Hazards of Secondary Bromadiolone Intoxications Evaluated using High-performance Liquid Chromatography with Electrochemical Detection
Abstract
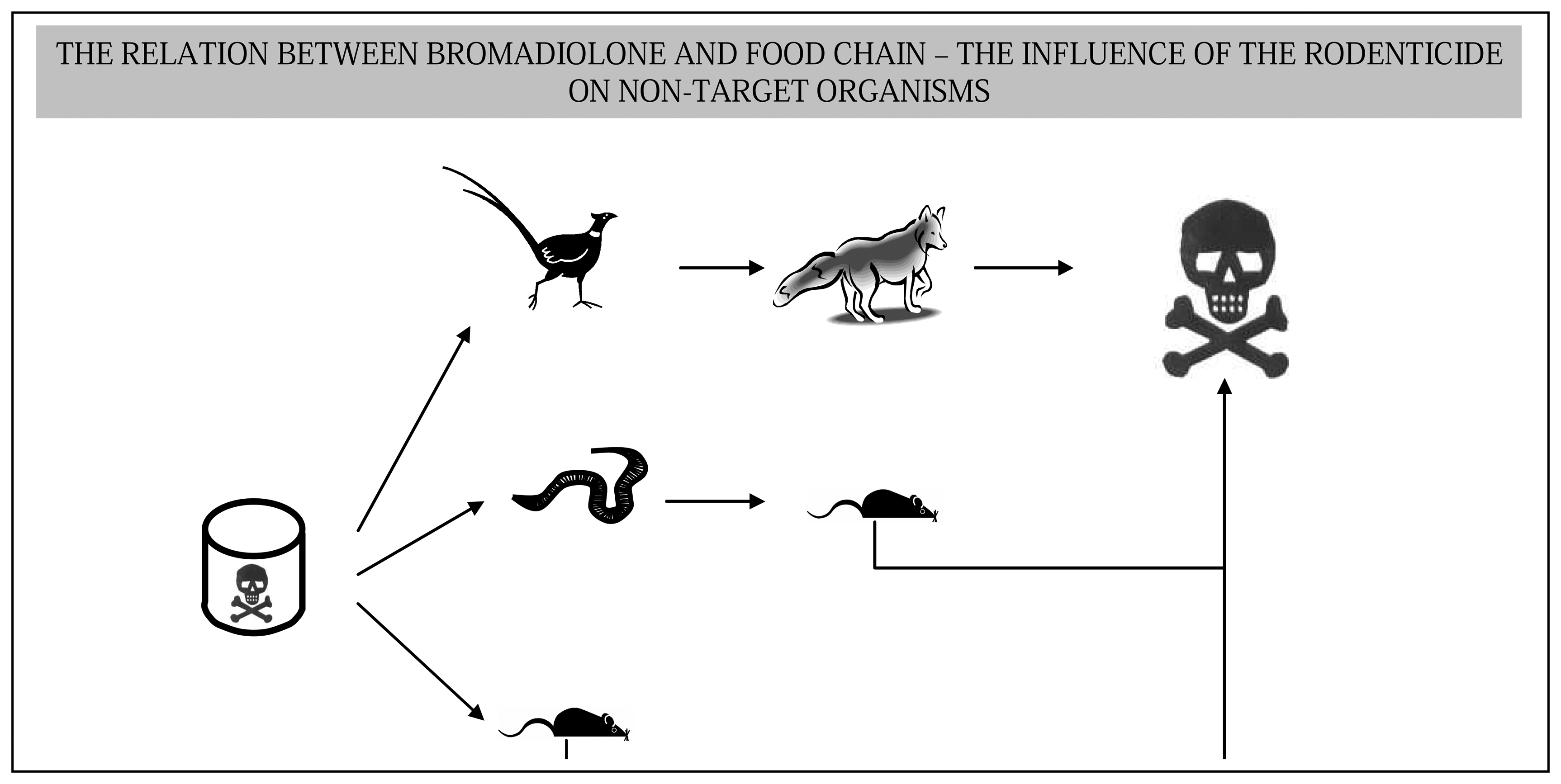
:1. Introduction
2. Experimental Section
2.1 Chemicals
2.2 Rodenticide-containing product
2.3 Biotests
2.3.1 Toxicity for earthworms – test in the artificial soil substrate
2.3.2 Primary and secondary intoxication of common voles
2.3.3 Hares
2.4 Preparation of biological samples
2.5 Electrochemical measurements in the stationary system
2.6 Electrochemical measurements in the flow system
3. Results
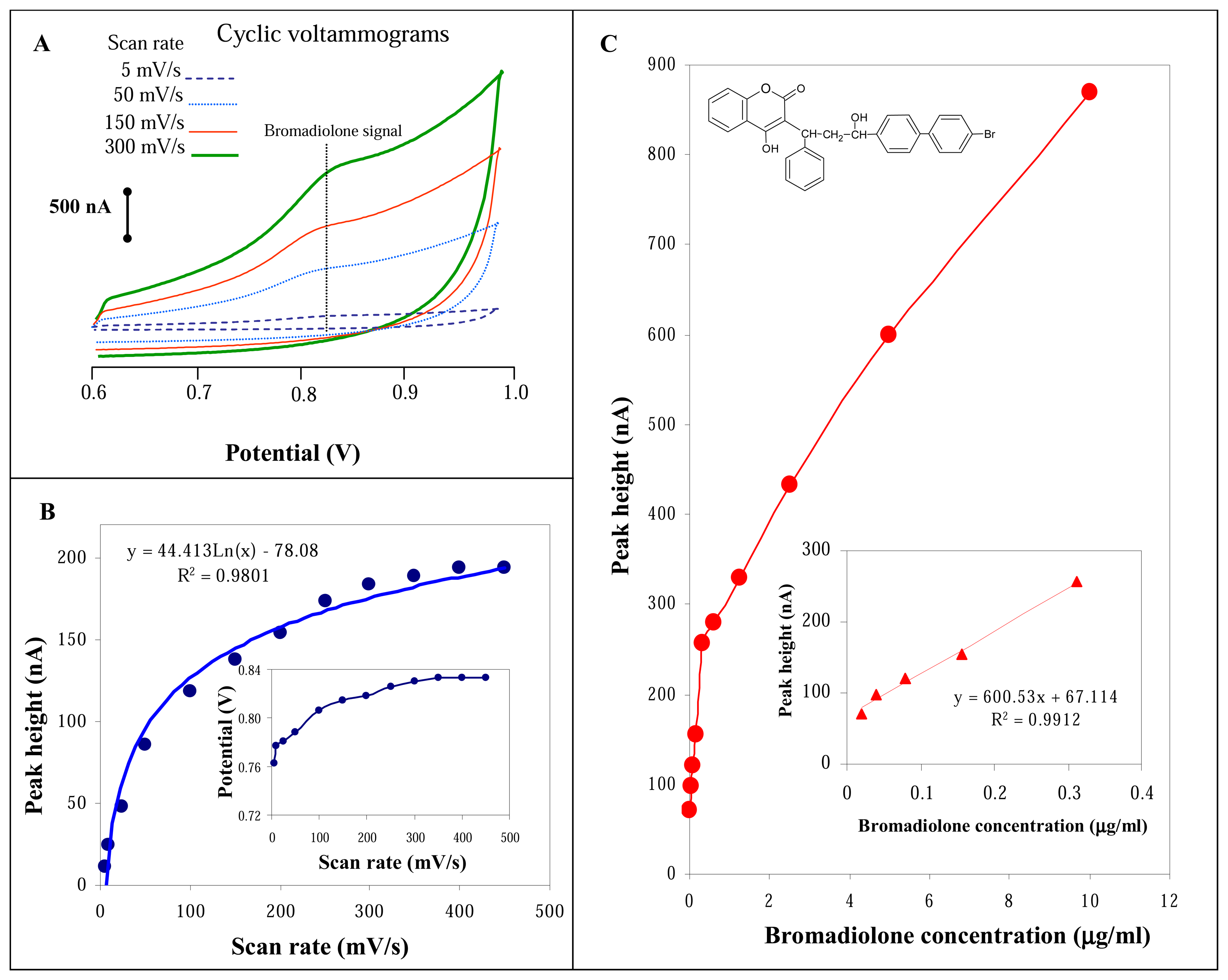
3.1 Detection of bromadiolone using cyclic voltammetry
3.2 Detection of bromadiolone using flow injection analysis with glassy carbon electrode
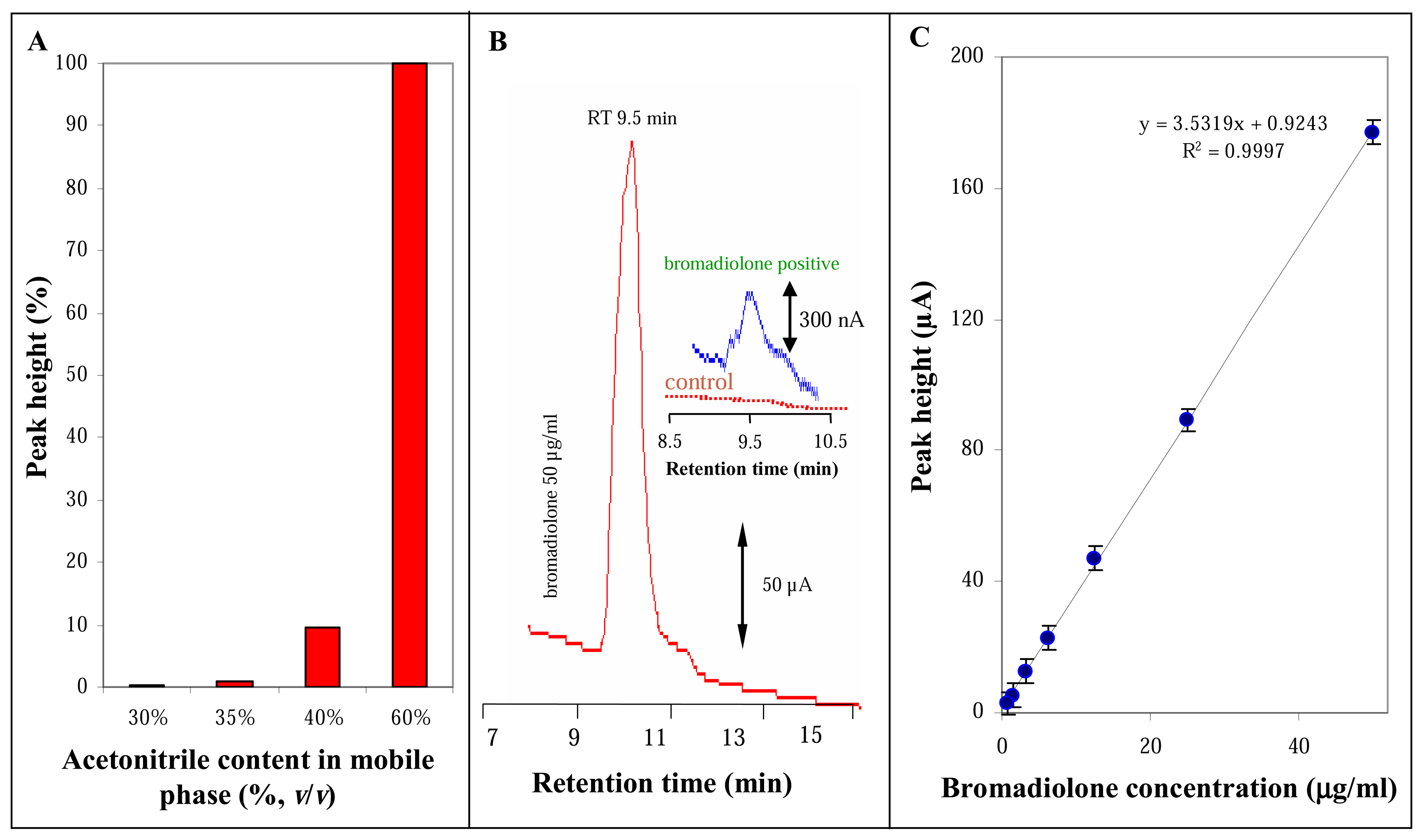
3.3 High performance liquid chromatographic analysis of bromadiolone
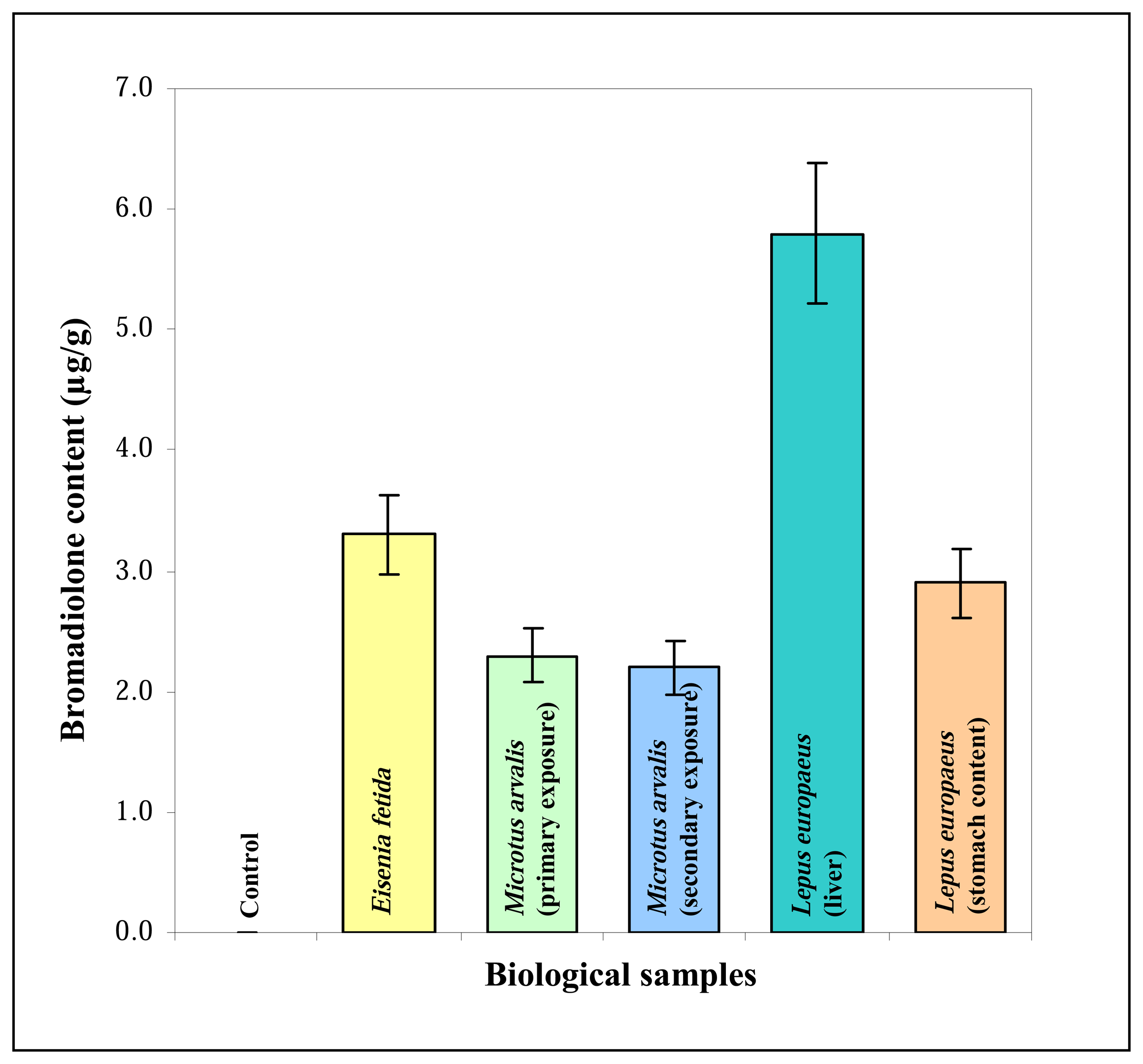
3.4 Bromadiolone exposure
3.4.1 Earthworms
3.4.2 Common voles
3.4.3 Hares
4. Discussion
Acknowledgments
References
- Kizek, R.; Vacek, J.; Trnkova, L.; Klejdus, B.; Kuban, V. Electrochemical biosensors in agricultural and environmental analysis. Chem. Listy 2003, 97, 1003–1006. [Google Scholar]
- Ulrich, R.; Raszyk, J. Variations in environmental contamination by polychlorinated biphenyls (PCB) and chlorinated pesticides (Lindane, DDT) on pig farms in Hodonin district in 1994 to 1999. Vet. Med. 2002, 47, 159–168. [Google Scholar]
- Cohen, S.Z.; Creeger, S.M.; Carsel, R.F.; Enfield, C.G. Potential Pesticide Contamination of Groundwater from Agricultural Uses. Acs Symposium Series 1984, 259, 297–325. [Google Scholar]
- Cope, R.B. Small animal anticoagulant rodenticide poisoning. Aust. Vet. Pract. 2004, 34, 50, -+. [Google Scholar]
- Jones, R.L.; Mangels, G. Review of the validation of models used in federal insecticide, fungicide, and rodenticide act environmental exposure assessments. Environ. Toxicol. Chem. 2002, 21, 1535–1544. [Google Scholar]
- Snyder, N.J.; Cartron, J.M.; van Wesenbeeck, I.; Carver, L.S.; Ritter, A.M. Electronic soil moisture measurements in federal insecticide, fungicide, and rodenticide act field dissipation and prospective groundwater studies; American Chemical Society, 2003; p. 368. [Google Scholar]
