Formation and Fluorimetric Characterization of Micelles in a Micro-flow Through System with Static Micro Mixer
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and micelle formation
2.2. Micro fluidic arrangement
2.3. Measurement principle
3. Results and Discussion
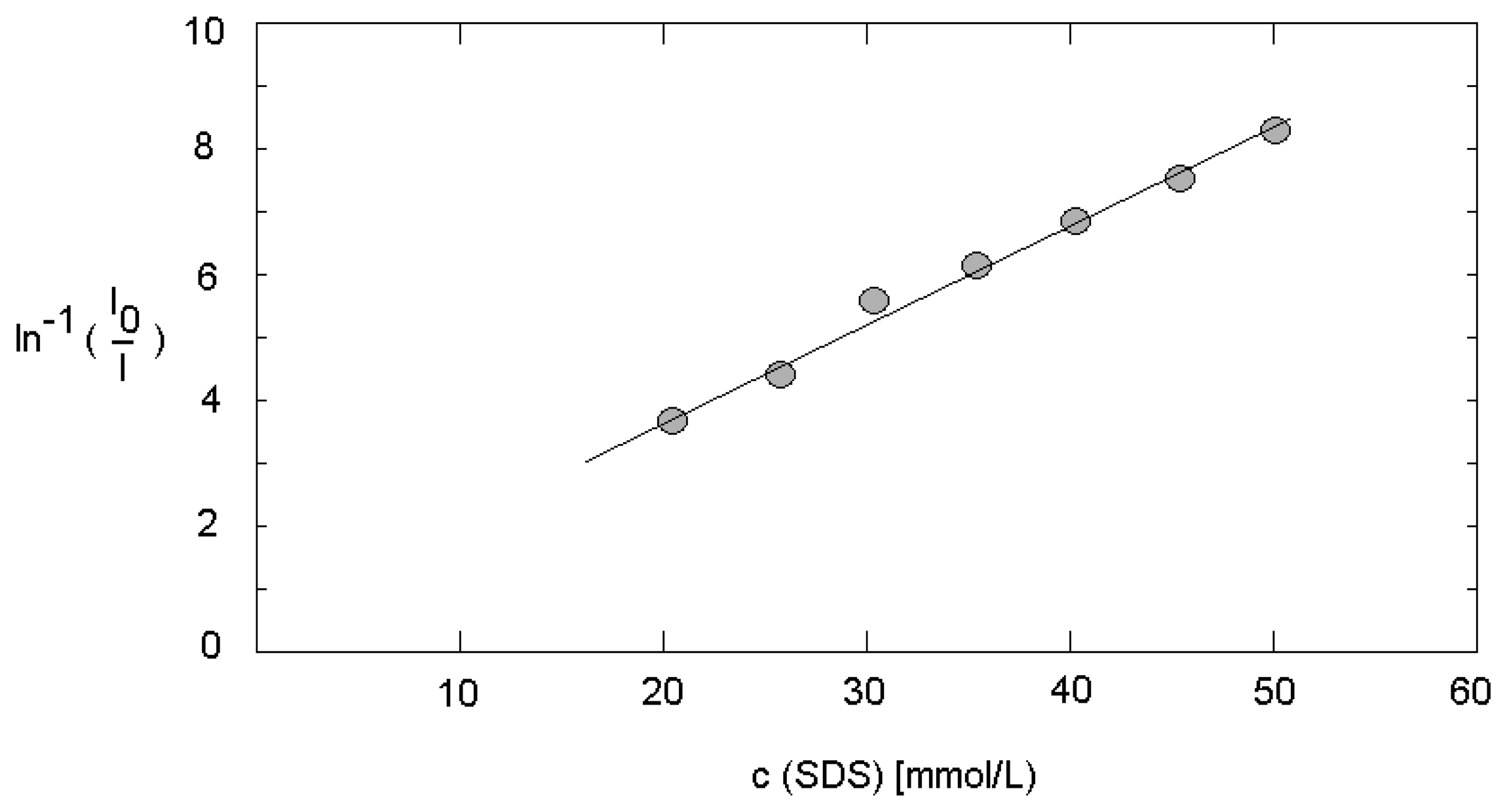
3.1. Reference measurements at static conditions
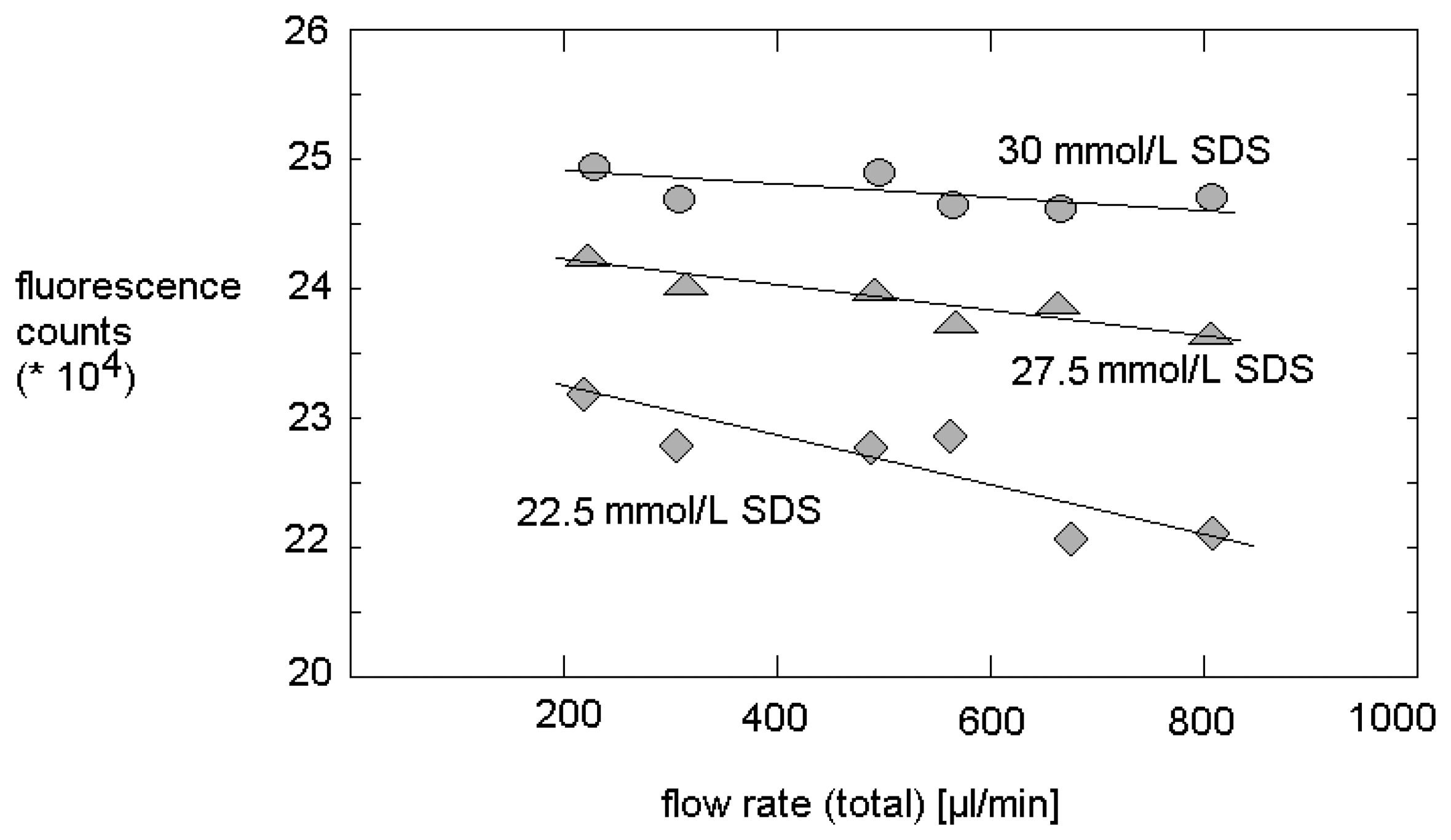
3.2. Characterization of micro fluidic arrangement
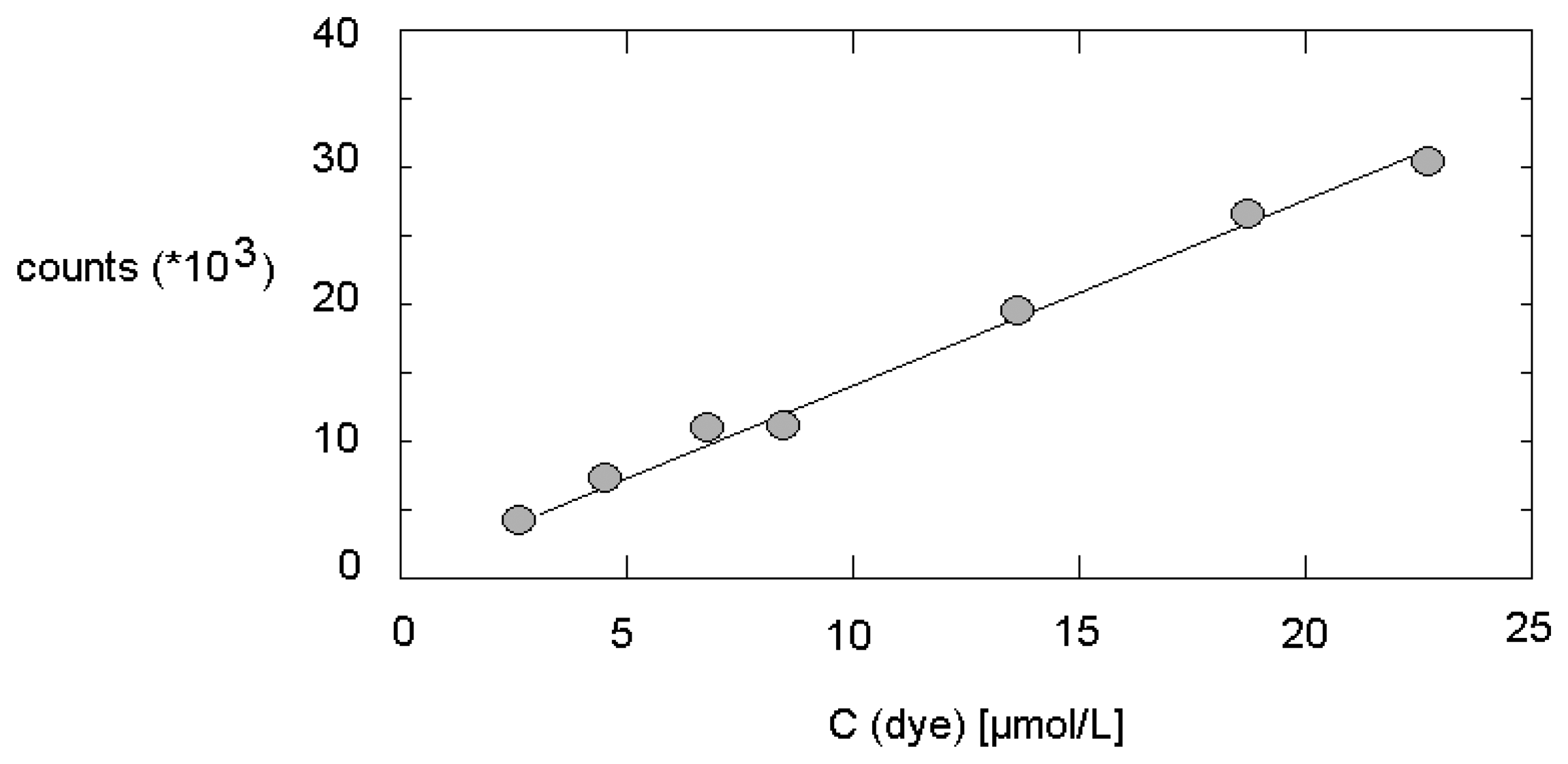
3.3. Fluorescence intensity and micelle formation under micro fluidic conditions
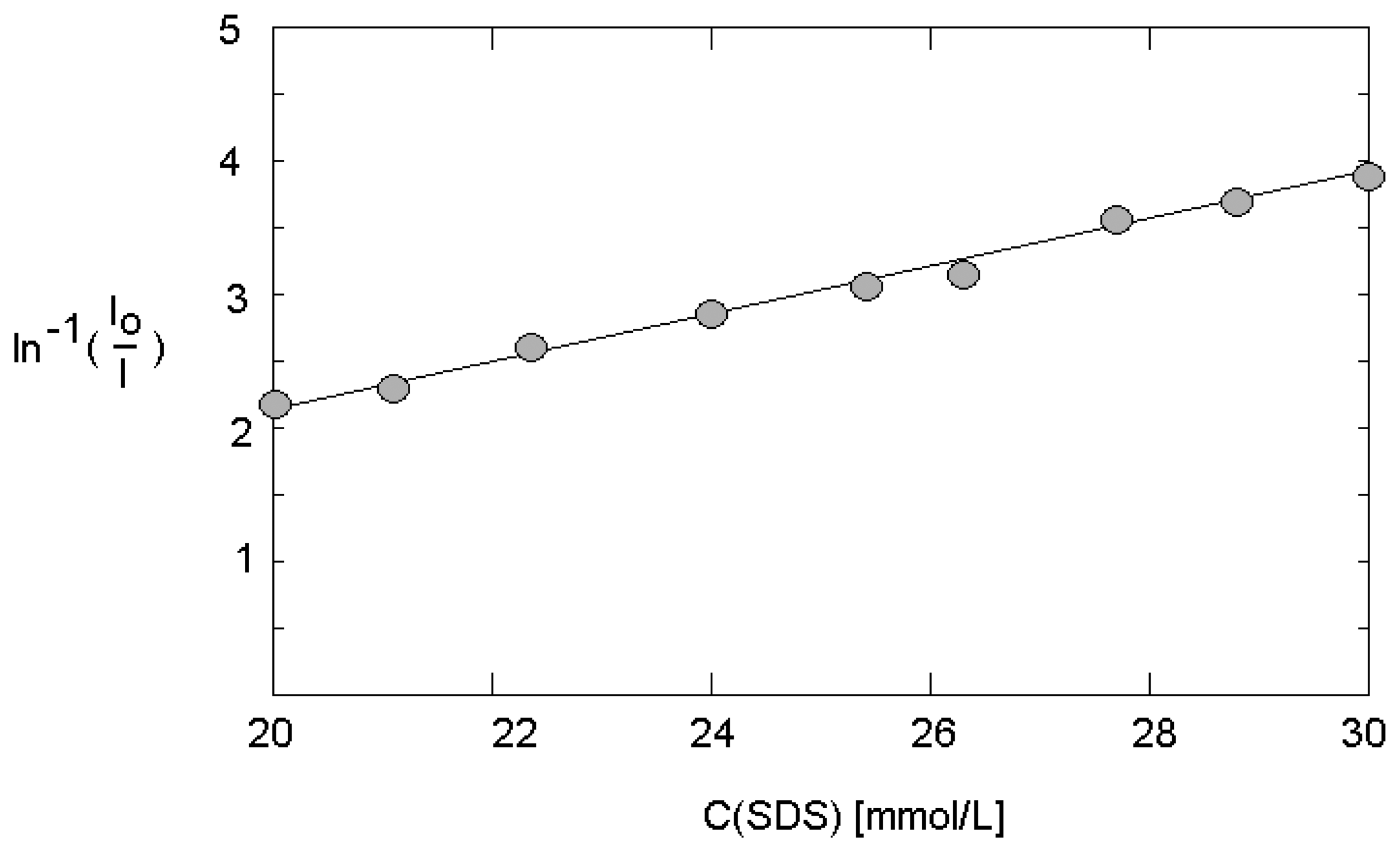
3.4. Fluorescence behavior in presence of quencher and determination of aggregation number at micro fluidic conditions
4. Conclusions
Acknowledgments
References and Notes
- Burns, J.R.; Ramshaw, C. The intensification of rapid reactions in multiphase systems using slug flow in capillaries. Lab on a Chip 2001, 1, 10–15. [Google Scholar]
- Thorsen, T.; Roberts, R.W.; Arnold, F.H.; Quake, S.R. Dynamic Pattern Formation in a Vesicle-Generating Microfluidic Device. Phys. Rev. Lett. 2001, 86, 4163–4166. [Google Scholar]
- Nisisako, T.; Torii, T.; Higuchi, T. Chemical reactions in microdroplets by electrostatic manipulation of droplets in liquid media. Lab on a Chip 2002, 2, 19–23. [Google Scholar]
