Selective Chemical Labeling of Proteins with Small Fluorescent Molecules Based on Metal-Chelation Methodology
Abstract
:1. Introduction
6. Conclusions
Acknowledgments
References
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar]
- Shaner, N.C.; Steinbach, P.A.; Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Meth. 2005, 2, 905–909. [Google Scholar]
- Lippincott-Schwartz, J.; Patterson, G.H. Development and use of fluorescent protein markers in living cells. Science 2003, 300, 87–91. [Google Scholar]
- Lisenbee, C.S.; Karnik, S.K.; Trelease, R.N. Overexpression and mislocalization of a tail-anchored GFP redefines the identity of peroxisomal ER. Traffic 2003, 4, 491–501. [Google Scholar]
- Andresen, M.; Schmitz-Salue, R.; Jakobs, S. Short tetracysteine tags to β-tubulin demonstrate the significance of small labels for live cell imaging. Mol. Biol. Cell 2004, 15, 5616–5622. [Google Scholar]
- Wang, L.; Schultz, P.G. Expanding the genetic code. Chem. Commun. 2002, 1–11. [Google Scholar]
- George, N.; Pick, H.; Vogel, H.; Johnsson, N.; Johnsson, K. Specific labeling of cell surface proteins with chemically diverse compounds. J. Am. Chem. Soc. 2004, 126, 8896–8897. [Google Scholar]
- Lin, C.W.; Ting, A.Y. Transglutaminase-catalyzed site-specific conjugation of small-molecule probes to proteins in vitro and on the surface of living cells. J. Am. Chem. Soc. 2006, 128, 4542–4543. [Google Scholar]
- Chen, I.; Howarth, M.; Lin, W.Y.; Ting, A.Y. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat. Meth. 2005, 2, 99–104. [Google Scholar]
- Duckworth, B.P.; Zhang, Z.; Hosokawa, A.; Distefano, M.D. Selective labeling of proteins by using protein farnesyltransferase. ChemBioChem 2007, 8, 98–105. [Google Scholar]
- Carrico, I.S.; Carlson, B.L.; Bertozzi, C.R. Introducing genetically encoded aldehydes into proteins. Nat. Chem. Biol. 2007, 3, 321–322. [Google Scholar]
- Keppler, A.; Gendreizig, S.; Gronemeyer, T.; Pick, H.; Vogel, H.; Johnsson, K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechnol. 2003, 21, 86–89. [Google Scholar]
- Keppler, A.; Pick, H.; Arrivoli, C.; Vogel, H.; Johnsson, K. Labeling of fusion proteins with synthetic fluorophores in live cells. Proc. Natl. Acad. Sci. USA 2004, 101, 9955–9959. [Google Scholar]
