Electroanalysis of Plant Thiols
Abstract
:1. Introduction
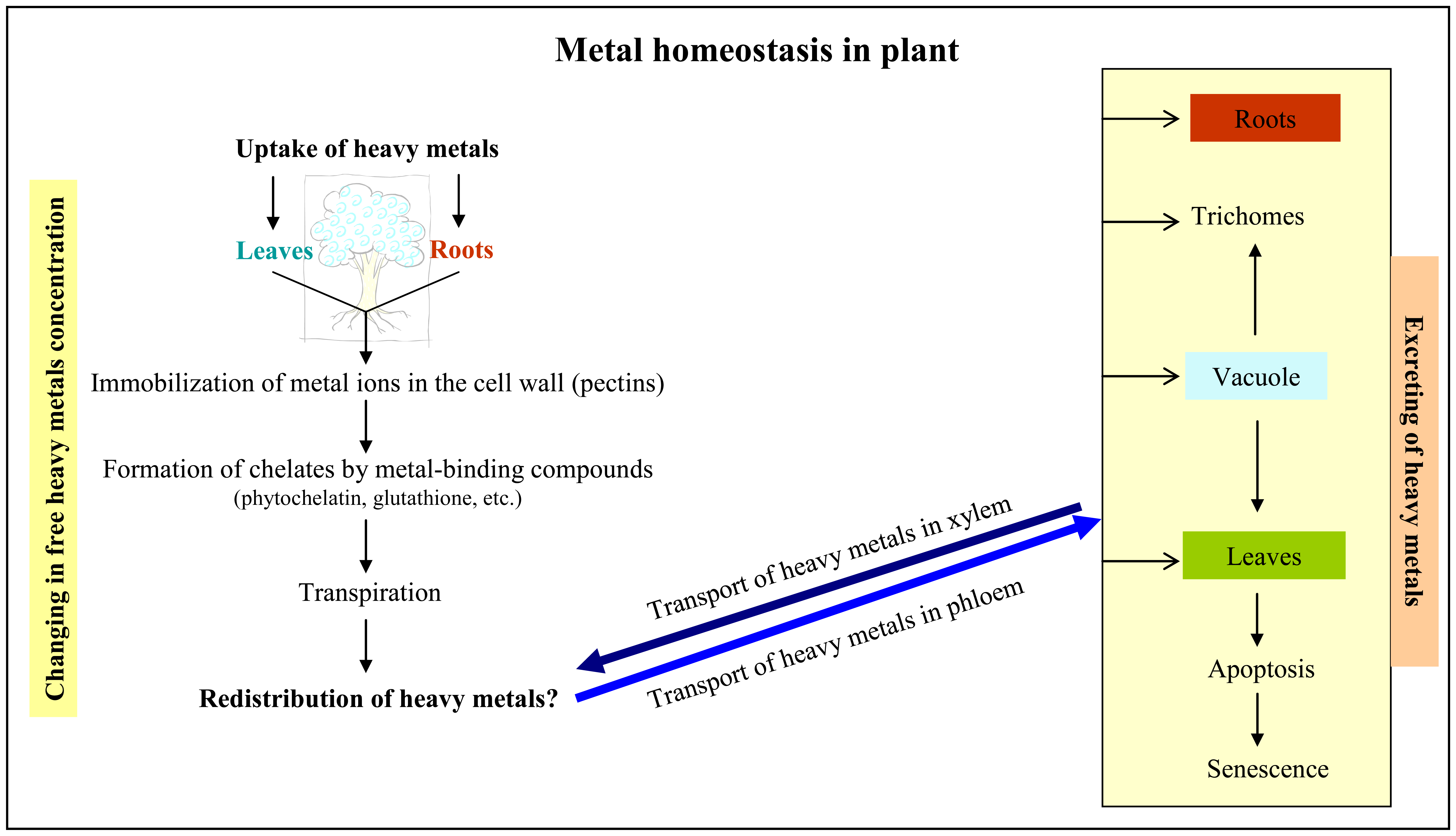
1.1 Uptake of heavy metals by plants
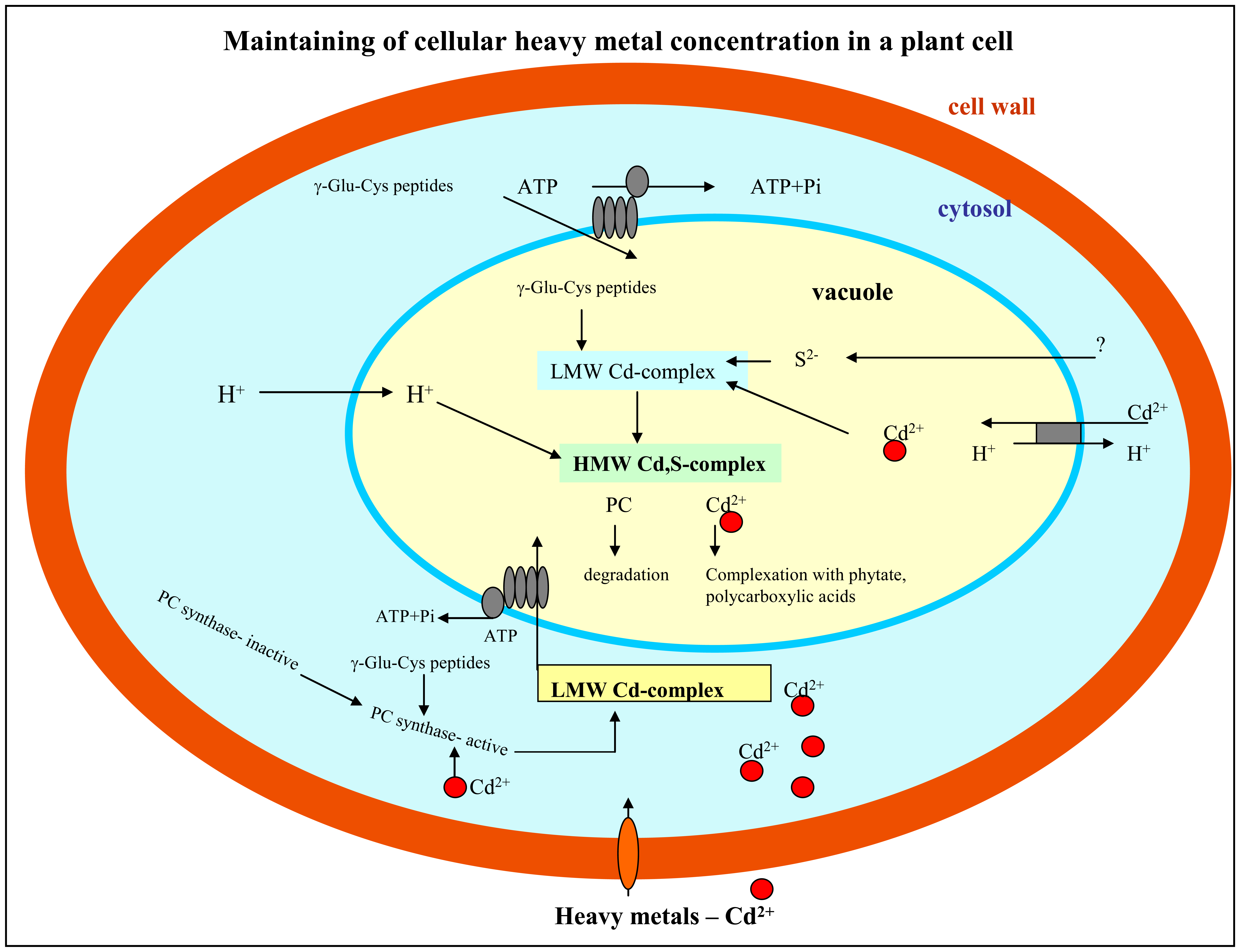
1.2 Response of plant cells on the presence of heavy metals
1.3 Analytical instruments used to study of thiols
1.4 The aims
2. Experimental
2.1 Chemicals
2.2 Electrochemical measurement
2.3 Cyclic voltammetry of thiols
2.4 Differential pulse anodic stripping voltammetry of cadmium(II) ions
2.5 Flow injection analysis coupled with CouloChem III electrochemical detector
2.6 High performance liquid chromatography coupled with CoulArray electrochemical detector
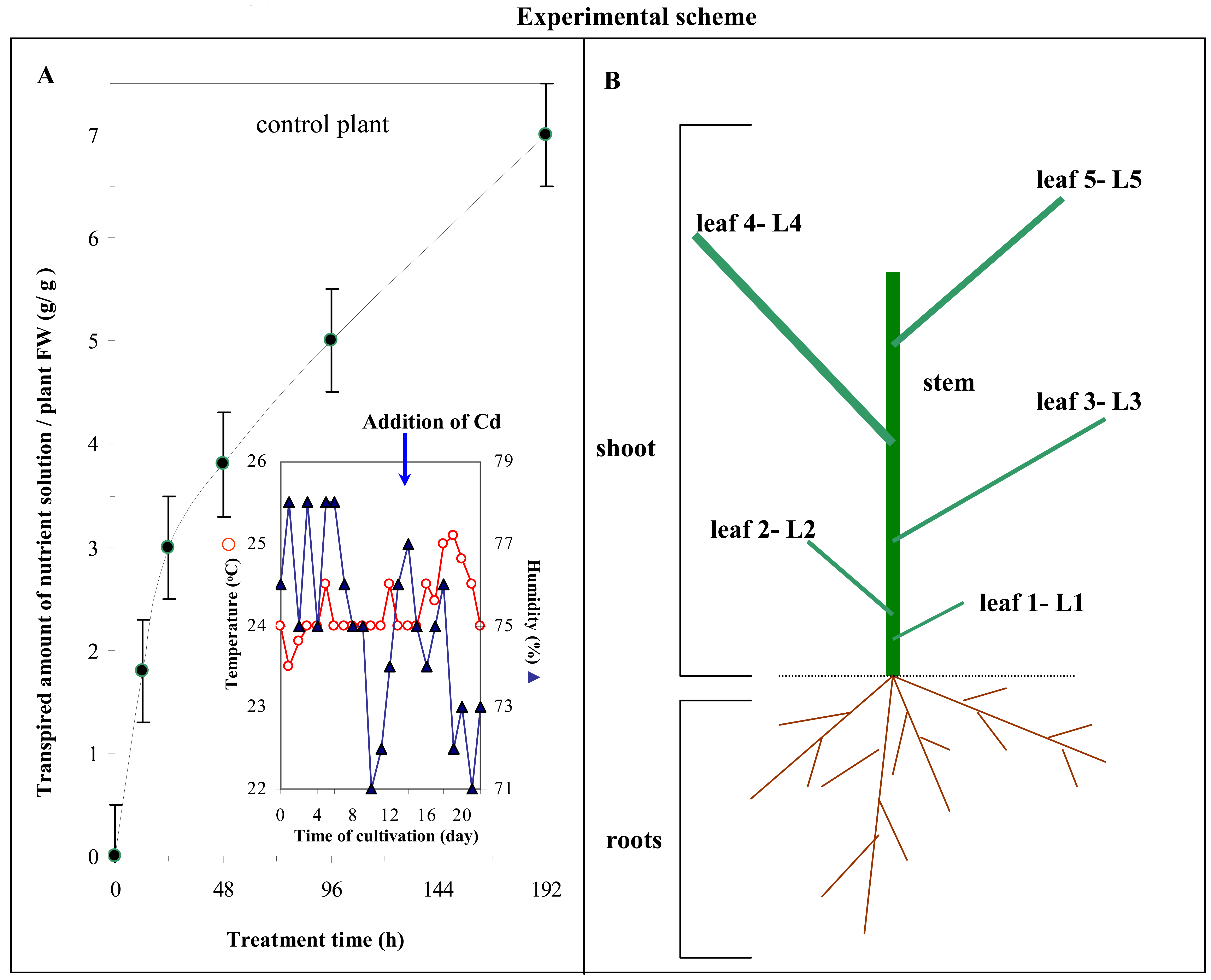
2.7 Plant material
2.8 Sample preparation for cadmium determination
2.9 Preparation of plant tissues for determination of thiols
2.10 Transpiration
2.11 Statistical analysis
3. Results and Discussion
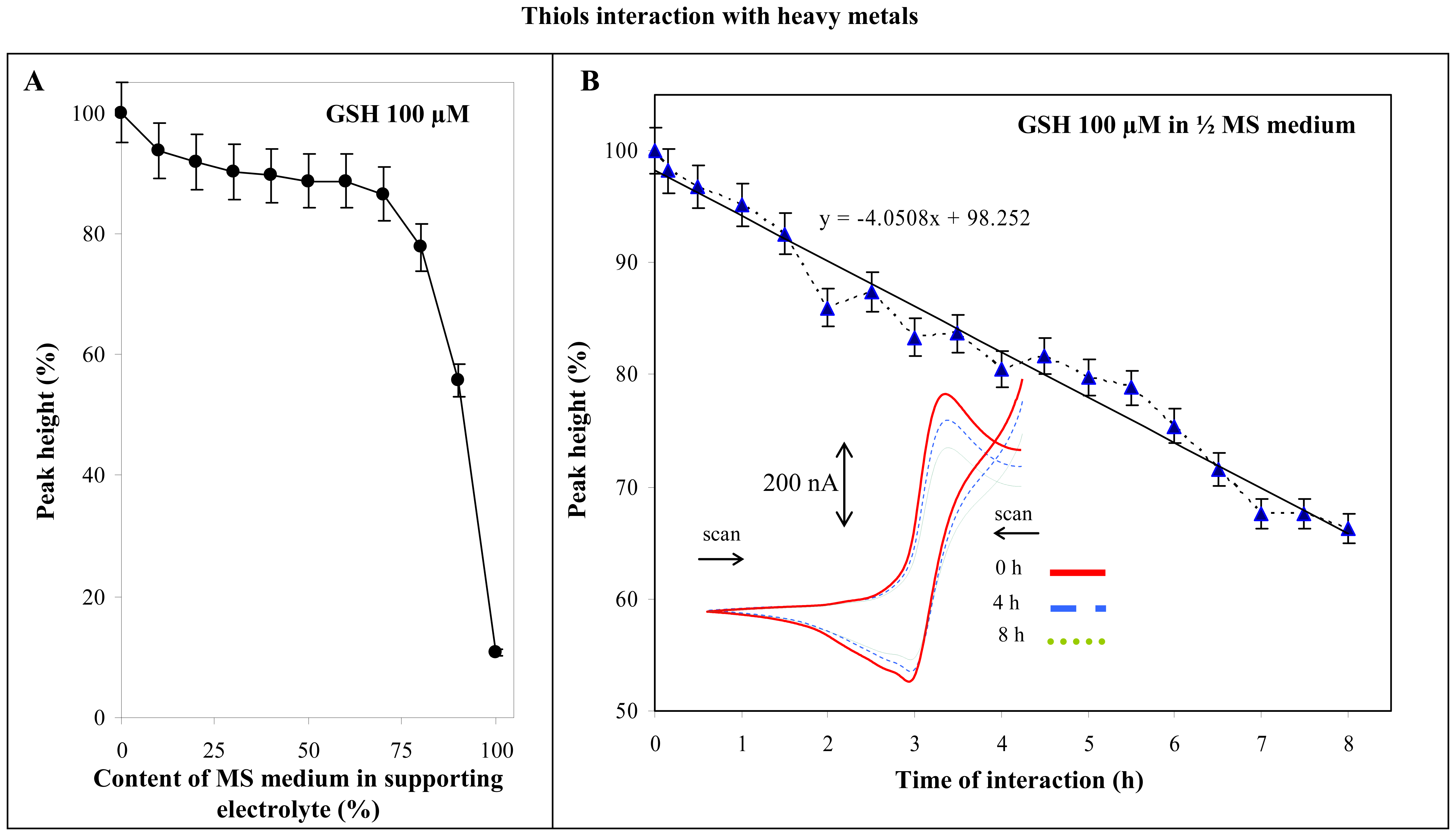
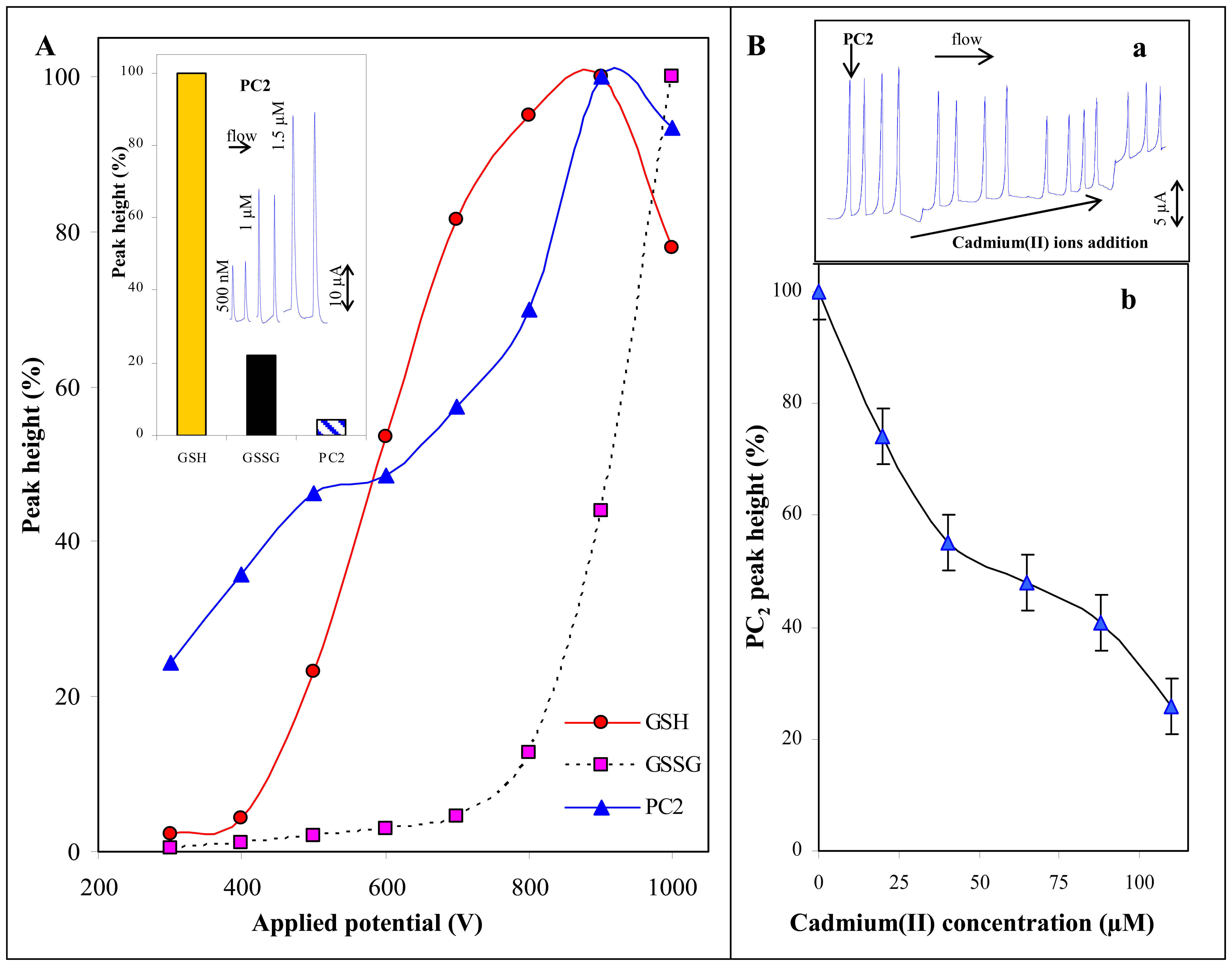
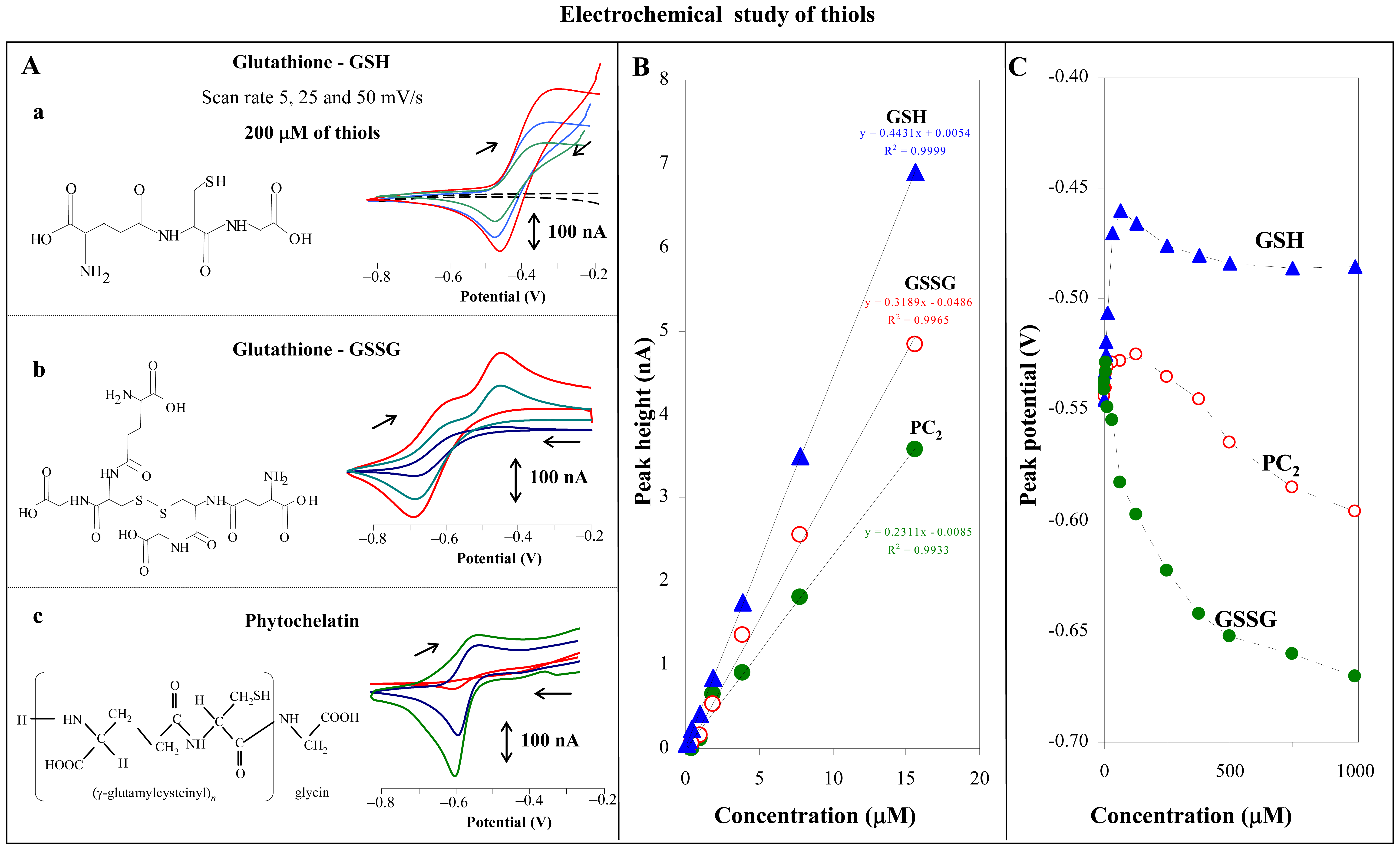
3.1 Investigation of interaction between heavy metals and thiols
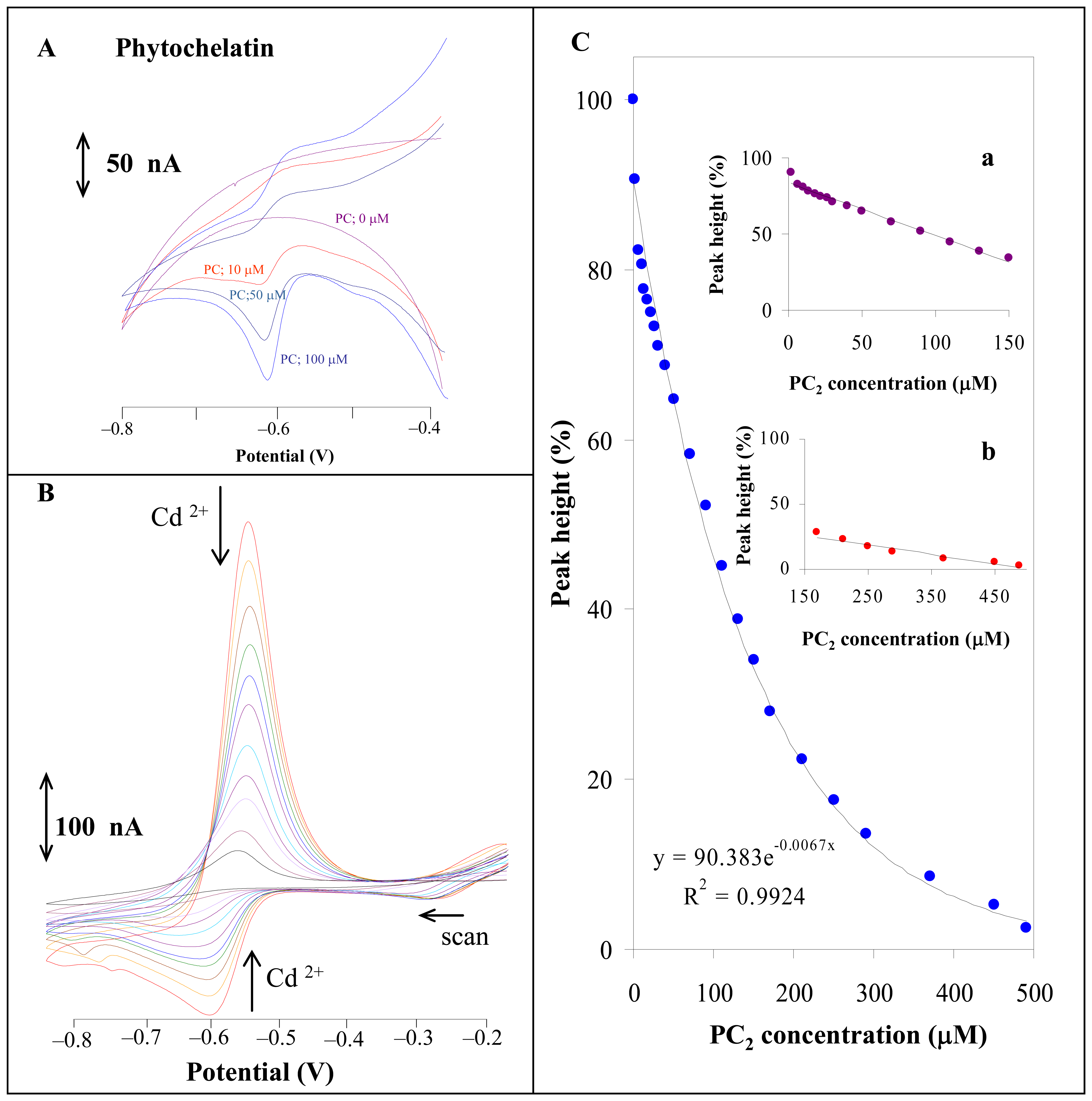
3.1.1 Phytochelatin and cadmium(II) ions
3.1.2 Glutahione and Murashige-Skoog medium
3.1.3 Thiols and cadmium(II) ions
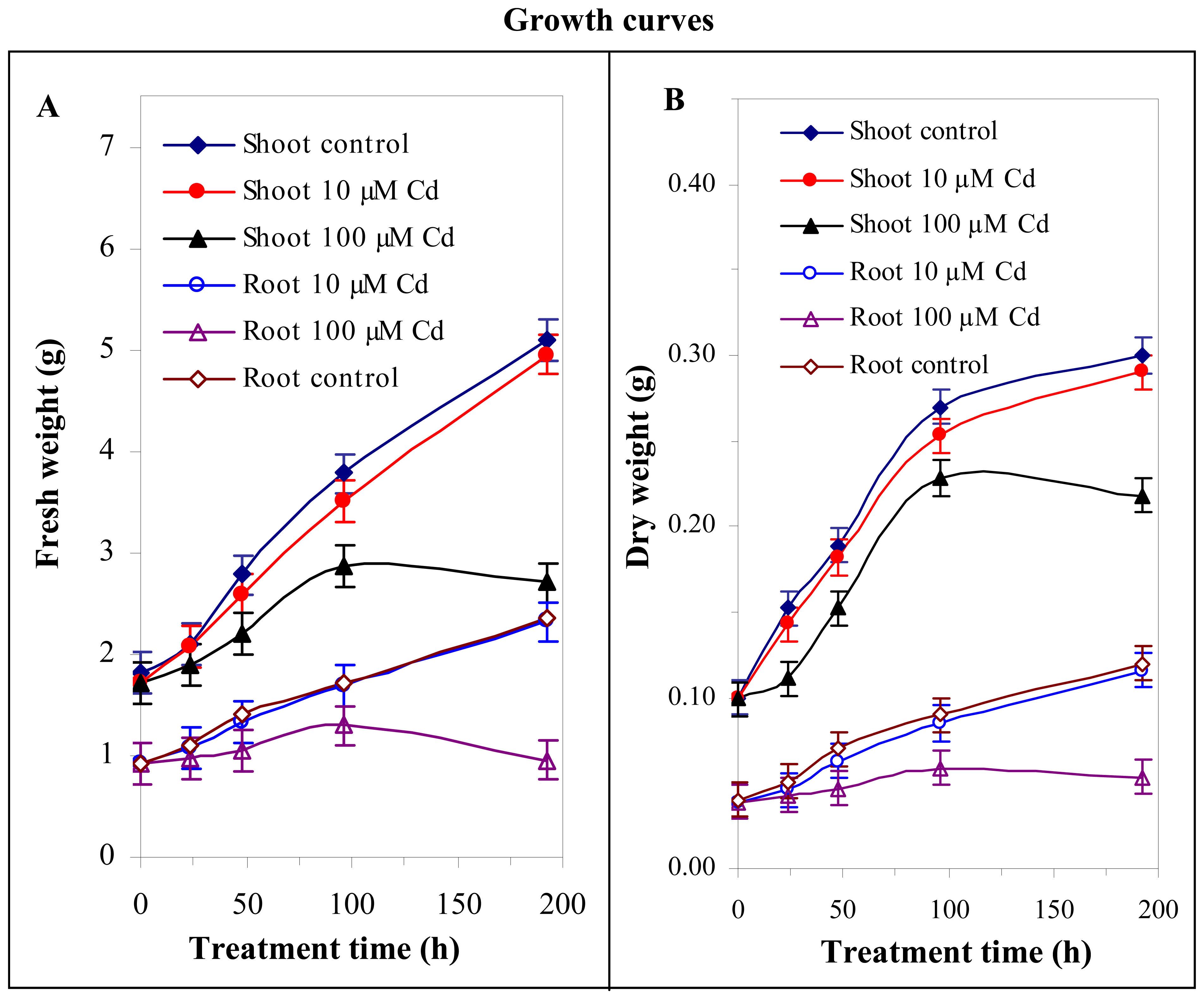
3.2 Effect of cadmium(II) ions on growth of maize plants
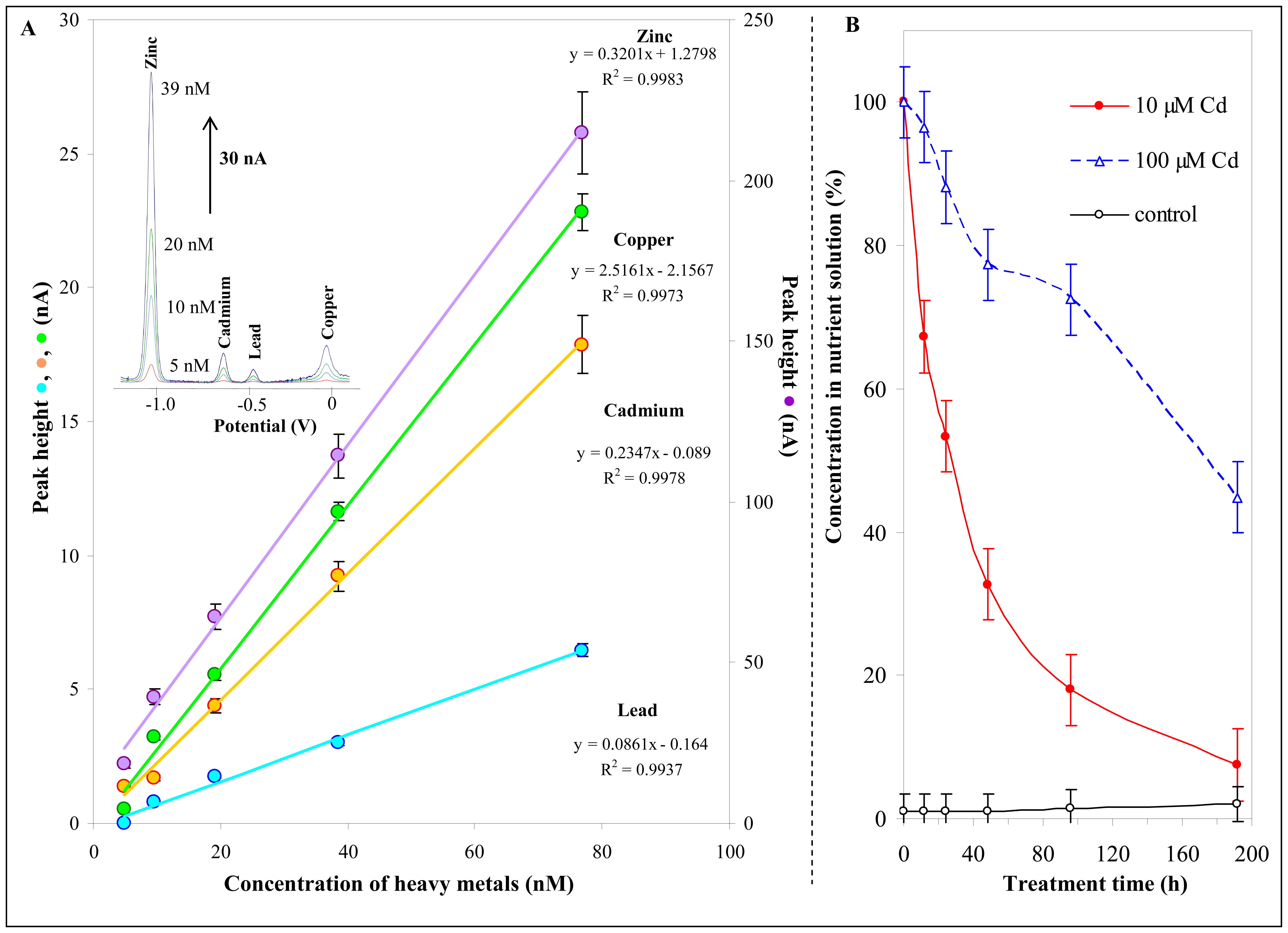
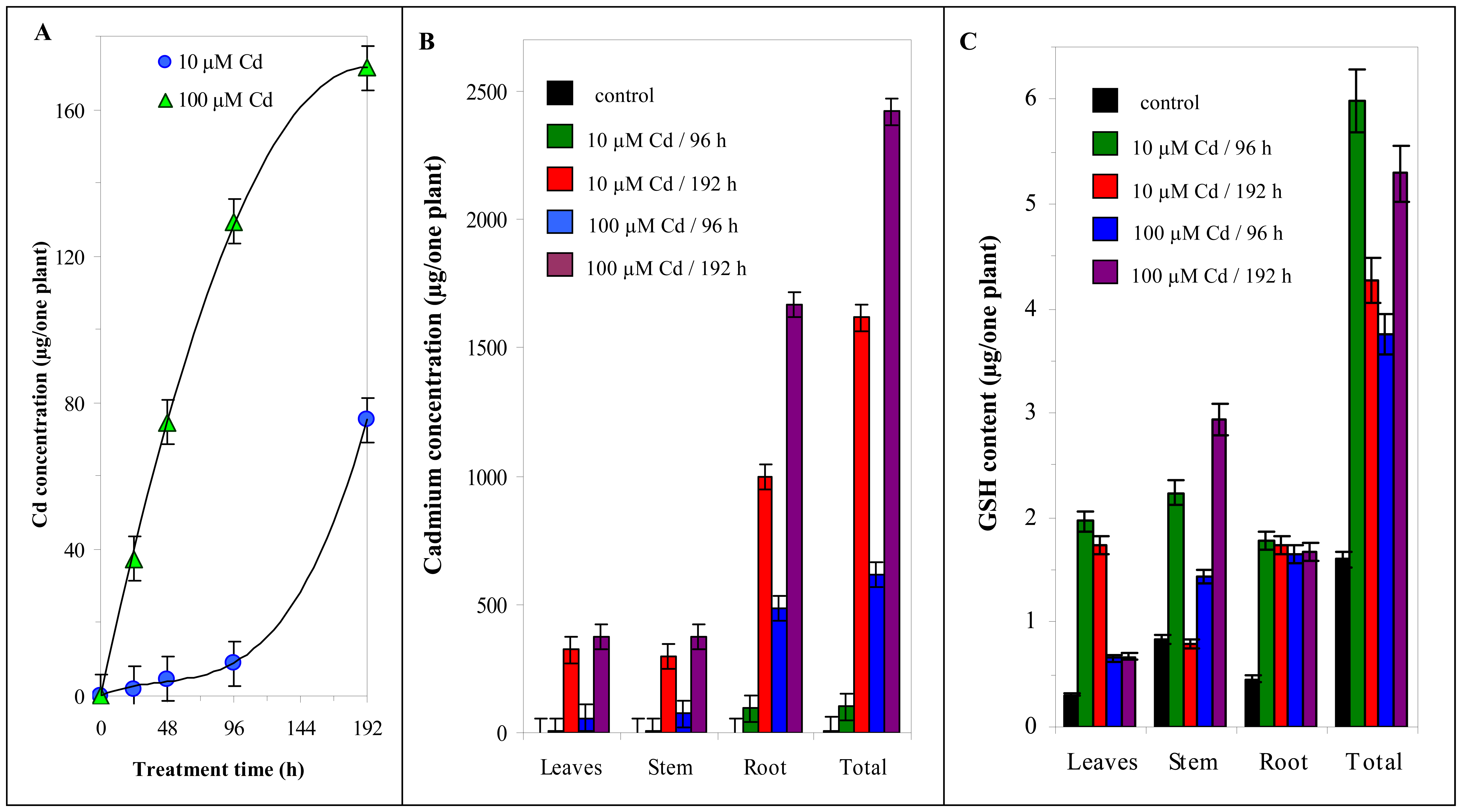
3.3 Cadmium concentration changes in nutrient solution
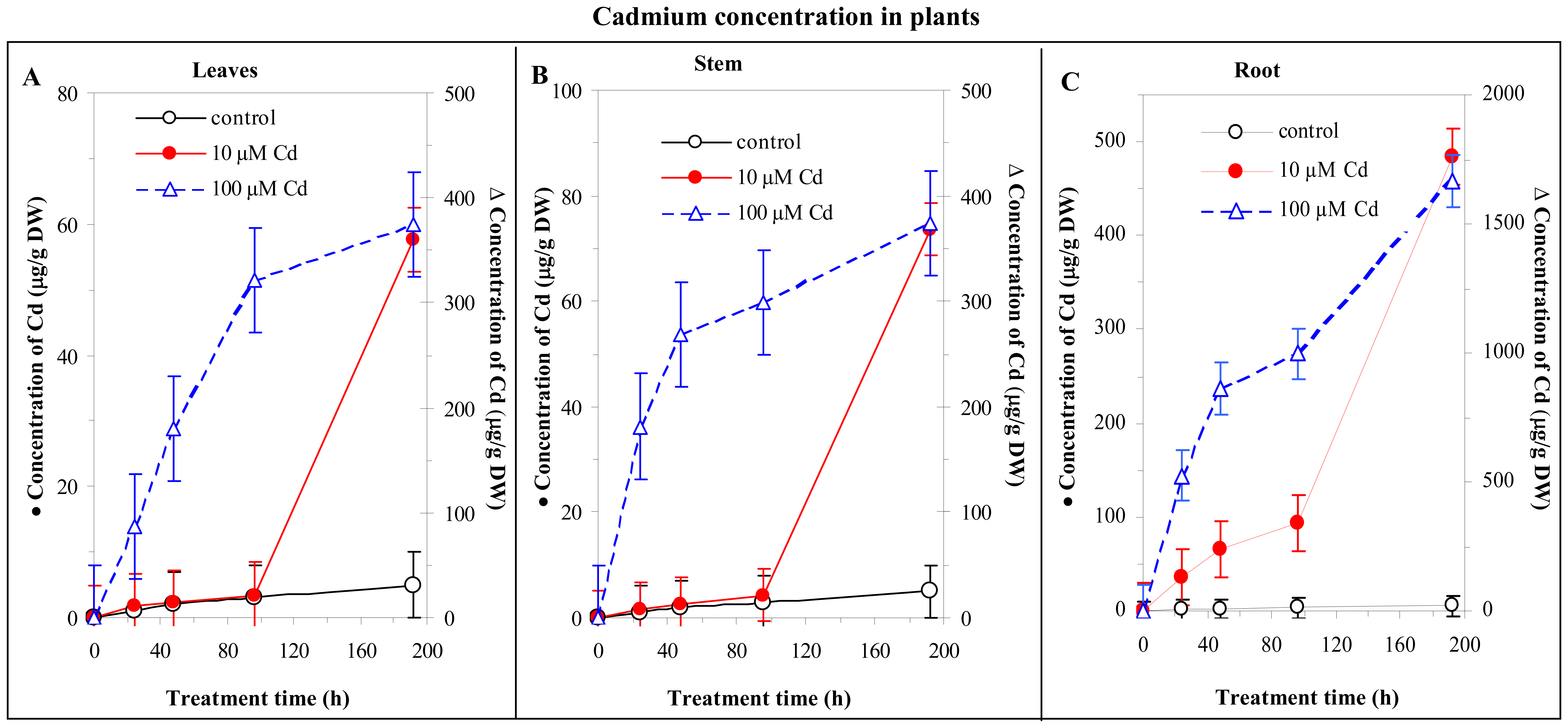
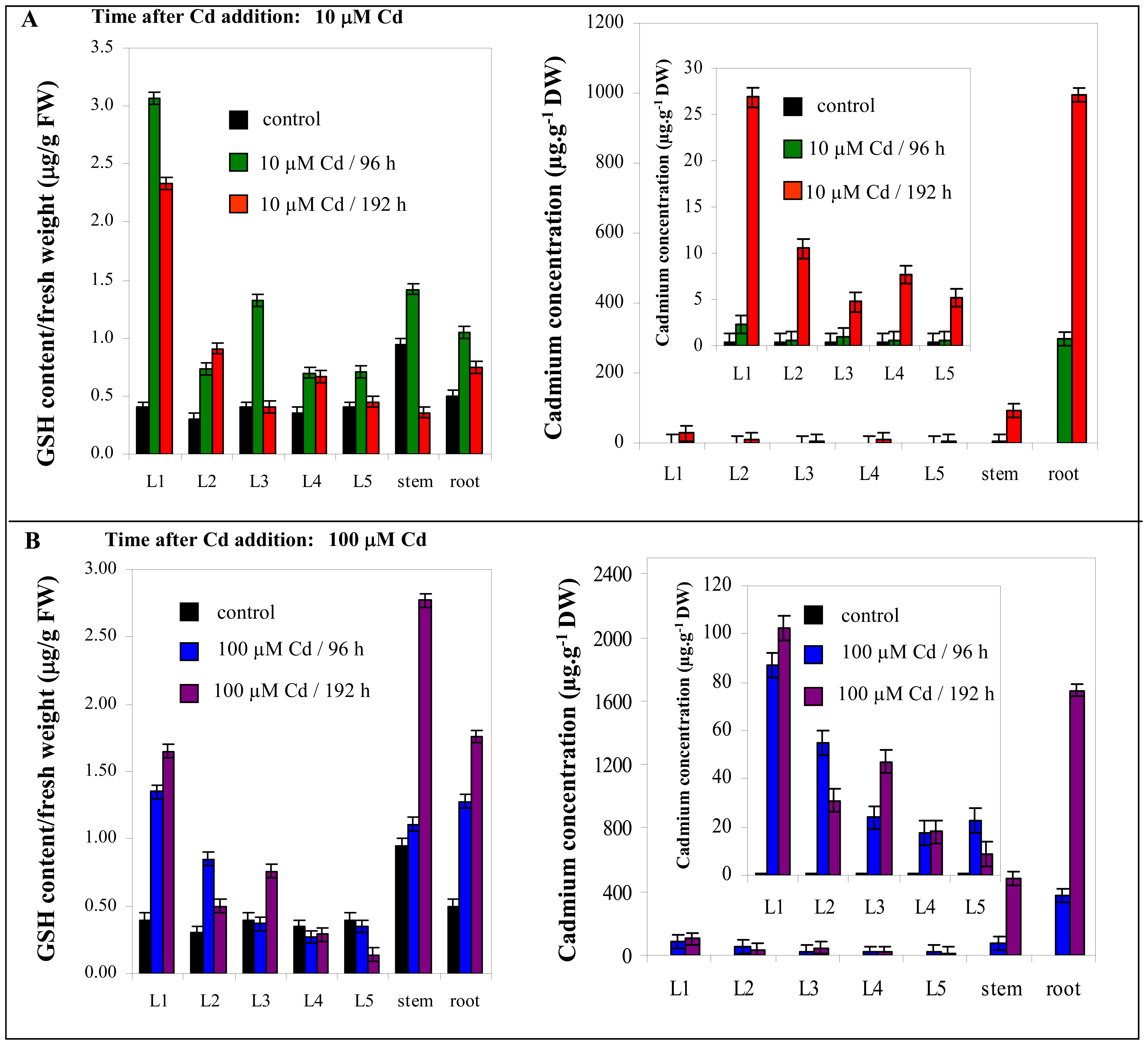
3.4 Cadmium concentration changes in plants
3.5 Electrochemical determination of thiols
3.5.1 Cyclic voltammetry of the thiols
3.5.2 Changes in content of thiols in maize plants
Conclusion
Acknowledgments
References
- Kizek, R.; Vacek, J.; Trnkova, L.; Klejdus, B.; Kuban, V. Electrochemical biosensors in agricultural and environmental analysis. Chem. Listy 2003, 97, 1003–1006. [Google Scholar]
- Zehnalek, J.; Adam, V.; Kizek, R. Influence of heavy metals on production of protecting compounds in agriculture plants. Lis. Cukrov. Reparske 2004, 120, 222–224. [Google Scholar]
- Zehnalek, J.; Vacek, J.; Kizek, R. Application of higher plants in phytoremetiation of heavy metals. Lis. Cukrov. Reparske 2004, 120, 220–221. [Google Scholar]
- Pulford, I.D.; Riddell-Black, D.; Stewart, C. Heavy metal uptake by willow clones from sewage sludge-treated soil: The potential for phytoremediation. Int. J. Phytoremediation 2002, 4, 59–72. [Google Scholar]
- Singh, K.P.; Mohan, D.; Sinha, S.; Dalwani, R. Impact assessment of treated/untreated wastewater toxicants discharged by sewage treatment plants on health, agricultural, and environmental quality in the wastewater disposal area. Chemosphere 2004, 55, 227–255. [Google Scholar]
- Shrotriya, N.; Joshi, J.K.; Mukhiya, Y.K.; Singh, V.P. Toxicity assessment of selected heavy-metals, herbicides and fertilizers in agriculture. Int. J. Environ. Stud. 1984, 22, 245–248. [Google Scholar]
- Endo, T.; Haraguchi, K.; Cipriano, F.; Simmonds, M.P.; Hotta, Y.; Sakata, M. Contamination by mercury and cadmium in the cetacean products from Japanese market. Chemosphere 2004, 54, 1653–1662. [Google Scholar]
- Bordajandi, L.R.; Gomez, G.; Abad, E.; Rivera, J.; Fernandez-Baston, M.D.; Blasco, J.; Gonzalez, M.J. Survey of persistent organochlorine contaminants (PCBs, PCDD/Fs, and PAHs), heavy metals (Cu, Cd, Zn, Pb, and Hg), and arsenic in food samples from Huelva (Spain): Levels and health implications. J. Agr. Food. Chem. 2004, 52, 992–1001. [Google Scholar]