- Pikula, J.; Treml, F.; Beklova, M.; Holesovska, Z.; Pikulova, J. Geographic information systems in epidemiology - Ecology of common vole and distribution of natural foci of tularaemia. Acta Vet. BRNO 2002, 71, 379–387. [Google Scholar]
- Pikula, J.; Treml, F.; Beklova, M.; Holesovska, Z.; Pikulova, J. Ecological conditions of natural foci of tularaemia in the Czech Republic. Eur. J. Epidemiol. 2003, 18, 1091–1095. [Google Scholar]
- Pikula, J.; Beklova, M.; Holesovska, Z.; Treml, F. Spatio-temporal aspects of tularemia in Southern Moravia, Czech Republic. Vet. Med. 2004, 49, 15–18. [Google Scholar]
- Pikula, J.; Beklova, M.; Holesovska, Z.; Treml, F. Prediction of possible distribution of tularemia in the Czech Republic. Vet. Med. 2004, 49, 61–64. [Google Scholar]
- Treml, F.; Pejcoch, M.; Holesovska, Z. Small mammals - natural reservoir of pathogenic leptospires. Vet. Med. 2002, 47, 309–314. [Google Scholar]
- Pikula, J.; Beklova, M.; Horakova, J.; Skocovska, B.; Vitula, F. Comparation of toxic effects of rodenticides for birds. Chem. Listy 2005, 99, S77–S79. [Google Scholar]
- Beklova, M.; Pikula, J.; Horakova, J.; Skocovska, B.; Vitula, F. Evaluation of environmental hazards of rodenticides. Chem. Listy 2005, 99, S79–S81. [Google Scholar]
- Berny, P.J.; de Oliveira, L.A.; Videmann, B.; Rossi, S. Assessment of ruminal degradation, oral bioavailability, and toxic effects of anticoagulant rodenticides in sheep. Am. J. Vet. Res. 2006, 67, 363–371. [Google Scholar]
- Stone, W.B.; Okoniewski, J.C.; Stedelin, J.R. Poisoning of wildlife with anticoagulant rodenticides in New York. J. Wildl. Dis. 1999, 35, 187–193. [Google Scholar]
- Berny, P.J.; Buronfosse, T.; Buronfosse, F.; Lamarque, F.; Lorgue, G. Field evidence of secondary poisoning of foxes (Vulpes vulpes) and buzzards (Buteo buteo) by bromadiolone, a 4-year survey. Chemosphere 1997, 35, 1817–1829. [Google Scholar]
- Fournier-Chambrillon, C.; Berny, P.J.; Coiffier, O.; Barbedienne, P.; Dasse, B.; Delas, G.; Galineau, H.; Mazet, A.; Pouzenc, P.; Rosoux, R.; Fournier, P. Evidence of secondary poisoning of free-ranging riparian mustelids by anticoagulant rodenticides in France: Implications for conservation of European mink (Mustela lutreola). J. Wildl. Dis. 2004, 40, 688–695. [Google Scholar]