- Los, G.; Learish, R.; Karassina, N.; Zimprich, C.; McDougall, M.G.; Encell, L.P.; Friedman-Ohana, R.; Wood, M.; Vidugiris, G.; Zimmerman, K.; Otto, P.; Berstock, S.; Klaubert, D.; Wood, K.V. Halotag™ technology: cell imaging and protein analysis. Cell Notes 2006, 14, 10–14. [Google Scholar]
- Miller, L.W.; Sable, J.; Goelet, P.; Sheetz, M.P.; Cornish, V.W. Methotrexate conjugates: a molecular in vivo protein tag. Angew. Chem. Int. Ed. Engl 2004, 43, 1672–1675. [Google Scholar]
- Miller, L.W.; Cai, Y.; Sheetz, M.P.; Cornish, V.W. In vivo protein labeling with trimethoprim conjugates: a flexible chemical tag. Nat. Meth. 2005, 2, 255–257. [Google Scholar]
- Marks, K.M.; Braun, P.D.; Nolan, G.P. A general approach for chemical labeling and rapid, spatially controlled protein inactivation. Proc. Natl. Acad. Sci. USA 2004, 101, 9982–9987. [Google Scholar]
- Chen, I.; Ting, A.Y. Site-specific labeling of proteins with small molecules in live cells. Curr. Opin. Biotechnol. 2005, 16, 35–40. [Google Scholar]
- Marks, K.M.; Nolan, G.P. Chemical labeling strategies for cell biology. Nat. Meth. 2006, 3, 591–596. [Google Scholar]
- Miller, L.W.; Cornish, V.W. Selective chemical labeling of proteins in living cells. Curr. Opin. Chem. Biol. 2005, 9, 56–61. [Google Scholar]
- O'Hare, H.M.; Johnsson, K.; Gautier, A. Chemical probes shed light on protein function. Curr. Opin. Sruct. Biol. 2007, 17, 488–494. [Google Scholar]
- Dragulescu-Andrasi, A.; Rao, J. Chemical labeling of protein in living cells. ChemBioChem. 2007, 8, 1099–1101. [Google Scholar]
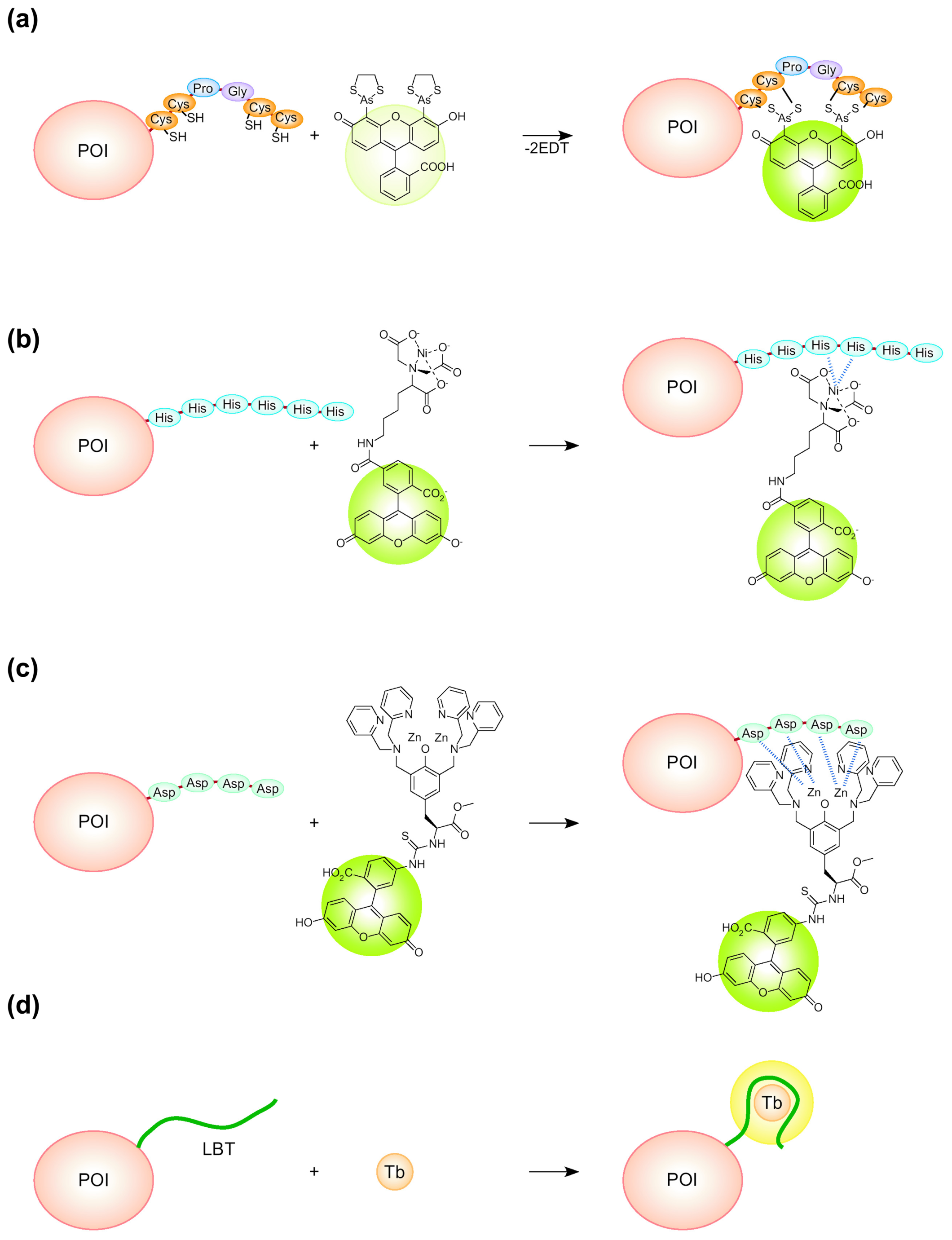
- Griffin, B.A.; Adams, S.R.; Tsien, R.Y. Specific covalent labeling of recombinant protein molecules inside live cells. Science 1998, 281, 269–272. [Google Scholar]
- Adams, S.R.; Campbell, R.E.; Gross, L.A.; Martin, B.R.; Walkup, G.K.; Yao, Y.; Llopis, J.; Tsien, R.Y. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 2002, 124, 6063–6076. [Google Scholar]
- Gaietta, G.; Deerinck, T.J.; Adams, S.R.; Bouwer, J.; Tour, O.; Laird, D.W.; Sosinsky, G.E.; Tsien, R.Y.; Ellisman, M.H. Multicolor and electron microscopic imaging of connexin trafficking. Science 2002, 296, 503–507. [Google Scholar]