- Nisisako, T.; Torii, T.; Higuchi, T. Droplet formation in a microchannel network. Lab on a Chip 2002, 2, 24–26. [Google Scholar]
- Link, D.R.; Anna, S.L.; Weitz, D.A.; Stone, H.A. Geometrically Mediated Breakup of Drops in Microfluidic Devices. Phys. Rev. Lett. 2004, 92, 054503. [Google Scholar]
- Nisisako, T.; Okushima, S.; Torii, T. Controlled formulation of monodisperse double emulsions in a multiple-phase microfluidic system. Soft Matter 2005, 1, 23–27. [Google Scholar]
- Köhler, J.M.; Kirner, T. Nanoliter segment formation in micro fluid devices for chemical and biological micro serial flow processes in dependence on flow rate and viscosity. Sensors and Actuators A 2005, 119, 19–27. [Google Scholar]
- Balasz, A.C.; Verberg, R.; Pooley, C.M.; Kusenok, O. Modeling the flow of complex fluids through heterogeneous channels. Soft Matter 2005, 1, 44–54. [Google Scholar]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A Microfluidic System for Controlling Reaction Networks in Time. Angew. Chem. 2003, 115, 792–796. [Google Scholar]
- Ismagilov, R.F. Integrierte Mikrofluidsysteme. Angew. Chem. 2003, 115, 4262–4264. [Google Scholar]
