Improvement of Aptamer Affinity by Dimerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Binding assay measurements
The linked thrombin-binding aptamer
The linked VEGF-binding aptamer
2.3. Thrombin activity inhibition assay using linked aptamers
3. Results and Discussion
3.1. The linked thrombin-binding aptamer
3.1.1. Design of the aptamer dimer
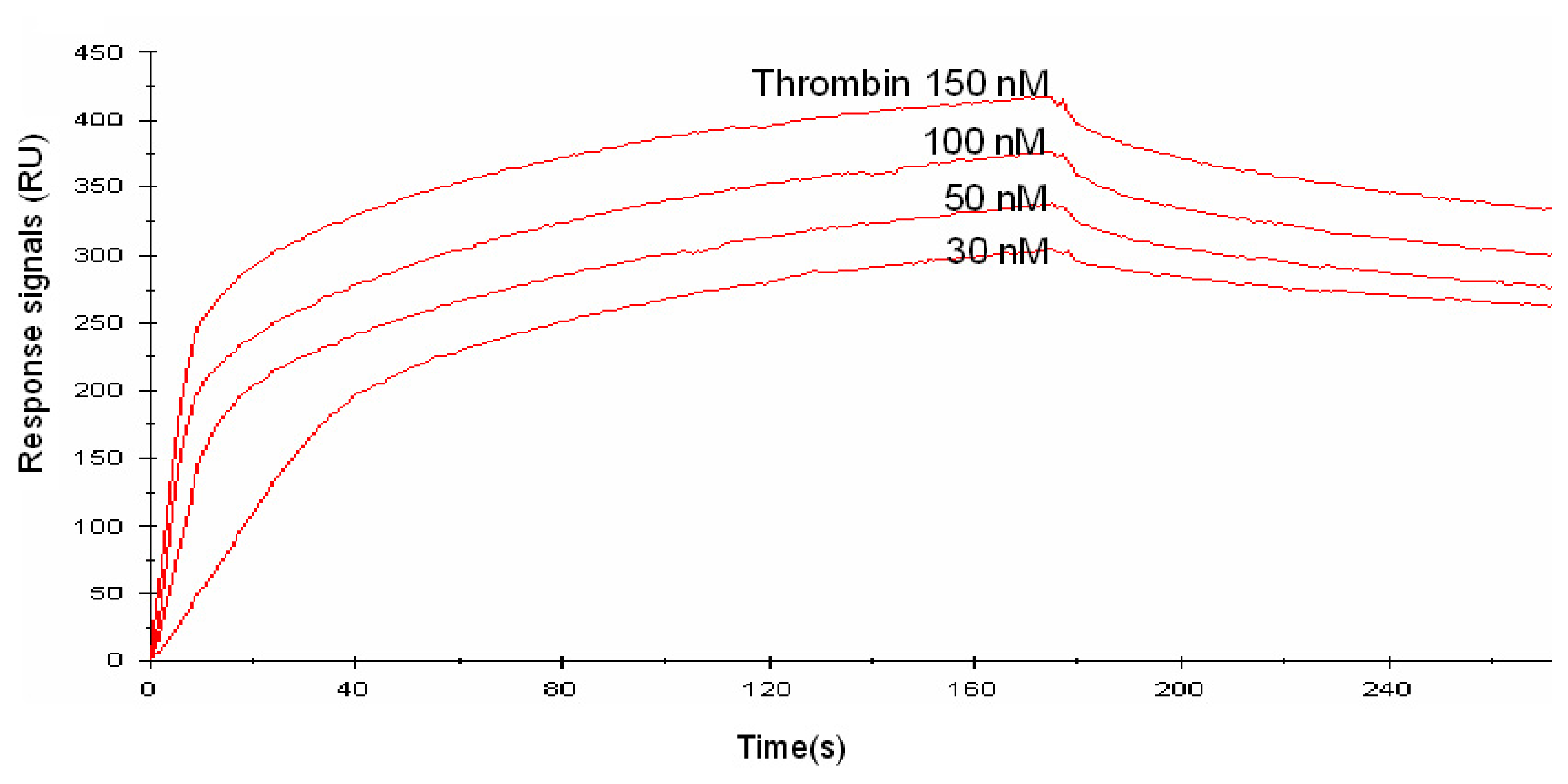
3.1.2. Binding assay using SPR measurement
3.1.3. The inhibitory activity of the linked aptamers
3.2. The linked VEGF-binding aptamer
3.2.1. Design of the aptamer dimer
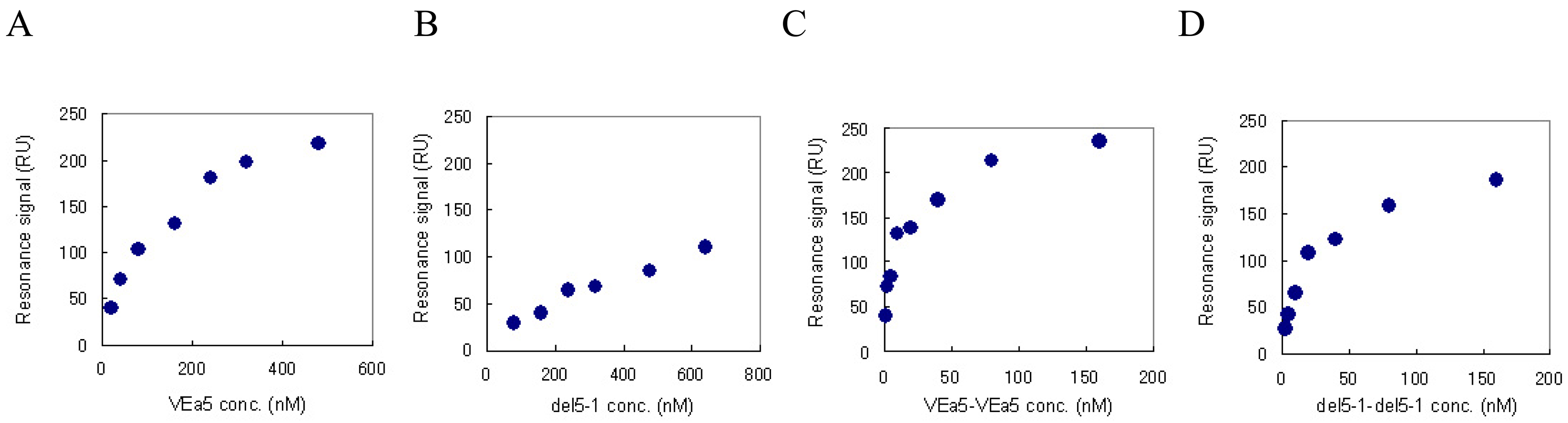
3.2.2. Binding assay using SPR measurement
4. Conclusion
Acknowledgments
References and Notes
- Jayasena, S.D. Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar]
- Ellington, A.D.; Szostak, J. W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar]
- Noma, T.; Ikebukuro, K. Aptamer selection based on inhibitory activity using an evolution-mimicking algorithm. Biochem. Biophys. Res. Commun. 2006, 347, 226–231. [Google Scholar]
- Fredriksson, S.; Gullberg, M.; Jarvius, J.; Olsson, C.; Pietras, K.; Gustafsdottir, S.M.; Ostman, A.; Landegren, U. Protein detection using proximity-dependent DNA ligation assays. Nat. Biotechnol. 2002, 20, 473–477. [Google Scholar]
- Yoshida, W.; Sode, K.; Ikebukuro, K. Homogeneous DNA sensing using enzyme-inhibiting DNA aptamers. Biochem. Biophys. Res. Commun. 2006, 348, 245–252. [Google Scholar]