- Fargasova, A. Toxicity comparison of some possible toxic metals (Cd, Cu, Pb, Se, Zn) on young seedlings of Sinapis alba L. Plant Soil Environ. 2004, 50, 33–38. [Google Scholar]
- Sharma, R.K.; Agrawal, M. Biological effects of heavy metals: An overview. J.Environ.Biol. 2005, 26, 301–313. [Google Scholar]
- Barocsi, A.; Csintalan, Z.; Kocsanyi, L.; Dushenkov, S.; Kuperberg, J.M.; Kucharski, R.; Richter, P.I. Optimizing phytoremediation of heavy metal-contaminated soil by exploiting plants stress adaptation. Int. J. Phytoremediation 2003, 5, 13–23. [Google Scholar]
- Bukvic, G.; Antunovic, M.; Popovic, S.; Rastija, M. Effect of P and Zn fertilisation on biomass yield and its uptake by maize lines (Zea mays L.). Plant Soil Environ. 2003, 49, 505–510. [Google Scholar]
- Garcia, G.; Zanuzzi, A.L.; Faz, A. Evaluation of heavy metal availability prior to an in situ soil phytoremediation program. Biodegradation 2005, 16, 187–194. [Google Scholar]
- Hanzlik, P.; Jehlicka, J.; Weishauptova, Z.; Sebek, O. Adsorption of copper, cadmium and silver from aqueous solutions onto natural carbonaceous materials. Plant Soil Environ. 2004, 50, 257–264. [Google Scholar]
- Smejkalova, M.; Mikanova, O.; Boruvka, L. Effects of heavy metal concentrations on biological activity of soil micro-organisms. Plant Soil Environ. 2003, 49, 321–326. [Google Scholar]
- Cohen-Shoel, N.; Ilzycer, D.; Gilath, I.; Tel-Or, E. The involvement of pectin in Sr-2+ biosorption by Azolla. Water Air Soil Pollut. 2002, 135, 195–205. [Google Scholar]
- Clemens, S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 2001, 212, 475–486. [Google Scholar]
- Clemens, S.; Simm, C. Schizosaccharomyces pombe as a model for metal homeostasis in plant cells: the phytochelatin-dependent pathway is the main cadmium detoxification mechanism. New Phytol. 2003, 159, 323–330. [Google Scholar]
- di Toppi, L.S.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar]
- Kruusma, J.; Benham, A.M.; Williams, J.A.G.; Kataky, R. An introduction to thiol redox proteins in the endoplasmic reticulum and a review of current electrochemical methods of detection of thiols. Analyst 2006, 131, 459–473. [Google Scholar]
- Brazdova, M.; Kizek, R.; Havran, L.; Palecek, E. Determination of glutathione-S-transferase traces in preparations of p53 C-terminal domain (aa320-393). Bioelectrochemistry 2002, 55, 115–118. [Google Scholar]
- Mannervik, B.; Danielson, U.H. Glutathione Transferases - Structure and Catalytic Activity. Crc Crit. Rev. Biochem. 1988, 23, 283–337. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases - First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Vitecek, J.; Petrlova, J.; Petrek, J.; Adam, V.; Potesil, D.; Havel, L.; Mikelova, R.; Trnkova, L.; Kizek, R. Electrochemical study of S-nitrosoglutathione and nitric oxide by carbon fibre NO sensor and cyclic voltammetry - possible way of monitoring of nitric oxide. Electrochim. Acta 2006, 51, 5087–5094. [Google Scholar]
- Mayer, B.; Pfeiffer, S.; Schrammel, A.; Koesling, D.; Schmidt, K.; Brunner, F. A new pathway of nitric oxide cyclic GMP signaling involving S-nitrosoglutathione. J. Biol. Chem. 1998, 273, 3264–3270. [Google Scholar]
- Clancy, R.M.; Levartovsky, D.; Leszczynskapiziak, J.; Yegudin, J.; Abramson, S.B. Nitric-Oxide Reacts with Intracellular Glutathione and Activates the Hexose-Monophosphate Shunt in Human Neutrophils - Evidence for S-Nitrosoglutathione as a Bioactive Intermediary. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 3680–3684. [Google Scholar]