- Shore, R.F.; Birks, J.D.S.; Afsar, A.; Wienburg, C.L.; Kitchener, A.C. Spatial and temporal analysis of second-generation anticoagulant rodenticide residues in polecats (Mustela putorius) from throughout their range in Britain, 1992-1999. Environ. Pollut. 2003, 122, 183–193. [Google Scholar]
- Revathi, K.; Yogananda, M. Effect of bromadiolone on haematology, liver and kidney in Mus musculus. J.Environ. Biol. 2006, 27, 135–140. [Google Scholar]
- Murphy, M.J.; Gerken, D.F. The anticoagulant rodenticides; W. B. Saunders Co, 1989; pp. 143–146. [Google Scholar]
- Deepa, S.; Mishra, A.K. Fluorescence spectroscopic study of serum albumin-bromadiolone interaction: fluorimetric determination of bromadiolone. J. Pharm. Biomed. Anal. 2005, 38, 556–563. [Google Scholar]
- Aprea, C.; Colosio, C.; Mammone, T.; Minoia, C.; Maroni, M. Biological monitoring of pesticide exposure: a review of analytical methods. J. Chromatogr. B 2002, 769, 191–219. [Google Scholar]
- Balinova, A. Strategies for chromatographic analysis of pesticide residues in water. J. Chromatogr. A 1996, 754, 125–135. [Google Scholar]
- Barcelo, D. A Review of Liquid-Chromatography in Environmental Pesticide Analysis. Chromatographia 1988, 25, 928–936. [Google Scholar]
- Cabras, P.; Angioni, A. Pesticide residues in grapes, wine, and their processing products. J. Agric. Food Chem. 2000, 48, 967–973. [Google Scholar]
- Pico, Y.; Font, G.; Molto, J.C.; Manes, J. Pesticide residue determination in fruit and vegetables by liquid chromatography-mass spectrometry. J. Chromatogr. A 2000, 882, 153–173. [Google Scholar]
- Torres, C.M.; Pico, Y.; Manes, J. Determination of pesticide residues in fruit and vegetables. J. Chromatogr. A 1996, 754, 301–331. [Google Scholar]
- Berny, P.J.; Buronfosse, T.; Lorgue, G. Anticoagulant Poisoning in Animals - a Simple New High-Performance Thin-Layer Chromatographic (Hptlc) Method for the Simultaneous Determination of 8 Anticoagulant Rodenticides in Liver Samples. J. Anal. Toxicol. 1995, 19, 576–580. [Google Scholar]
- Welling, P.G.; Lee, K.P.; Khanna, U.; Wagner, J.G. Comparison of Plasma Concentrations of Warfarin Measured by Both Simple Extraction and Tlc Methods. J. Pharm. Sci. 1970, 59, 1621, -&. [Google Scholar]