- Stroffekava, K.; Proenza, C.; Beam, K.G. The protein-labeling reagent FLASH-EDT2 binds not only to CCXXCC motifs but also non-specifically to endogenous cysteine-rich proteins. Pflugers Archiv. Eur. J. Physiol 2001, 442, 859–866. [Google Scholar]
- Poskanzer, K.E.; Marek, K.W.; Sweeney, S.T.; Davis, G.W. Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature 2003, 426, 559–563. [Google Scholar]
- Ju, W.; Morishita, W.; Tsui, J.; Gaietta, G.; Deerinck, T.J.; Adams, S.R.; Garner, C.C.; Tsien, R.Y.; Ellisman, M.H.; Malenka, R.C. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat. Neurosci. 2004, 7, 244–253. [Google Scholar]
- Hoffmann, C.; Gaietta, G.; Bunemann, M.; Adams, S.R.; Oberdorff-Maass, S.; Behr, B.; Vilardaga, J.P.; Tsien, R.Y.; Eisman, M.H.; Lohse, M.J. A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat. Meth. 2005, 2, 171–176. [Google Scholar]
- Gaietta, G.M.; Giepmans, B.N.G.; Deerinck, T.J.; Smith, W.B.; Ngan, L.; Llopis, J.; Adams, S.R.; Tsien, R.Y.; Ellisman, M.H. Golgi twins in late mitosis revealed by genetically encoded tags for live cell imaging and correlated electron microscopy. Proc. Natl. Acad. Sci. 2006, 103, 17777–17782. [Google Scholar]
- Nakanishi, J.; Nakajima, T.; Sato, M.; Ozawa, T.; Tohda, K.; Umezawa, Y. Imaging of conformational changes of proteins with a new environment-sensitive fluorescent probe designed for site-specific labeling of recombinant proteins in live cells. Anal. Chem. 2001, 73, 2920–2928. [Google Scholar]
- Spagnuolo, C.C.; Vermeij, R.J.; Jares-Erijman, E.A. Improved photostable FRET-competent biarsenical-tetracysteine probes based on fluorinated fluoresceins. J. Am. Chem. Soc. 2006, 128, 12040–12041. [Google Scholar]
- Cao, H.; Xiong, Y.; Wang, T.; Chen, B.; Squier, T.C.; Mayer, M.U. A red Cy3-based biarsenical fluorescent probe targeted to a complementary binding peptide. J. Am. Chem. Soc. 2007, 129, 8672–8673. [Google Scholar]
- Cao, H.; Chen, B.; Squier, T.C.; Mayer, M.U. CrAsH: a biarsenical multi-use affinity probe with low non-specific fluorescence. Chem. Commun. 2006, 2601–2603. [Google Scholar]
- Bhunia, A.K.; Miller, S.C. Labeling tetracysteine-tagged proteins with a SplAsH of color: a modular approach to bis-arsenical fluorophores. ChemBioChem 2007, 8, 1642–1645. [Google Scholar]