- Lu, S.C. Regulation of hepatic glutathione synthesis: current concepts and controversies. Faseb J. 1999, 13, 1169–1183. [Google Scholar]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar]
- Cobbett, C.S. Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr. Opin. Plant Biol. 2000, 3, 211–216. [Google Scholar]
- Rauser, W.E. Phytochelatins and Related Peptides - Structure, Biosynthesis, and Function. Plant Physiol. 1995, 109, 1141–1149. [Google Scholar]
- Rauser, W.E. Phytochelatins. Annu. Rev. Biochem. 1990, 59, 61–86. [Google Scholar]
- Grill, E.; Loffler, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the Heavy-Metal-Binding Peptides of Plants, Are Synthesized from Glutathione by a Specific Gamma-Glutamylcysteine Dipeptidyl Transpeptidase (Phytochelatin Synthase). Proc. Natl. Acad. Sci. U. S. A. 1989, 86, 6838–6842. [Google Scholar]
- Grill, E.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, a Class of Heavy-Metal-Binding Peptides from Plants, Are Functionally Analogous to Metallothioneins. Proc. Natl. Acad. Sci. U. S. A. 1987, 84, 439–443. [Google Scholar]
- Ramos, J.; Clemente, M.R.; Naya, L.; Loscos, J.; Perez-Rontome, C.; Sato, S.; Tabata, S.; Becana, M. Phytochelatin synthases of the model legume Lotus japonicus. A small multigene family with differential response to cadmium and alternatively spliced variants. Plant Physiol. 2007, 143, 1110–1118. [Google Scholar]
- Kawakami, S.K.; Gledhill, M.; Achterberg, E.P. Determination of phytochelatins and glutathione in phytoplankton from natural waters using HPLC with fluorescence detection. Trac-Trends Anal. Chem. 2006, 25, 133–142. [Google Scholar]
- Bramanti, E.; Toncelli, D.; Morelli, E.; Lampugnani, L.; Zamboni, R.; Miller, K.E.; Zemetra, J.; D'Ulivo, A. Determination and characterization of phytochelatins by liquid chromatography coupled with on line chemical vapour generation and atomic fluorescence spectrometric detection. J. Chromatogr. A 2006, 1133, 195–203. [Google Scholar]
- Bramanti, E.; Lomonte, C.; Onor, M.; Zamboni, R.; D'Ulivo, A.; Raspi, G. Mercury speciation by liquid chromatography coupled with on-line chemical vapour generation and atomic fluorescence spectrometric detection (LC-CVGAFS). Talanta 2005, 66, 762–768. [Google Scholar]
- Kang, S.H.; Kim, J.W.; Chung, D.S. Determination of homocysteine and other thiols in human plasma by capillary electrophoresis. J. Pharmaceut. Biomed. 1997, 15, 1435–1441. [Google Scholar]
- Kand'ar, R.; Zakova, P.; Lotkova, H.; Kucera, O.; Cervinkova, Z. Determination of reduced and oxidized glutathione in biological samples using liquid chromatography with fluorimetric detection. J. Pharm. Biomed. Anal. 2007, 43, 1382–1387. [Google Scholar]
- Hiraku, Y.; Murata, M.; Kawanishi, S. Determination of intracellular glutathione and thiols by high performance liquid chromatography with a gold electrode at the femtomole level: comparison with a spectroscopic assay. BBA-Gen. Subjects 2002, 1570, 47–52. [Google Scholar]
- Norris, R.L.; Eaglesham, G.K.; Shaw, G.R.; Smith, M.J.; Chiswell, R.K.; Seawright, A.A.; Moore, M.R. A sensitive and specific assay for glutathione with potential application to glutathione disulphide, using high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2001, 762, 17–23. [Google Scholar]
- Petrlova, J.; Mikelova, R.; Stejskal, K.; Kleckerova, A.; Zitka, O.; Petrek, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Kizek, R. Simultaneous determination of eight biologically active thiol compounds using gradient elution-liquid chromatography with Coul-Array detection. J. Sep. Sci. 2006, 29, 1166–1173. [Google Scholar]