- Fauconnet, V.; Pouliquen, H.; Pinault, L. Reversed-phase HPLC determination of eight anticoagulant rodenticides in animal liver. J. Anal. Toxicol. 1997, 21, 548–553. [Google Scholar]
- Hunter, K. High-Performance Liquid-Chromatographic Strategies for the Determination and Confirmation of Anticoagulant Rodenticide Residues in Animal-Tissues. J. Chromatogr. 1985, 321, 255–272. [Google Scholar]
- Marek, L.J.; Koskinen, W.C. Multiresidue analysis of seven anticoagulant rodenticides by LC/ES/MS/MS. Abstr. Pap. Am. Chem. Soc. 2005, 229, U79–U79. [Google Scholar]
- Marek, L.J.; Koskinen, W.C. Multiresidue analysis of seven anticoagulant rodenticides by high-performance liquid chromatography/electrospray/mass spectrometry. J. Agric. Food Chem. 2007, 55, 571–576. [Google Scholar]
- Kollroser, M.; Schober, C. Determination of coumarin-type anticoagulants in human plasma by HPLC-electrospray ionization tandem mass spectrometry with an ion trap detector. Clin. Chem. 2002, 48, 84–91. [Google Scholar]
- Passaro, V.M.N.; Dell'Olio, F.; Casamassima, B.; De Leonardis, F. Guided-wave optical biosensors. Sensors 2007, 7, 508–536. [Google Scholar]
- Jaffrezic-Renault, N.; Martelet, C.; Chevolot, Y.; Cloarec, J.P. Biosensors and bio-bar code assays based on biofunctionalized magnetic microbeads. Sensors 2007, 7, 589–614. [Google Scholar]
- Pohanka, M.; Pavlis, O.; Skladal, P. Rapid characterization of monoclonal antibodies using the piezoelectric immunosensor. Sensors 2007, 7, 341–353. [Google Scholar]
- Wang, C.Y.; Wang, Z.X.; Zhu, A.P.; Hu, X.Y. Voltammetric determination of dopamine in human serum with amphiphilic chitosan modified glassy carbon electrode. Sensors 2006, 6, 1523–1536. [Google Scholar]
- Prieto-Simon, B.; Campas, M.; Andreescu, S.; Marty, J.L. Trends in flow-based biosensing systems for pesticide assessment. Sensors 2006, 6, 1161–1186. [Google Scholar]
- Chailapakul, O.; Ngamukot, P.; Yoosamran, A.; Siangproh, W.; Wangfuengkanagul, N. Recent electrochemical and optical sensors in flow-based analysis. Sensors 2006, 6, 1383–1410. [Google Scholar]
- Diculescu, V.C.; Paquim, A.M.C.; Brett, A.M.O. Electrochemical DNA sensors for detection of DNA damage. Sensors 2005, 5, 377–393. [Google Scholar]
- Labuda, J.; Bubnicova, K.; Kovalova, L.; Vanickova, M.; Mattusch, J.; Wennrich, R. Voltammetric detection of damage to DNA by arsenic compounds at a DNA biosensor. Sensors 2005, 5, 411–423. [Google Scholar]