- Yoshida, W.; Sode, K.; Ikebukuro, K. Aptameric enzyme subunit for biosensing based on enzymatic activity measurement. Anal. Chem. 2006, 78, 3296–3303. [Google Scholar]
- Adams, G.P.; Schier, R. Generating improved single-chain Fv molecules for tumor targeting. J. Immunol. Methods 1999, 231, 249–260. [Google Scholar]
- Viti, F.; Tarli, L.; Giovannoni, L.; Zardi, L.; Neri, D. Increased binding affinity and valence of recombinant antibody fragments lead to improved targeting of tumoral angiogenesis. Cancer Res. 1999, 59, 347–352. [Google Scholar]
- Neri, D.; Momo, M.; Prospero, T.; Winter, G. High-affinity antigen binding by chelating recombinant antibodies (CRAbs). J. Mol. Biol. 1995, 246, 367–373. [Google Scholar]
- Hasegawa, H.; Sode, K.; Ikebukuro, K. Selection of DNA aptamers against VEGF(165) using a protein competitor and the aptamer blotting method. Biotechnol. Lett. 2008. [Google Scholar]
- Nakahara, H.; Song, J.; Sugimoto, M.; Hagihara, K.; Kishimoto, T.; Yoshizaki, K.; Nishimoto, N. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 1521–1529. [Google Scholar]
- Voorzanger-Rousselot, N.; Garnero, P. Biochemical markers in oncology. Part I: molecular basis. Part II: clinical uses. Cancer Treat. Rev. 2007, 33, 230–283. [Google Scholar]
- Bock, L.C.; Griffin, L. C.; Latham, J. A.; Vermaas, E. H.; Toole, J. J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 1992, 355, 564–566. [Google Scholar]
- Tasset, D.M.; Kubik, M. F.; Steiner, W. Oligonucleotide inhibitors of human thrombin that bind distinct epitopes. J Mol Biol 1997, 272, 688–698. [Google Scholar]