- Shestopalov, I.; Tice, J.D.; Ismagilov, R.F. Multi-step synthesis of nanoparticles performed on millisecond time scale in a microfluidic droplet-based system. Lab on a Chip 2004, 4, 316–321. [Google Scholar]
- Zheng, B.; Tice, J.D.; Ismagilov, R.F. Formation of Droplets of Alternating Composition in Microfluidic Channels and Applications to Indexing of Concentrations in Droplet-Based Assays. Anal. Chem. 2004, 76, 4977–4982. [Google Scholar]
- Köhler, J.M.; Henkel, T.; Grodrian, A.; Kirner, T.; Roth, M.; Martin, K.; Metze, J. Digital reaction technology by micro segmented flow—components, concepts and applications. Chem. Engn. J. 2004, 101, 201–216. [Google Scholar]
- Henkel, T.; Bermig, T.; Kielpinski, M.; Grodrian, A.; Metze, J.; Köhler, J.M. Chip modules for generation and manipulation of fluid segments for micro serial flow processes. Chem. Engn. J. 2004, 101, 439–445. [Google Scholar]
- Günther, P.M.; Möller, F.; Henkel, T.; Köhler, J.M.; Groβ, G.A. Formation of Monomeric and Novolak Azo Dyes in Nanofluid Segments by Use of a Double Injector Chip Reactor. Chem. Eng. Technol. 2005, 28, 520–527. [Google Scholar]
- Martin, K.; Henkel, T.; Baier, V.; Grodrian, A.; Schön, T.; Roth, M.; Köhler, J.M.; Metze, J. Generation of larger numbers of separated microbial populations by cultivation in segmented-flow microdevices. Lab on a Chip 2003, 3, 202–207. [Google Scholar]