- Trnkova, L.; Jelen, F.; Petrlova, J.; Adam, V.; Potesil, D.; Kizek, R. Elimination voltammetry with linear scan as a new detection method for DNA sensors. Sensors 2005, 5, 448–464. [Google Scholar]
- Adam, V.; Zehnalek, J.; Petrlova, J.; Potesil, D.; Sures, B.; Trnkova, L.; Jelen, F.; Vitecek, J.; Kizek, R. Phytochelatin modified electrode surface as a sensitive heavy-metal ion biosensor. Sensors 2005, 5, 70–84. [Google Scholar]
- Lei, Y.; Mulchandani, P.; Chen, W.; Mulchandani, A. Biosensor for direct determination of fenitrothion and EPN using recombinant Pseudomonas putida JS444 with surface expressed organophosphorus hydrolase. 1. Modified Clark oxygen electrode. Sensors 2006, 6, 466–472. [Google Scholar]
- Schoning, M.J. “Playing around” with field-effect sensors on the basis of EIS structures, LAPS and ISFETs. Sensors 2005, 5, 126–138. [Google Scholar]
- Huang, X.J.; Liu, J.H.; Pi, Z.X.; Yu, Z.L. Detecting pesticide residue by using modulating temperature over a single SnO2-based gas sensor. Sensors 2003, 3, 361–370. [Google Scholar]
- Schoning, M.J.; Arzdorf, M.; Mulchandani, P.; Chen, W.; Mulchandani, A. Towards a capacitive enzyme sensor for direct determination of organophosphorus pesticides: Fundamental studies and aspects of development. Sensors 2003, 3, 119–127. [Google Scholar]
- Babula, P.; Huska, D.; Hanustiak, P.; Baloun, J.; Krizkova, S.; Adam, V.; Hubalek, J.; Havel, L.; Zemlicka, M.; Horna, A.; Beklova, M.; Kizek, R. Flow injection analysis coupled with carbon electrodes as the tool for analysis of naphthoquinones with respect to their content and functions in biological samples. Sensors 2006, 11, 1466–1482. [Google Scholar]
- Prasek, J.; Adamek, M.; Hubalek, J.; Adam, V.; Trnkova, L.; Kizek, R. New hydrodynamic electrochemical arrangement for cadmium ions detection using thick-film chemical sensor electrodes. Sensors 2006, 11, 1498–1512. [Google Scholar]
- Supalkova, V.; Huska, D.; Diopan, V.; Hanustiak, P.; Zitka, O.; Stejskal, K.; Baloun, J.; Pikula, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Beklova, M.; Kizek, R. Electroanalysis of plant thiols. Sensors 2007, 7, 932–959. [Google Scholar]