- Muller, J.; Wulffen, B.; Potzsch, B.; Mayer, G. Multidomain targeting generates a high-affinity thrombin-inhibiting bivalent aptamer. Chembiochem. 2007, 8, 2223–2226. [Google Scholar]
- Stauffer, M.E.; Skelton, N. J.; Fairbrothe, W. J. Refinement of the solution structure of the heparin-binding domain of vascular endothelial growth factor using residual dipolar couplings. J. Biomol. NMR 2002, 23, 57–61. [Google Scholar]
- Muller, Y. A.; Li, B.; Christinger, H. W.; Wells, J. A.; Cunningham, B. C.; de Vos, A. M. Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc. Natl. Acad. Sci. USA 1997, 94, 7192–7197. [Google Scholar]
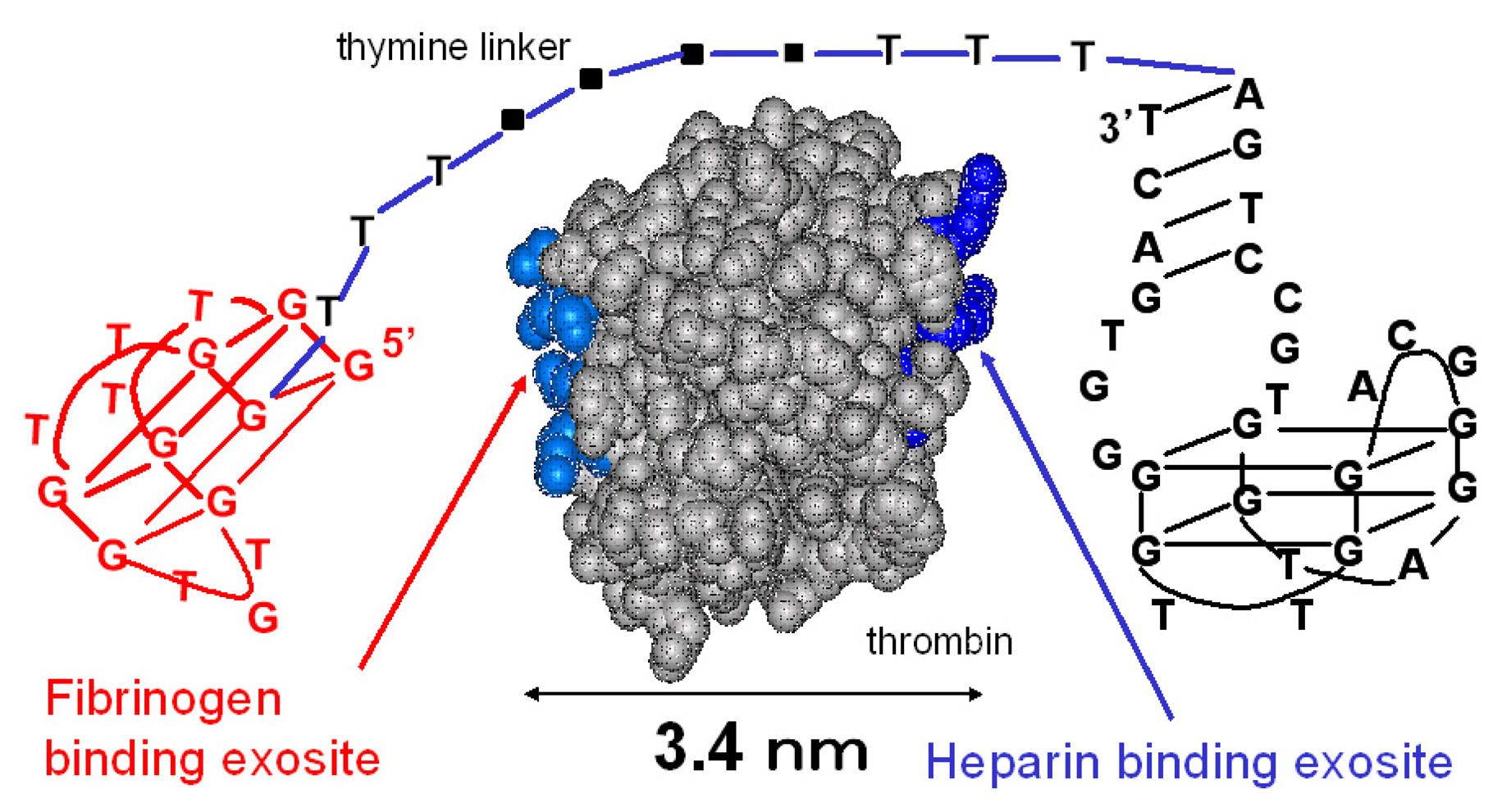

| aptamers | Sequences (5'-3') |
|---|---|
| Thrombin-binding aptamer | |
| 15-mer | GGTTGGTGTGGTTGG |
| 29-mei | AGTCCGTGGTAGGGCAGGTTGGGGTGACT |
| Linker 0 | GGTTGGTGTGGTTGG--------------------AGTCCGTGGTAGGGCAGGTTGGGGTGACT |
| Linker 5 | GGTTGGTGTGGTTGG-------TTTTT--------AGTCCGTGGTAGGGCAGGTTGGGGTGACT |
| Linker 10 | GGTTGGTGTGGTTGG-----TTTTTTTTTT-----AGTCCGTGGTAGGGCAGGTTGGGGTGACT |
| Linker 20 | GGTTGGTGTGGTTGGTTTTTTTTTTTTTTTTTTTTAGTCCGTGGTAGGGCAGGTTGGGGTGACT |
| VEGF-binding aptamer | |
| VEa5 | ATACCAGTCTATTCAATTGGGCCCGTCCGTATGGTGGGTGTGCTGGCCAGATAGTATGTGCAATCA |
| VEa5-VEa5 | [VEa5]--------------------[VEa5] |
| VEa5-T10-VEa5 | [VEa5]----- TTTTTTTTTT-----[VEa5] |
| VEa5-T20-VEa5 | [VEa5] TTTTTTTTTTTTTTTTTTTT[VEa5] |