- Martin, B.R.; Giepmans, B.N.G.; Adams, S.R.; Tsien, R.Y. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat. Biotechnol. 2005, 23, 1308–1314. [Google Scholar]
- Chen, B.; Cao, H.; Yan, P.; Mayer, M.U.; Squier, T.C. Identification of an orthogonal peptide binding motif for biarsenical multiuse affinity probes. Bioconjugate Chem. 2007, 18, 1259–1265. [Google Scholar]
- Wang, T.; Yan, P.; Squier, T.C.; Mayer, M.U. Prospecting the proteome: identification of naturally occurring binding motifs for biarsenical probes. ChemBioChem 2007, 8, 1937–1940. [Google Scholar]
- Tour, O.; Adams, S.R.; Kerr, R.A.; Meijer, R.M.; Sejnowski, T.J.; Tsien, R.W.; Tsien, R.Y. Nat. Chem. Biol. 2007, 3, 423–431.
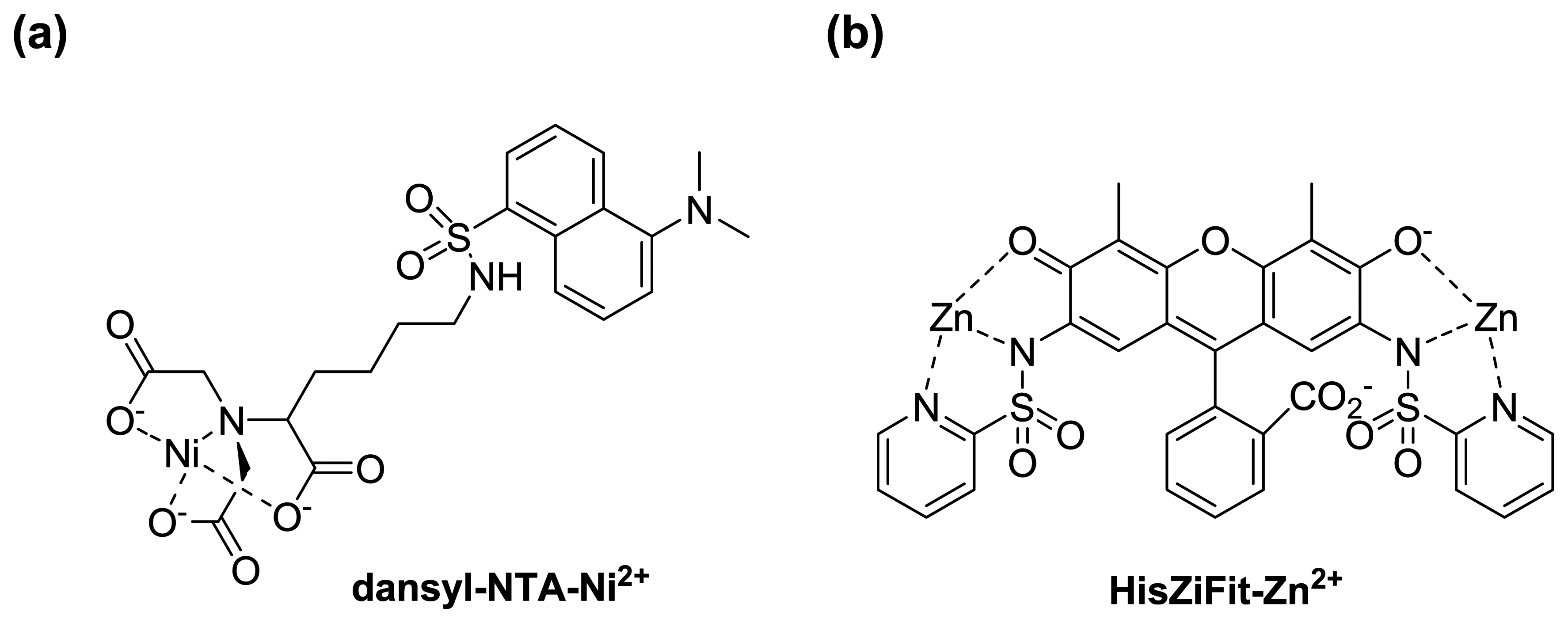
- Kapanidis, A.N.; Ebright, Y.W.; Ebright, R.H. Site-specific incorporation of fluorescent probes into protein: hexahistidine-tag-mediated fluorescent labeling with (Ni2+:nitrilotriacetic acid)n-fluorochrome conjugates. J. Am. Chem. Soc. 2001, 123, 12123–1215. [Google Scholar]
- Amano, H.; Ohuchi, Y.; Katayama, Y.; Maeda, M. A new fluorescent reagent for the detection of proteins having histidine-tag (his-tag). Anal. Sci. 2001, 17 suppl., i1469–i1471. [Google Scholar]
- Guignet, E.G.; Hovius, R.; Vogel, H. Reversible site-selective labeling of membrane proteins in living cells. Nat. Biotechnol. 2004, 22, 440–444. [Google Scholar]
- Goldsmith, C.R.; Jaworski, J.; Sheng, M.; Lippard, S.J. Selective labeling of extracellular proteins containing polyhistidine sequences by a fluorescein-nitrilotriacetic acid conjugate. J. Am. Chem. Soc. 2006, 128, 418–419. [Google Scholar]
- Lata, S.; Reichel, A.; Brock, R.; Tamplé, R.; Piehler, J. High-affinity adaptors for switchable recognition of histidine-tagged proteins. J. Am. Chem. Soc. 2005, 127, 10205–10215. [Google Scholar]
- Lata, S.; Gavutis, M.; Tampé, R.; Piehler, J. Specific and stable fluorescence labeling of histidine-tagged proteins for dissecting multi-protein complex formation. J. Am. Chem. Soc. 2006, 128, 2365–2372. [Google Scholar]