- Supalkova, V.; Petrek, J.; Baloun, J.; Adam, V.; Bartusek, K.; Trnkova, L.; Beklova, M.; Diopan, V.; Havel, L.; Kizek, R. Multi-instrumental investigation of affecting of early somatic embryos of Spruce by cadmium(II) and lead(II) ions. Sensors 2007, 7, 743–759. [Google Scholar]
- Supalkova, V.; Petrek, J.; Havel, L.; Krizkova, S.; Petrlova, J.; Adam, V.; Potesil, D.; Babula, P.; Beklova, M.; Horna, A.; Kizek, R. Electrochemical sensors for detection of acetylsalicylic acid. Sensors 2006, 11, 1483–1497. [Google Scholar]
- Vitecek, J.; Petrlova, J.; Adam, V.; Havel, L.; Kramer, K.J.; Babula, P.; Kizek, R. A fluorimetric sensor for detection of one living cell. Sensors 2007, 7, 222–238. [Google Scholar]
- Adam, V.; Krizkova, S.; Zitka, O.; Trnkova, L.; Petrlova, J.; Beklova, M.; Kizek, R. A determination of apo-metallothionein using adsorptive transfer stripping technique in connection with differential pulse voltammetry. Electroanalysis 2007, 19, 339–347. [Google Scholar]
- Adam, V.; Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Trnkova, L.; Jelen, F.; Kizek, R. Study of metallothionein modified electrode surface behaviour in the presence of heavy metal ions -biosensor. Electroanalysis 2005, 17, 1649–1657. [Google Scholar]
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin electrochemical biosensor. Electrochim. Acta 2006, 51, 5169–5173. [Google Scholar]
- Beklova, M.; Krizkova, S.; Supalkova, V.; Mikelova, R.; Adam, V.; Pikula, J.; Kizek, R. Determination of bromadiolone in pheasants and foxes by differential pulse voltammetry. Int. J. Environ. Anal. Chem. 2007, 87, 459–469. [Google Scholar]
- OECD guideline for testing chemicals No. 207. Earthworm, acute toxicity tests; Organisation for Economics Cooperation and Development, 1984.
- Kizek, R.; Masarik, M.; Kramer, K.J.; Potesil, D.; Bailey, M.; Howard, J.A.; Klejdus, B.; Mikelova, R.; Adam, V.; Trnkova, L.; Jelen, F. An analysis of avidin, biotin and their interaction at attomole levels by voltammetric and chromatographic techniques. Anal. Bioanal. Chem. 2005, 381, 1167–1178. [Google Scholar]
- Masarik, M.; Kizek, R.; Kramer, K.J.; Billova, S.; Brazdova, M.; Vacek, J.; Bailey, M.; Jelen, F.; Howard, J.A. Application of avidin-biotin technology and adsorptive transfer stripping square-wave voltammetry for detection of DNA hybridization and avidin in transgenic avidin maize. Anal. Chem. 2003, 75, 2663–2669. [Google Scholar]
