.8 | 22 | 200 |
| Bz | 4.60 | 5.24 | 8.07 | 7.73 | 34.0 | 37.2 | 32.6 | 42.5 | 7.1 | 63 | ||
| Source of Variation | Sum of Squares (×10-7) | Degrees of Freedom | Mean Square (×10-7) | F0 | P-value |
|---|---|---|---|---|---|
| FS | 2.45 | 1 | 2.45 | 1838 | <0.01 |
| FW | 0.05 | 1 | 0.05 | 37.5094 | <0.01 |
| FL | 0.0055 | 1 | 0.0055 | 4.1335 | 0.065 |
| Error | 0.004 | 3 | 0.0013 | - | - |
| Total | 2.5095 | 6 | - | - | - |
| Source of Variation | Sum of Squares (×10-7) | Degrees of Freedom | Mean Square (×10-7) | F0 | P-value |
|---|---|---|---|---|---|
| FS | 0.0221 | 1 | 0.0221 | 16.6377 | <0.01 |
| FW | 0.0889 | 1 | 0.0889 | 66.7502 | <0.01 |
| FL | 0.0821 | 1 | 0.0221 | 16.6377 | <0.01 |
| Error | 0.004 | 3 | 0.0013 | - | - |
| Total | 1.3334 | 6 | - | - | - |
| Factor | NDT-Aunm | MUA-Aunm |
|---|---|---|
| FS | -3.5 | -0.333 |
| FW | 0.5 | -0.667 |
| FL | -0.166 | -0.333 |
| MUA-Aunm | NDT-Aunm | MUA-+NDT-Aunm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
| Eigenvalue | 3.9898 | 0.0095 | 0.0006 | 3.799 | 0.1935 | 0.0072 | 5.902 | 1.0377 | 0.0539 |
| Proportion | 0.997 | 0.002 | 0 | 0.95 | 0.048 | 0.002 | 0.843 | 0.148 | 0.008 |
| Cumulative | 0.997 | 1 | 1 | 0.95 | 0.998 | 1 | 0.843 | 0.991 | 0.999 |
© 2006 by MDPI ( http://www.mdpi.org) Reproduction is permitted for noncommercial purposes.
Share and Cite
Wang, L.; Kariuki, N.N.; Schadt, M.; Mott, D.; Luo, J.; Zhong, C.-J.; Shi, X.; Zhang, C.; Hao, W.; Lu, S.; et al. Sensing Arrays Constructed from Nanoparticle Thin Films and Interdigitated Microelectrodes. Sensors 2006, 6, 667-679. https://doi.org/10.3390/s6060667
Wang L, Kariuki NN, Schadt M, Mott D, Luo J, Zhong C-J, Shi X, Zhang C, Hao W, Lu S, et al. Sensing Arrays Constructed from Nanoparticle Thin Films and Interdigitated Microelectrodes. Sensors. 2006; 6(6):667-679. https://doi.org/10.3390/s6060667
Chicago/Turabian StyleWang, Lingyan, Nancy N. Kariuki, Mark Schadt, Derrick Mott, Jin Luo, Chuan-Jian Zhong, Xiajing Shi, Chen Zhang, Weibing Hao, Susan Lu, and et al. 2006. "Sensing Arrays Constructed from Nanoparticle Thin Films and Interdigitated Microelectrodes" Sensors 6, no. 6: 667-679. https://doi.org/10.3390/s6060667
APA StyleWang, L., Kariuki, N. N., Schadt, M., Mott, D., Luo, J., Zhong, C.-J., Shi, X., Zhang, C., Hao, W., Lu, S., Kim, N., & Wang, J.-Q. (2006). Sensing Arrays Constructed from Nanoparticle Thin Films and Interdigitated Microelectrodes. Sensors, 6(6), 667-679. https://doi.org/10.3390/s6060667