- Tinazli, A.; Tang, J.; Valiokas, R.; Picuric, S.; Lata, S.; Piehler, J.; Liedberg, B.; Tampé, R. High-affinity chelator thiols for switchable and oriented immobilization of histidine-tagged proteins: a generic platform for protein chip technologies. Chem. Eur. J. 2005, 11, 5249–5259. [Google Scholar]
- Lata, S.; Gavutis, M.; Piehler, J. Monitoring the dynamics of ligand-receptor complexes on model membranes. J. Am. Chem. Soc. 2006, 128, 6–7. [Google Scholar]
- Klenkar, G.; Valiokas, R.; Lundström, I.; Tinazli, A.; Tampé, R.; Piehler, J.; Liedberg, B. Piezo dispensed microarray of multivalent chelating thiols for dissecting complex protein-protein interactions. Anal. Chem. 2006, 78, 3643–3650. [Google Scholar]
- Valiokas, R.; Klenkar, G.; Tinazli, A.; Tampé, R.; Liedberg, B.; Piehler, J. Differential protein assembly on micropatterned surfaces with tailored molecular and surface multivalency. ChemBioChem 2006, 7, 1325–1329. [Google Scholar]
- Krishnan, B.; Szymanska, A.; Gierasch, L.M. Site-specific fluorescent labeling of poly-histidine sequences using a metal-chelating cysteine. Chem. Biol. Drug. Des. 2007, 69, 31–40. [Google Scholar]
- Meredith, G.D.; Wu, H.Y.; Allbritton, N.L. Targeted protein functionalization using His-tags. Bioconjugate Chem. 2004, 15, 969–982. [Google Scholar]
- Soh, N.; Seto, D.; Nakano, K.; Imato, T. Methodology of reversible protein labeling for ratiometric fluorescent measurement. Mol. BioSyst. 2006, 2, 128–131. [Google Scholar]
- Hauser, C.T.; Tsien, R.Y. A hexahistidine-Zn2+-dye label reveals STIM1 surface exposure. Proc. Natl. Acad. Sci. USA 2007, 104, 3693–3697. [Google Scholar]
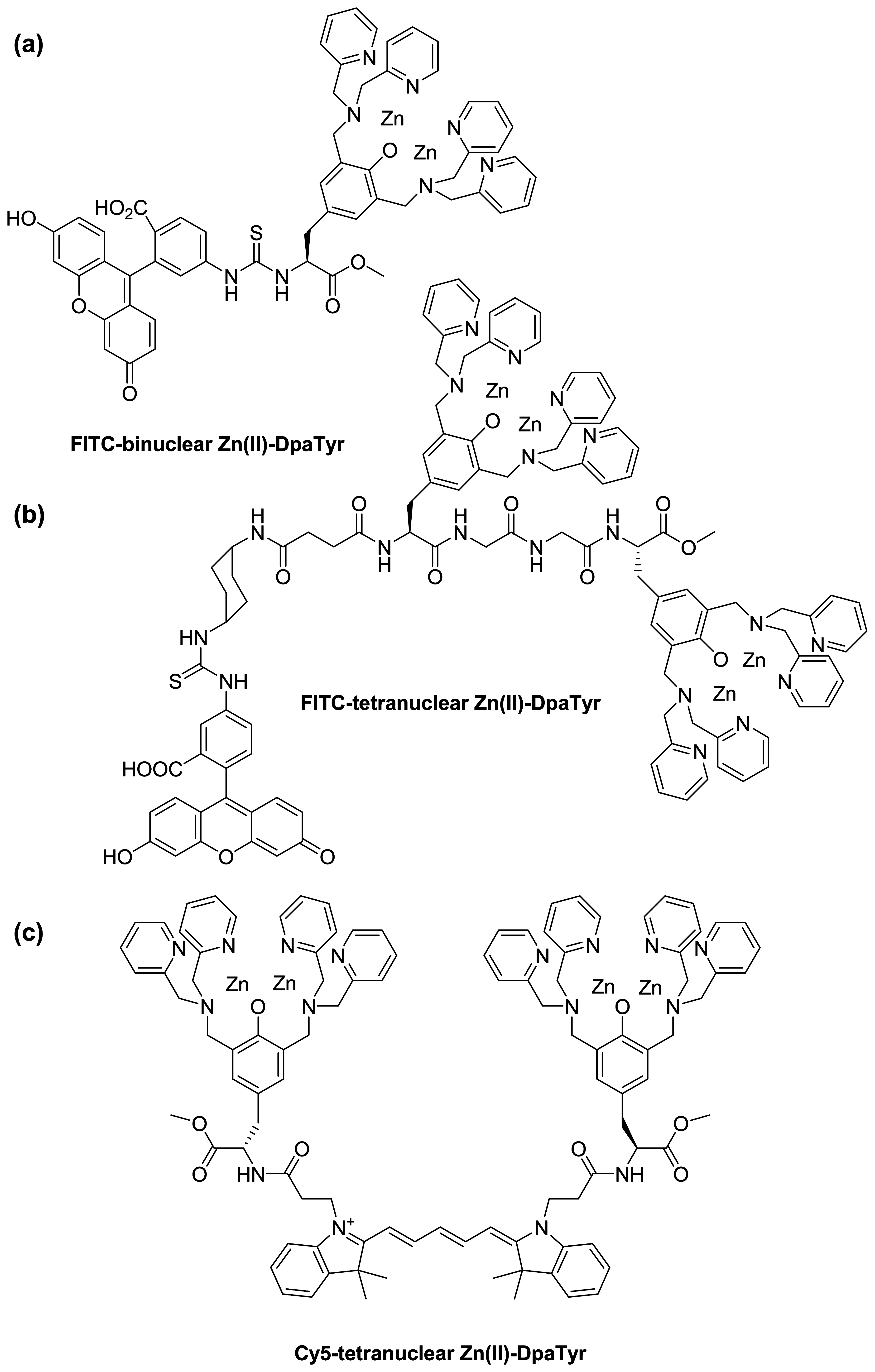
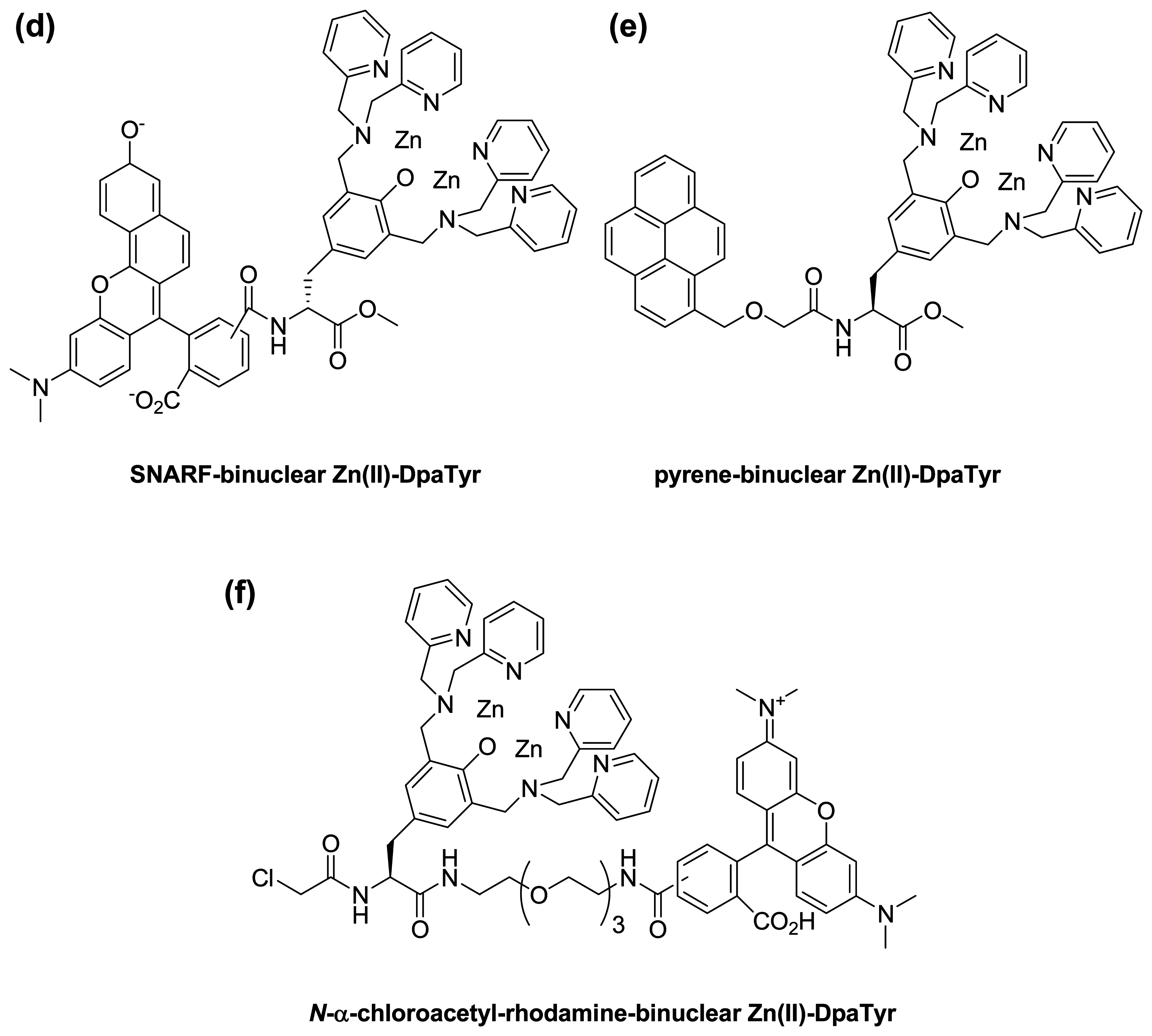
- Ojida, A.; Honda, K.; Shinmi, D.; Kiyonaka, S.; Mori, Y.; Hamachi, I. Oligo-Asp Tag/Zn(II) complex probe as a new pair for labeling and fluorescence imaging of proteins. J. Am. Chem. Soc. 2006, 128, 10452–10459. [Google Scholar]
- Honda, K.; Nakata, E.; Ojida, A.; Hamachi, I. Ratiometric fluorescence detection of a tag fused protein using the dual-emission artificial molecular probe. Chem. Commun. 2006, 4024–4026. [Google Scholar]
- Honda, K.; Fujishima, S.-H.; Ojida, A.; Hamachi, I. Pyrene excimer-based dual-emission detection of a oligoaspartate tag-fused protein by using a ZnII-DpaTyr probe. ChemBioChem 2007, 8, 1370–1372. [Google Scholar]