- Grodrian, A.; Metze, J.; Henkel, T.; Martin, K.; Roth, M.; Köhler, J.M. Segmented flow generation by chip reactors for highly parallelized cell cultivation. Biosensors & Bioelectronics 2004, 19, 1421–1428. [Google Scholar]
- Brösing, A.; Köhler, J.M. Kolloquium Heiligenstadt 2004, 12, 381.
- Hessel, V.; Löwe, H. Mikroverfahrenstechnik: Komponenten - Anlagenkonzeption – Anwender-akzeptanz - Teil 1. Chemie Ingenieur Technik 2002, 74, 17–30. [Google Scholar]
- Hessel, V.; Löwe, H. Mikroverfahrenstechnik: Komponenten - Anlagenkonzeption – Anwender-akzeptanz - Teil 2. Chemie Ingenieur Technik 2002, 74, 185–207. [Google Scholar]
- Jongen, N.; Donnet, M.; Bowen, P.; Lemaître, J.; Hofmann, H.; Schenk, R.; Hofmann, C.; Aoun-Habbache, M.; Guillemet-Fritsch, S.; Sarrias, J.; Rousset, A.; Viviani, M.; Buscaglia, M.T.; Buscaglia, V.; Nanni, P.; Testino, A.; Herguijuela, J.R. Development of a Continuous Segmented Flow Tubular Reactor and the “Scale-out” Concept - In Search of Perfect Powders. Chem. Eng. Technol. 2003, 26, 303–305. [Google Scholar]
- Schenk, R.; Hessel, V.; Werner, B.; Ziogas, A.; Hofmann, C.; Donnet, M.; Jongen, N. Micromixers as a tool for powder production. Chem. Eng. Trans. 2002, 1, 909–914. [Google Scholar]