- Petrlova, J.; Potesil, D.; Mikelova, R.; Blastik, O.; Adam, V.; Trnkova, L.; Jelen, F.; Prusa, R.; Kukacka, J.; Kizek, R. Attomole voltammetric determination of metallothionein. Electrochim. Acta 2006, 51, 5112–5119. [Google Scholar]
- Potesil, D.; Mikelova, R.; Adam, V.; Kizek, R.; Prusa, R. Change of the protein p53 electrochemical signal according to its structural form - Quick and sensitive distinguishing of native, denatured, and aggregated form of the “guardian of the genome”. Protein J. 2006, 25, 23–32. [Google Scholar]
- Potesil, D.; Petrlova, J.; Adam, V.; Vacek, J.; Klejdus, B.; Zehnalek, J.; Trnkova, L.; Havel, L.; Kizek, R. Simultaneous femtomole determination of cysteine, reduced and oxidized glutathione, and phytochelatin in maize (Zea mays L.) kernels using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A 2005, 1084, 134–144. [Google Scholar]
- Spadaro, A.; Bousquet, E.; Santagati, N.A.; Vittorio, F.; Ronsisvalle, G. Simple analysis of glutathione in human colon carcinoma cells and epidermoid human larynx carcinoma cells by HPLC with electrochemical detection. Chromatographia 2005, 62, 11–15. [Google Scholar]
- Salimi, A.; Hallaj, R. Catalytic oxidation of thiols at preheated glassy carbon electrode modified with abrasive immobilization of multiwall carbon nanotubes: applications to amperometric detection of thiocytosine, L-cysteine and glutathione. Talanta 2005, 66, 967–975. [Google Scholar]
- Zhang, W.; Wan, F.L.; Zhu, W.; Xu, H.H.; Ye, X.Y.; Cheng, R.Y.; Jin, L.T. Determination of glutathione and glutathione disulfide in hepatocytes by liquid chromatography with an electrode modified with functionalized carbon nanotubes. J. Chromatogr. B 2005, 818, 227–232. [Google Scholar]
- Hiraku, Y.; Murata, M.; Kawanishi, S. Determination of intracellular glutathione and thiols by high performance liquid chromatography with a gold electrode at the femtomole level: comparison with a spectroscopic assay. Biochim. Biophys. Acta-Gen. Subj. 2002, 1570, 47–52. [Google Scholar]
- Zhang, S.; Sun, W.L.; Xian, Y.Z.; Zhang, W.; Jin, L.T.; Yamamoto, K.; Tao, S.G.; Jin, J.Y. Multichannel amperometric detection system for liquid chromatography to assay the thiols in human whole blood using the platinum microelectrodes chemically modified by copper tetraaminophthalocyanine. Anal. Chim. Acta 1999, 399, 213–221. [Google Scholar]
- Zitka, O.; Stejskal, K.; Kleckerova, A.; Adam, V.; Beklova, M.; Horna, A.; Supalkova, V.; Havel, L.; Kizek, R. Utilizing electrochemical techniques for detection of biological samples. Chem. Listy 2007, 101, 225–231. [Google Scholar]
- Xu, F.; Wang, L.; Gao, M.N.; Jin, L.T.; Jin, J.Y. Amperometric determination of glutathione and cysteine on a Pd-IrO2 modified electrode with high performance liquid chromatography in rat brain microdialysate. Anal. Bioanal. Chem. 2002, 372, 791–794. [Google Scholar]
- Klejdus, B.; Zehnalek, J.; Adam, V.; Petrek, J.; Kizek, R.; Vacek, J.; Trnkova, L.; Rozik, R.; Havel, L.; Kuban, V. Sub-picomole high-performance liquid chromatographic/mass spectrometric determination of glutathione in the maize (Zea mays L.) kernels exposed to cadmium. Anal. Chim. Acta 2004, 520, 117–124. [Google Scholar]
- Petrek, J.; Vitecek, J.; Vlasinova, H.; Kizek, R.; Kramer, K.J.; Adam, V.; Klejdus, B.; Havel, L. Application of computer imaging, stripping voltammetry and mass spectrometry to study the effect of lead (Pb-EDTA) on the growth and viability of early somatic embryos of Norway spruce (Picea abies/L./Karst.). Anal. Bioanal. Chem. 2005, 383, 576–586. [Google Scholar]