- Nonaka, H.; Tsukiji, S.; Ojida, A.; Hamachi, I. Non-enzymatic covalent protein labeling using a reactive tag. J. Am. Chem. Soc. 2007, 129, 15777–15779. [Google Scholar]
- MacManus, J.P.; Hogue, C.W.; Marsden, B.J.; Sikorska, M.; Szabo, A.G. Terbium luminescence in synthetic peptide loops from calcium-binding proteins with different energy donors. J. Biol. Chem. 1990, 265, 10358–10366. [Google Scholar]
- Franz, K.J.; Nitz, M.; Imperiali, B. Lanthanide-binding tags as versatile protein coexpression probes. ChemBioChem 2003, 4, 265–271. [Google Scholar]
- Nitz, M.; Franz, K.J.; Maglathlin, R.L.; Imperiali, B. Powerful combinatorial screen to identify high-affinity terbium(III)-binding peptides. ChemBioChem 2003, 4, 272–276. [Google Scholar]
- Martin, L.J.; Sculimbrene, B.R.; Nitz, M.; Imperiali, B. Rapid combinatorial screening of peptide libraries for the selection of lanthanide-binding tags (LBTs). QSAR Comb. Sci. 2005, 24, 1149–1157. [Google Scholar]
- Nitz, M.; Sherawat, M.; Franz, K.J.; Peisach, E.; Allen, K.N.; Imperiali, B. Structural origin of the high affinity of a chemically evolved lanthanide-binding peptide. Angew. Chem. Int. Ed. 2004, 43, 3682–3685. [Google Scholar]
- Sculimbrene, B.R.; Imperiali, B. Lanthanide-binding tags as luminescent probes for studying protein interactions. J. Am. Chem. Soc. 2006, 128, 7346–7352. [Google Scholar]
- Wöhnert, J.; Franz, K.J.; Nitz, M.; Imperiali, B.; Schwalbe, H. Protein alignment by a coexpressed lanthanide-binding tag for the measurement of residual dipolar couplings. J. Am. Chem. Soc. 2003, 125, 13338–13339. [Google Scholar]
- Martin, L.J.; Hähnke, M.J.; Nitz, M.; Wöhnert, J.; Silvaggi, N.R.; Allen, K.N.; Schwalbe, H.; Imperiali, B. Double-lanthanide-binding tags: design, photophysical properties, and NMR applications. J. Am. Chem. Soc. 2007, 129, 7106–7113. [Google Scholar]
- Silvaggi, N.R.; Martin, L.J.; Schwalbe, H.; Imperiali, B.; Allen, K.N. Double-lanthanide-binding tags for macromolecular crystallographic structure determination. J. Am. Chem. Soc. 2007, 129, 7114–7120. [Google Scholar]
- Soh, N.; Yoshida, K.; Nakajima, H.; Nakano, K.; Imato, T.; Fukaminato, T.; Irie, M. Chem. Commun. 2007, 5206–5208.