- Chan, E.M.; Mathies, R.A.; Alivisatos, A.P. Size-Controlled Growth of CdSe Nanocrystals in Microfluidic Reactors. Nano Letters 2003, 3, 199–201. [Google Scholar]
- Fritzsche, W. DNA-gold conjugates for the detection of specific molecular interactions. Rev. Mol. Biotechnol. 2001, 82, 37–46. [Google Scholar]
- Wagner, J.; Kirner, T.; Mayer, G.; Albert, J.; Köhler, J.M. Generation of metal nanoparticles in a microchannel reactor. Chem. Eng. J. 2004, 101, 251–260. [Google Scholar]
- Wagner, J.; Köhler, J.M. Continuous Synthesis of Gold Nanoparticles in a Microreactor. Nano Letters 2005, 5, 685–691. [Google Scholar]
- Köhler, J.M.; Wagner, J.; Albert, J. Formation of isolated and clustered Au nanoparticles in the presence of polyelectrolyte molecules using a flow-through Si chip reactor. J. Mater. Chem. 2005, 15, 1924–1930. [Google Scholar]
- Chang, Z.; Liu, G.; Tian, Y.; Zhang, Z. Preparation of micron-size monodisperse poly(vinyl acetate) microspheres with γ-rays-initiated dispersion polymerization in microreactor. Materials Letters 2004, 58, 522–524. [Google Scholar]
- Günther, P.M.; Wagner, J.; Groβ, G.A.; Köhler, J.M. Proceedings of the 9th Internatl. Conf. on Miniaturized Systems μ-TAS. Boston, Oct 9-13 2005; pp. 918–920.