- El-Zohri, M.H.A.; Cabala, R.; Frank, H. Quantification of phytochelatins in plants by reversed-phase HPLC-ESI-MS-MS. Anal. Bioanal. Chem. 2005, 382, 1871–1876. [Google Scholar]
- Gomez-Ariza, J.L.; Garcia-Barrera, T.; Lorenzo, F.; Bernal, V.; Villegas, M.J.; Oliveira, V. Use of mass spectrometry techniques for the characterization of metal bound to proteins (metallomics) in biological systems. Anal. Chim. Acta 2004, 524, 15–22. [Google Scholar]
- Rellan-Alvarez, R.; Hernandez, L.E.; Abadia, J.; Alvarez-Fernandez, A. Direct and simultaneous determination of reduced and oxidized glutathione and homoglutathione by liquid chromatographyelectrospray/mass spectrometry in plant tissue extracts. Anal. Biochem. 2006, 356, 254–264. [Google Scholar]
- Kupper, H.; Mijovilovich, A.; Meyer-Klaucke, W.; Kroneck, P.M.H. Tissue- and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol. 2004, 134, 748–757. [Google Scholar]
- Sestakova, I.; Mader, P. Voltammetry on mercury and carbon electrodes as a tool for studies of metallothionein interactions with metal ions. Cell. Mol. Biol. 2000, 46, 257–267. [Google Scholar]
- Alberich, A.; Arino, C.; Diaz-Cruz, J.M.; Esteban, M. Multivariate curve resolution applied to the simultaneous analysis of electrochemical and spectroscopic data: Study of the Cd(II)/glutathione-fragment system by voltammetry and circular dichroism spectroscopy. Anal. Chim. Acta 2007, 584, 403–409. [Google Scholar]
- Huska, D.; Zitka, O.; Adam, V.; Beklova, M.; Krizkova, S.; Zeman, L.; Horna, A.; Havel, L.; Zehnalek, J.; Kizek, R. A sensor for investigating the interaction between biologically important heavy metals and glutathione. Czech J. Anim. Sci. 2007, 52, 37–43. [Google Scholar]
- Cruz, B.H.; Diaz-Cruz, J.M.; Sestakova, I.; Velek, J.; Arino, C.; Esteban, M. Differential pulse voltammetric study of the complexation of Cd(II) by the phytochelatin (gamma-Glu-Cys)(2)Gly assisted by multivariate curve resolution. J. Electroanal. Chem. 2002, 520, 111–118. [Google Scholar]
- Adam, V.; Zehnalek, J.; Petrlova, J.; Potesil, D.; Sures, B.; Trnkova, L.; Jelen, F.; Vitecek, J.; Kizek, R. Phytochelatin modified electrode surface as a sensitive heavy-metal ion biosensor. Sensors 2005, 5, 70–84. [Google Scholar]
- Krizkova, S.; Adam, V.; Petrlova, J.; Zitka, O.; Stejskal, K.; Zehnalek, J.; Sures, B.; Trnkova, L.; Beklova, M.; Kizek, R. A suggestion of electrochemical biosensor for study of Platinum(II)-DNA interactions. Electroanalysis 2007, 19, 331–338. [Google Scholar]
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin electrochemical biosensor. Electrochim. Acta 2006, 51, 5169–5173. [Google Scholar]
- Prusa, R.; Petrlova, J.; Kukacka, J.; Adam, V.; Sures, B.; Beklova, M.; Kizek, R. Study of interaction of glutathiones and metallothionein with cytostatics. Clin. Chem. 2006, 52, A175–A175. [Google Scholar]
- Vasilescu, I.; Litescu, S.; Radu, G.L. Electrochemical study of pH influence on the behaviour of the cystine-cysteine system. Rev. Chim. 2006, 57, 955–959. [Google Scholar]
- Serrano, N.; Sestakova, I.; Diaz-Cruz, J.M.; Arino, C. Adsorptive accumulation in constant current stripping chronopotentiometry as an alternative for the electrochemical study of metal complexation by thiol-containing peptides. J. Electroanal. Chem. 2006, 591, 105–117. [Google Scholar]