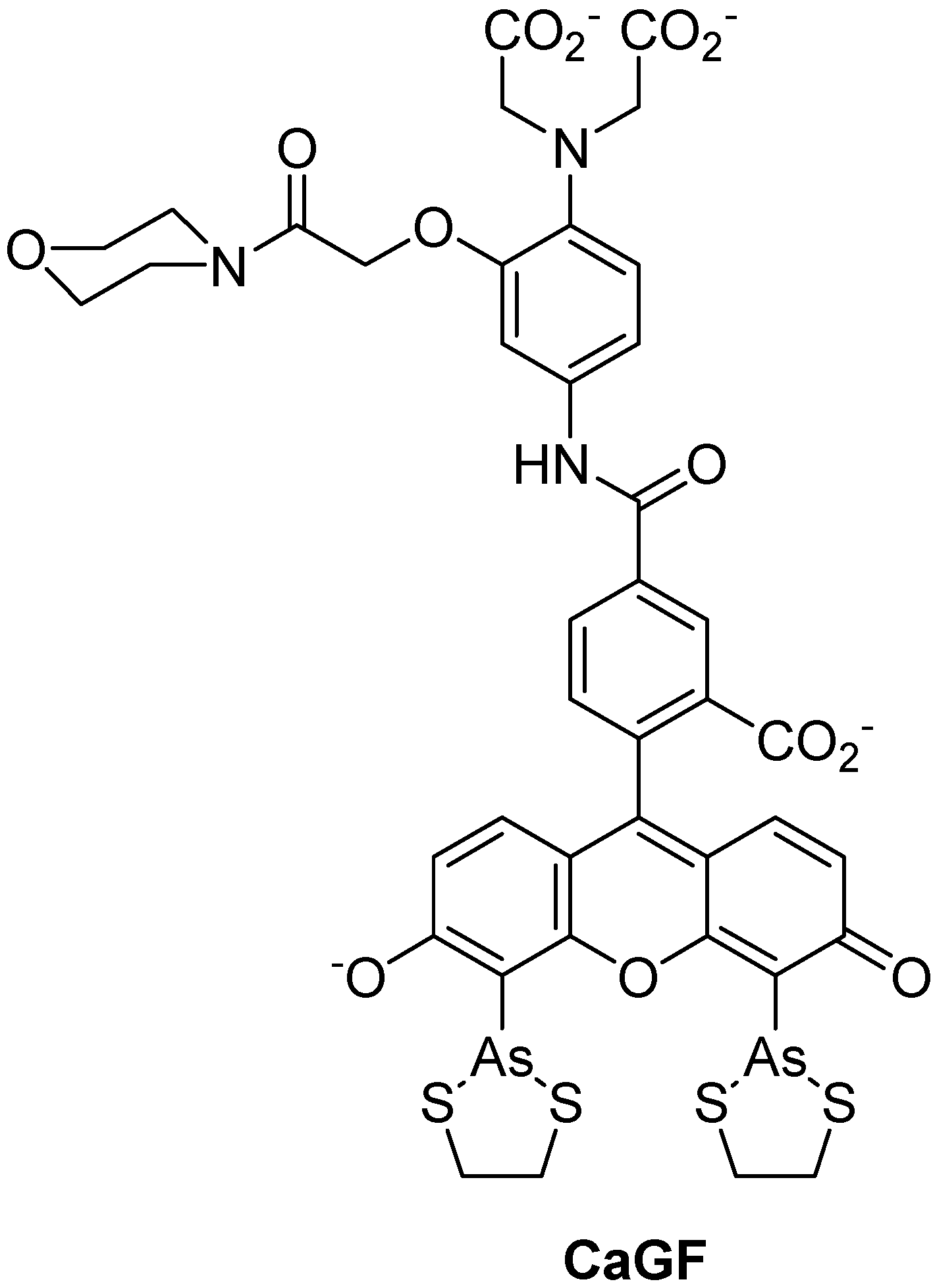