- Noorgard, K.; Weygand, M.J.; Kjaer, K.; Brust, M.; Bjornholm, T. Adaptive chemistry of bifunctional gold nanoparticles at the air/water interface. A synchrotron X-ray study of giant amphiphiles. Faraday Discussions 2004, 125, 221–233. [Google Scholar]
- Doty, R.C.; Tsikhudo, T.R.; Brust, M.; Fernig, D. Extremely Stable Water-Soluble Ag Nanoparticles. Chemistry of Materials 2005, 17, 4630–4635. [Google Scholar]
- Sönnichsen, K. Presented at 3rd Ilmenauer Workshop on Micro Laboratory Techniques; Ilmenau, Germany, 2006. [Google Scholar]
- Dörfler, H.-D. Grenzflächen- und Kolloidchemie; Wiley/VCH: Weinheim, 1994. [Google Scholar]
- Moroi, Y. Micelles: Theoretical and Applied Aspects; Springer: New York, 1992. [Google Scholar]
- Turro, N.; Yekta, A. Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles. J. Am. Chem. Soc. 1978, 100, 5951–5952. [Google Scholar]
- Quina, F.H.; Nassar, P.M.; Bonilha, J.B.S.; Bales, B.L. Growth of Sodium Dodecyl Sulfate Micelles with Detergent Concentration. J. Phys. Chem. 1995, 99, 17028–17031. [Google Scholar]
- Schwesinger, N.; Frank, T.; Wurmus, H. A modular microfluid system with an integrated micromixer. J. Micromech. Microeng. 1996, 6, 99–102. [Google Scholar]
- Hardt, S.; Drese, K.S.; Hessel, V.; Schönfeld, F. Passive micromixers for applications in the microreactor and mTAS fields. Microfluid Nanofluid 2005, 1, 108–118. [Google Scholar]
- Kirner, T.; Albert, J.; Günther, M.; Mayer, G.; Reinhäckel, K.; Köhler, J.M. Static micromixers for modular chip reactor arrangements in two-step reactions and photochemical activated processes. Chem. Eng. J. 2004, 101, 65–74. [Google Scholar]
- Draeger, Ch. Mesoskopische Mizellen aus amphiphilen Bipyridiyliumkomplexen mit Ruthenmium (II), Osmium (II) und Palladium (II). Ph.D. Thesis, Techn. Univ., Berlin, 2001. [Google Scholar]


© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Schuch, M.; Groß, G.A.; Köhler, J.M. Formation and Fluorimetric Characterization of Micelles in a Micro-flow Through System with Static Micro Mixer. Sensors 2007, 7, 2499-2509. https://doi.org/10.3390/s7112499
Schuch M, Groß GA, Köhler JM. Formation and Fluorimetric Characterization of Micelles in a Micro-flow Through System with Static Micro Mixer. Sensors. 2007; 7(11):2499-2509. https://doi.org/10.3390/s7112499
Chicago/Turabian StyleSchuch, Michael, G. Alexander Groß, and J. Michael Köhler. 2007. "Formation and Fluorimetric Characterization of Micelles in a Micro-flow Through System with Static Micro Mixer" Sensors 7, no. 11: 2499-2509. https://doi.org/10.3390/s7112499
APA StyleSchuch, M., Groß, G. A., & Köhler, J. M. (2007). Formation and Fluorimetric Characterization of Micelles in a Micro-flow Through System with Static Micro Mixer. Sensors, 7(11), 2499-2509. https://doi.org/10.3390/s7112499