- Petrlova, J.; Masarik, M.; Potesil, D.; Adam, V.; Trnkova, L.; Kizek, R. Zeptomole detection of streptavidin using carbon paste electrode and square wave voltammetry. Electroanalysis 2007, 19, 1177–1182. [Google Scholar]
- Billova, S.; Kizek, R.; Jelen, F.; Novotna, P. Square-wave voltammetric determination of cefoperazone in a bacterial culture, pharmaceutical drug, milk, and urine. Anal. Bioanal. Chem. 2003, 377, 362–369. [Google Scholar]
- Klejdus, B.; Vacek, J.; Adam, V.; Zehnalek, J.; Kizek, R.; Trnkova, L.; Kuban, V. Determination of isoflavones in soybean food and human urine using liquid chromatography with electrochemical detection. J. Chromatogr. B 2004, 806, 101–111. [Google Scholar]
- Potesil, D.; Mikelova, R.; Adam, V.; Kizek, R.; Prusa, R. Change of the protein p53 electrochemical signal according to its structural form - Quick and sensitive distinguishing of native, denatured, and aggregated form of the “guardian of the genome”. Protein J. 2006, 25, 23–32. [Google Scholar]
- Potesil, D.; Petrlova, J.; Adam, V.; Vacek, J.; Klejdus, B.; Zehnalek, J.; Trnkova, L.; Havel, L.; Kizek, R. Simultaneous femtomole determination of cysteine, reduced and oxidized glutathione, and phytochelatin in maize (Zea mays L.) kernels using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A 2005, 1084, 134–144. [Google Scholar]
- Kula, H.; Larink, O. Tests on the Earthworms Eisenia fetida and Aporrectodea caliginosa; John Wiley & Sons, 1998; p. 281. [Google Scholar]
- Atterby, H.; Kerins, G.M.; MacNiccoll, A.D. Whole-carcass residues of the rodenticide difenacoum in anticoagulant-resistant and -susceptible rat strains (Rattus norvegicus). Environ. Toxicol. Chem. 2005, 24, 318–323. [Google Scholar]
- Paoletti, M.G.; Sommaggio, D.; Favretto, M.R.; Petruzzelli, G.; Pezzarossa, B.; Barbafieri, M. Earthworms as useful bioindicators of agroecosystem sustainability in orchards and vineyards with different inputs. Appl. Soil Ecol. 1998, 10, 137–150. [Google Scholar]
- Dell'Omo, G.; Turk, A.; Shore, R.E. Secondary poisoning in the common shrew (Sorex araneus) fed earthworms exposed to an organophosphate pesticide. Environ. Toxicol. Chem. 1999, 18, 237–240. [Google Scholar]