| del5-1 | ATACCAGTCTATTCAATTGGGCCCGTCCGTATGGTGGGTGTGCTGGCCAG |
| del5-1-del5-1 | [del5-1]--------------------[del5-1] |
| del5-1-T10-del5-1 | [del5-1]----- TTTTTTTTTT-----[del5-1] |
| del5-1-T20-del5-1 | [del5-1] TTTTTTTTTTTTTTTTTTTT[del5-1] |
| kon1 (1/M-s) | kon2 (1/M-s) | koff1 (1/s) | koff2 (1/s) | Kd1 (nM) | Kd2 (nM) | |
|---|---|---|---|---|---|---|
| 15-mer | 1.89 × 105 | 3.83× 10-3 | 20.2 | |||
| 29-mer | 4.40 × 10s | 1.54 × 10-3 | 3.5 | |||
| Linker 0 | 5.96 × 104 | 5.80 × 104 | 8.57 × 10-6 | 8.62 × 10-6 | 0.14 | 0.15 |
| Linker 5 | 4.89 × 105 | 6.11 × 104 | 2.91 × 10-6 | 1.54 × 10-5 | 0.06 | 0.25 |
| Linker 10 | 5.51 × 104 | 3.69 × 105 | 4.09 × 10-5 | 1.91 × 10-4 | 0.74 | 0.52 |
| Linker 20 | 4.91 × 104 | 5.02 × 104 | 1.74 × 10-5 | 1.76 × 10-5 | 0.35 | 0.35 |
| Aptamers | kd (nM) |
|---|---|
| VEa5 | 116.28 |
| VEa5-VEa5 | 6.24 |
| del5-1 | 476.19 |
| del5-1-del5-1 | 17.15 |
© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Hasegawa, H.; Taira, K.-i.; Sode, K.; Ikebukuro, K. Improvement of Aptamer Affinity by Dimerization. Sensors 2008, 8, 1090-1098. https://doi.org/10.3390/s8021090
Hasegawa H, Taira K-i, Sode K, Ikebukuro K. Improvement of Aptamer Affinity by Dimerization. Sensors. 2008; 8(2):1090-1098. https://doi.org/10.3390/s8021090
Chicago/Turabian StyleHasegawa, Hijiri, Ken-ichi Taira, Koji Sode, and Kazunori Ikebukuro. 2008. "Improvement of Aptamer Affinity by Dimerization" Sensors 8, no. 2: 1090-1098. https://doi.org/10.3390/s8021090