- Tang, H.; Chen, J.H.; Nie, L.H.; Yao, S.Z.; Kuang, Y.F. Electrochemical oxidation of glutathione at well-aligned carbon nanotube array electrode. Electrochim. Acta 2006, 51, 3046–3051. [Google Scholar]
- Dorcak, V.; Sestakova, I. Electrochemical behavior of phytochelatins and related peptides at the hanging mercury drop electrode in the presence of cobalt(II) ions. Bioelectrochemistry 2006, 68, 14–21. [Google Scholar]
- Han, H.Y.; Tachikawa, H. Electrochemical determination of thiols at single-wall carbon nanotubes and PQQ modified electrodes. Front. Biosci. 2005, 10, 931–939. [Google Scholar]
- Mladenov, M.; Mirceski, V.; Gjorgoski, I.; Jordanoski, B. Redox kinetic measurements of glutathione at the mercury electrode by means of square-wave voltammetry. The role of copper, cadmium and zinc ions. Bioelectrochemistry 2004, 65, 69–76. [Google Scholar]
- Budnikov, G.K.; Ziyatdinova, G.K.; Valitova, Y.R. Electrochemical determination of glutathione. J. Anal. Chem. 2004, 59, 573–576. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Jelen, F. Cyclic voltammetric study of the redox system of glutathione using the disulfide bond reductant tris(2-carboxyethyl)phosphine. Bioelectrochemistry 2004, 63, 19–24. [Google Scholar]
- Vacek, J.; Petrek, J.; Kizek, R.; Havel, L.; Klejdus, B.; Trnkova, L.; Jelen, F. Electrochemical determination of lead and glutathione in a plant cell culture. Bioelectrochemistry 2004, 63, 347–351. [Google Scholar]
- Calvo-Marzal, P.; Chumbimuni-Torres, K.Y.; Hoehr, N.F.; Neto, G.D.; Kubota, L.T. Determination of reduced glutathione using an amperometric carbon paste electrode chemically modified with TTF-TCNQ. Sens. Actuator B-Chem. 2004, 100, 333–340. [Google Scholar]
- Salimi, A.; Pourbeyram, S. Renewable sol-gel carbon ceramic electrodes modified with a Ru-complex for the amperometric detection of L-cysteine and glutathione. Talanta 2003, 60, 205–214. [Google Scholar]
- Seymour, E.H.; Wilkins, S.J.; Lawrence, N.S.; Compton, R.G. Electrochemical detection of glutathione: An electrochemically initiated reaction pathway. Anal. Lett. 2002, 35, 1387–1399. [Google Scholar]
- Zeng, B.Z.; Ding, X.G.; Zhao, F.Q. Voltammetric response of glutathione and 3-mercaptopropionic acid self-assembled monolayer modified gold electrodes to Cu(II). Electroanalysis 2002, 14, 651–656. [Google Scholar]
- Supalkova, V.; Petrek, J.; Baloun, J.; Adam, V.; Bartusek, K.; Trnkova, L.; Beklova, M.; Diopan, V.; Havel, L.; Kizek, R. Multi-instrumental investigation of affecting of early somatic embryos of Spruce by cadmium(II) and lead(II) ions. Sensors 2007, 7, 743–759. [Google Scholar]
- Richter, O. Beiträge zur Ernährungsphysiologie der Kulturgräser. I. Uber das grosse Eisenbedürfnis der Reispflanze (Oriza sativa L.). Sitz.-Ber. Akad. Wiss. 1926, 203–242. [Google Scholar]
- Hoagland, D.R.; Snyder, W.C. Nutrition of strawberry under controlled conditions. (a) Effects of deficies of boron and certain other elements, (b) susceptibility to injury from sodium salts. Proc. Amer. Soc. Horticult. Sci. 1933, 1930, 288–294. [Google Scholar]
- Zaidi, P.H.; Rafique, S.; Singh, N.N. Response of maize (Zea mays L.) genotypes to excess soil moisture stress:morpho-physiological effects and basis of tolerance. Europ. J. Agronomy 2003, 19, 383–399. [Google Scholar]
- Rauser, W.E.; Meuwly, P. Retention of cadmium in roots of maize seedlings - role of complexation phytochelatins and related thiol peptides. Plant Physiol. 1995, 109, 195–202. [Google Scholar]
- di Toppi, S.L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar]