- Brown, P.; Charlton, A.; Cuthbert, M.; Barnett, L.; Ross, L.; Green, M.; Gillies, L.; Shaw, K.; Fletcher, M. Identification of pesticide poisoning in wildlife. J. Chromatogr. A 1996, 754, 463–478. [Google Scholar]
- Guan, F.; Ishii, A.; Seno, H.; Watanabe, K.; Kumazawa, T.; Suzuki, O. A method for simultaneous determination of five anticoagulant rodenticides in whole blood by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 1999, 21, 179–185. [Google Scholar]
- Guan, F.Y.; Ishii, A.; Seno, H.; Watanabe-Suzuki, K.; Kumazawa, T.; Suzuki, O. Use of an ion-pairing reagent for high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry determination of anionic anticoagulant rodenticides in body fluids. J. Chromatogr. B 1999, 731, 155–165. [Google Scholar]
- Obryan, S.M.; Constable, D.J.C. Quantification of Brodifacoum in Plasma and Liver-Tissue by Hplc. J. Anal. Toxicol. 1991, 15, 144–147. [Google Scholar]
- Park, S.W.; Seo, B.S.; Kim, E.H.; Kim, D.H.; Paeng, K.J. Purification and determination procedure of coumarin derivatives. J. Forensic Sci. 1996, 41, 685–688. [Google Scholar]

© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Krizkova, S.; Beklova, M.; Pikula, J.; Adam, V.; Horna, A.; Kizek, R. Hazards of Secondary Bromadiolone Intoxications Evaluated using High-performance Liquid Chromatography with Electrochemical Detection. Sensors 2007, 7, 1271-1286. https://doi.org/10.3390/s7071271
Krizkova S, Beklova M, Pikula J, Adam V, Horna A, Kizek R. Hazards of Secondary Bromadiolone Intoxications Evaluated using High-performance Liquid Chromatography with Electrochemical Detection. Sensors. 2007; 7(7):1271-1286. https://doi.org/10.3390/s7071271
Chicago/Turabian StyleKrizkova, Sona, Miroslava Beklova, Jiri Pikula, Vojtech Adam, Ales Horna, and René Kizek. 2007. "Hazards of Secondary Bromadiolone Intoxications Evaluated using High-performance Liquid Chromatography with Electrochemical Detection" Sensors 7, no. 7: 1271-1286. https://doi.org/10.3390/s7071271
APA StyleKrizkova, S., Beklova, M., Pikula, J., Adam, V., Horna, A., & Kizek, R. (2007). Hazards of Secondary Bromadiolone Intoxications Evaluated using High-performance Liquid Chromatography with Electrochemical Detection. Sensors, 7(7), 1271-1286. https://doi.org/10.3390/s7071271