- Strouhal, M.; Kizek, R.; Vecek, J.; Trnkova, L.; Nemec, M. Electrochemical study of heavy metals and metallothionein in yeast Yarrowia lipolytica. Bioelectrochemistry 2003, 60, 29–36. [Google Scholar]
- Waalkes, M.P. Cadmium carcinogenesis in rewiew. J. Inorg. Biochem. 2000, 79, 241–244. [Google Scholar]
- Kosobrukhov, A.; Knyazeva, I.; Mudrik, V. Plantago major plants responses to increase content of lead in soil: Growth and photosynthesis. Plant Growth Reg. 2004, 42, 145–151. [Google Scholar]
- Prasad, M.N.V. Cadmium toxicity and tolerance in vascular plants. Environ. Exp. Bot. 1995, 35, 525–545. [Google Scholar]
- Fojtova, M.; Kovarik, A. Genotoxic effect of cadmium is associated with apoptotic changes in tobacco cells. Plant Cell Environ. 2000, 23, 531–537. [Google Scholar]
- Cobbett, C.S.; Goldsbrough, P.B. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar]
- Stolt, J.P.; Sneller, F.E.C.; Bryngelsson, T.; Lundborg, T.; Schat, H. Phytochelatin and cadmium accumulation in wheat. Environ. Exp. Bot. 2003, 49, 21–28. [Google Scholar]
- Cosio, C.; Martinoia, E.; Keller, C. Hyperaccumulation of cadmium and zinc in Thlaspi caerulescens and Arabidopsis halleri at the leaf cellular level. Plant Physiol. 2004, 134, 716–725. [Google Scholar]
- Grill, E.; Winnacker, E.L.; Zenk, M.H. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science 1985, 320, 674–676. [Google Scholar]
- Urdaneta, A.; Jimenez, A.R.; Izquierdo, D.; Paramio, M.T. Effect of the addition of glutathione and glucose to the culture medium on embryo development of IVM-IVF prepubertal goat oocytes. Zygote 2003, 11, 131–138. [Google Scholar]
- Inoue, Y.; Moriyasu, Y. Degradation of membrane phospholipids in plant cells cultured in sucrose-free medium. Autophagy 2006, 2, 244–246. [Google Scholar]
- Zechmann, B.; Muller, M.; Zellnig, G. Intracellular adaptations of glutathione content in Cucurbita pepo L. induced by treatment with reduced glutathione and buthionine sulfoximine. Protoplasma 2006, 227, 197–209. [Google Scholar]
- Nunez-Vergara, L.J.; Squella, J.A.; Olea-Azar, C.; Bollo, S.; Navarrete-Encina, P.A.; Sturm, J.C. Nitrosobenzene: electrochemical, UV-visible and EPR spectroscopic studies on the nitrosobenzene free radical generation and its interaction with glutathione. Electrochim. Acta 2000, 45, 3555–3561. [Google Scholar]
- Vogeli-Lange, R.; Wagner, G.J. Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves. Plant Physiol. 1990, 92, 1086–1093. [Google Scholar]
- Gupta, D.K.; Tohoyama, H.; Joho, M.; Inouhe, M. Possible roles of phytochelatins and glutathione metabolism in cadmium tolerance in chickpea roots. J. Plant Res. 2002, 115, 429–437. [Google Scholar]
- Mazen, A.M.A. Calcium oxalate deposits in leaves of Corchorus olitotius as related to accumulation of toxic metals. Russ. J. Plant Physiol. 2004, 51, 281–285. [Google Scholar]
- Potters, G.; De Gara, L.; Asard, H.; Horemans, N. Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant. Physiol. Bioch. 2002, 40, 537–548. [Google Scholar]
- Wang, Y.; Fang, J.; Leonard, S.S.; Rao, K.M.K. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 1434–1443. [Google Scholar]
- Lux, A.; Sottnikova, A.; Opatrna, J.; Greger, M. Differencens in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiol Plantarum 2004, 120, 537–545. [Google Scholar]
- Lai, H.-Y.; Chen, Z.-S. Effects of EDTA on solubility of cadmium, zinc, and lead and their uptake by rainbow pink and vetiver grass. Chemosphere 2004, 55, 421–430. [Google Scholar]
- Romero-Puertas, M.C.; Palma, J.M.; Gomez, M.; Del Rio, L.A.; Sandalio, L.M. Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ. 2002, 2002, 677–686. [Google Scholar]
- Bartolf, M.; Brennan, E.; Price, C.A. Partial characterization of a cadmium-binding protein from the roots of cadmium-treated tomato. Plant Physiol. 1980, 66, 438–441. [Google Scholar]
- Carrier, P.; Baryla, A.; Havaux, M. Cadmium distribution and microlocalization in oilseed rape (Brassica napus) after long-term growth on cadmium-contaminated soil. Planta 2003, 216, 939–950. [Google Scholar]
- Heyrovsky, M.; Mader, P.; Vavricka, S.; Vesela, V.; Fedurco, M. The anodic reaction at mercury electrodes due to cystein. J. Electroanal. Chem. 1997, 430, 103–117. [Google Scholar]
- Vogeli-Lange, R.; Wagner, G.J. Relationship between cadmium, glutathione and cadmium-binding peptides (phytochelatins) in leaves of intact tobacco seedlings. Plant Sci. 1996, 114, 11–18. [Google Scholar]
- Kahle, H. Response of roots of trees to heavy metals. Environ. Exp. Bot. 1993, 33, 99–119. [Google Scholar]
- Inouhe, M.; Ito, R.; Ito, S.; Sasada, N.; Tohoyama, H.; Joho, M. Azuki bean cells are hypersensitive to cadmium and do not synthesize phytochelatins. Plant Physiol. 2000, 123, 1029–1036. [Google Scholar]
- Inouhe, M.; Ninomiya, S.; Tohoyama, H.; Joho, M.; Murayama, T. Different characteristics of roots in the cadmium-tolerance and Cd-binding complex formation between mono- and dicotyledonous plants. J. Plant. Res. 1994, 107, 201–207. [Google Scholar]
- Chen, Y.X.; He, Y.F.; Luo, Y.M.; Yu, Y.L.; Lin, Q.; Wong, M.H. Physiological mechanism of plant roots exposed to cadmium. Chemosphere 2003, 50, 789–793. [Google Scholar]
- Leopold, I.; Gunther, D.; Schmidt, J.; Neumann, D. Phytochelatins and heavy metal tolerance. Phytochemistry 1999, 50, 1323–1328. [Google Scholar]
- Hu, S.X.; Lau, K.W.K.; Wu, M. Cadmium sequestration in Chlamydomonas reinhardtii. Plant Sci. 2001, 161, 987–996. [Google Scholar]
- Tsuji, T.; Hirayanagi, N.; Iwabe, O.; Namba, T.; Taqawa, M.; Miyamoto, S.; Miyasaka, H.; Takagi, M.; Hirata, K.; Miyamoto, K. Regulation of phytochelatin synthesis by zinc and cadmium in marine green alga, Dunaliella tertiolecta. Phytochemistry 2003, 62, 453–459. [Google Scholar]
| Abbreviations | |
|---|---|
| GSH | reduced glutathione |
| GSSG | oxidized glutathione |
| PC2 | phytochelatin2 |
| M-PC complex | metal-phytochelatin complex |
| LMW | low molecular weight |
| HMW | high molecular weight |
| MS medium | Murashige-Skoog medium |
| HMDE | hanging mercury drop electrode |
| CV | cyclic voltammetry |
| DPASV | differential pulse anodic stripping voltammetry |
| SH | sulfhydryl group |
| FIA | flow injection analysis |
| HPLC | high performance liquid chromatography |
| ED | electrochemical detection |
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Supalkova, V.; Huska, D.; Diopan, V.; Hanustiak, P.; Zitka, O.; Stejskal, K.; Baloun, J.; Pikula, J.; Havel, L.; Zehnalek, J.; et al. Electroanalysis of Plant Thiols. Sensors 2007, 7, 932-959. https://doi.org/10.3390/s7060932
Supalkova V, Huska D, Diopan V, Hanustiak P, Zitka O, Stejskal K, Baloun J, Pikula J, Havel L, Zehnalek J, et al. Electroanalysis of Plant Thiols. Sensors. 2007; 7(6):932-959. https://doi.org/10.3390/s7060932
Chicago/Turabian StyleSupalkova, Veronika, Dalibor Huska, Vaclav Diopan, Pavel Hanustiak, Ondrej Zitka, Karel Stejskal, Jiri Baloun, Jiri Pikula, Ladislav Havel, Josef Zehnalek, and et al. 2007. "Electroanalysis of Plant Thiols" Sensors 7, no. 6: 932-959. https://doi.org/10.3390/s7060932