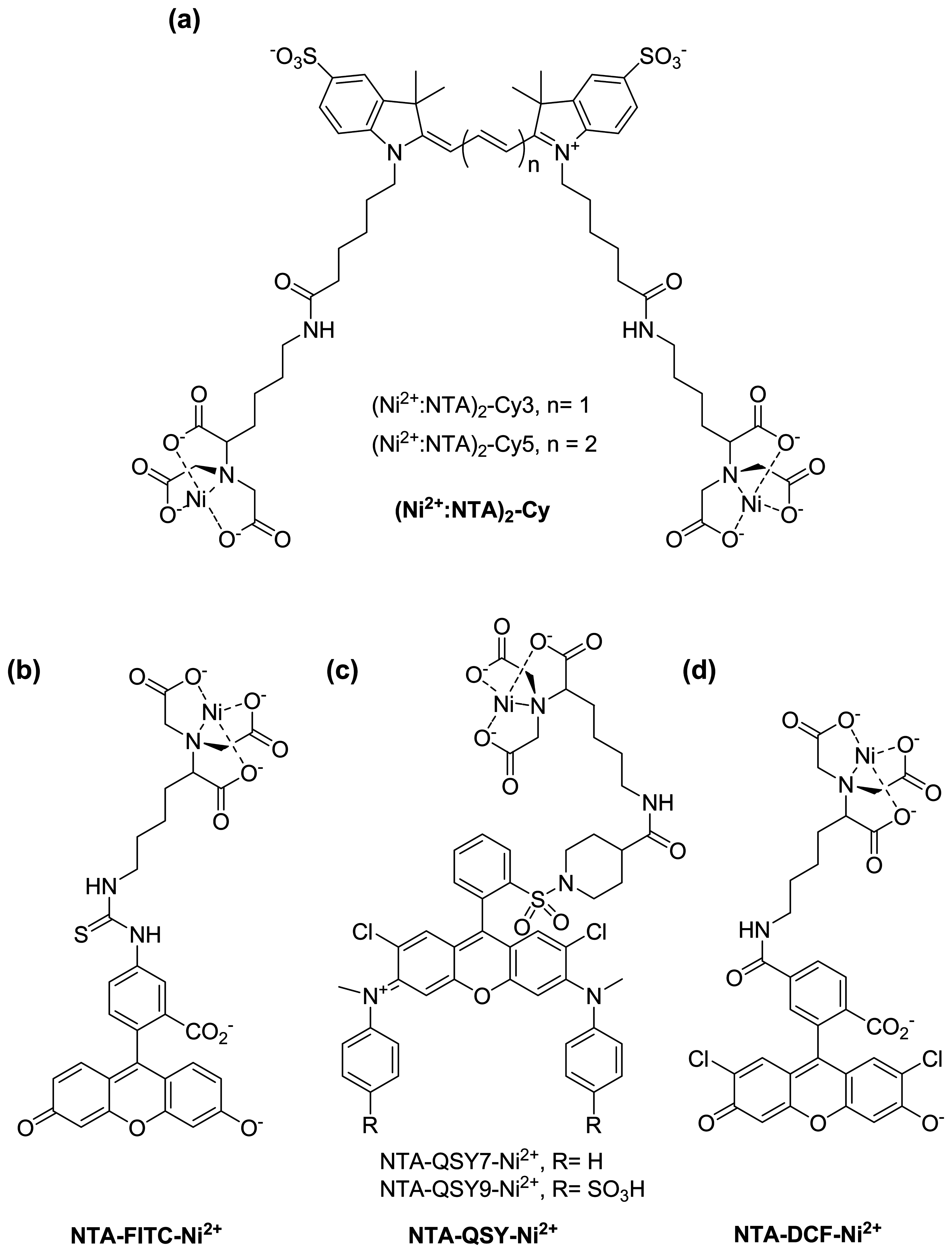
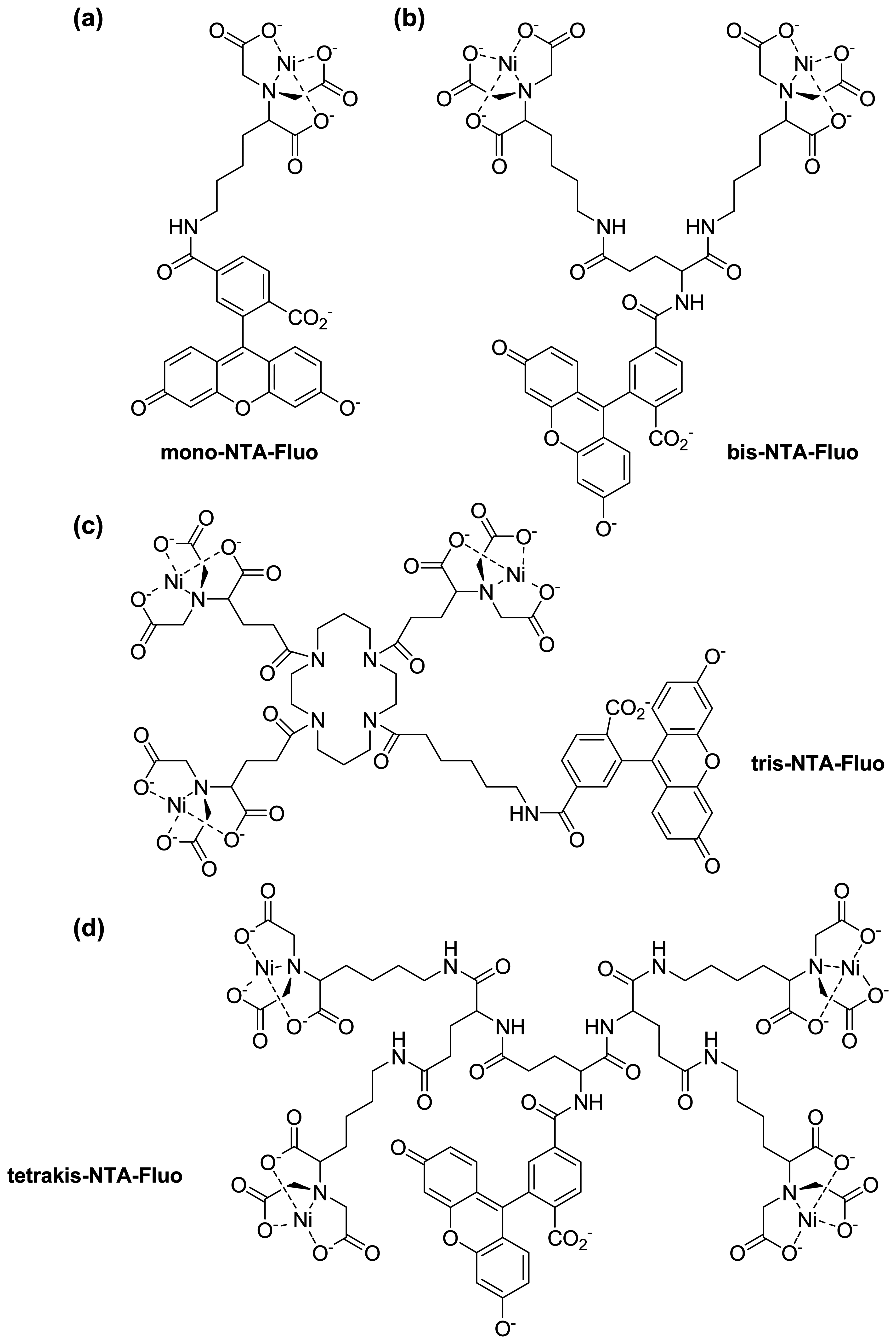
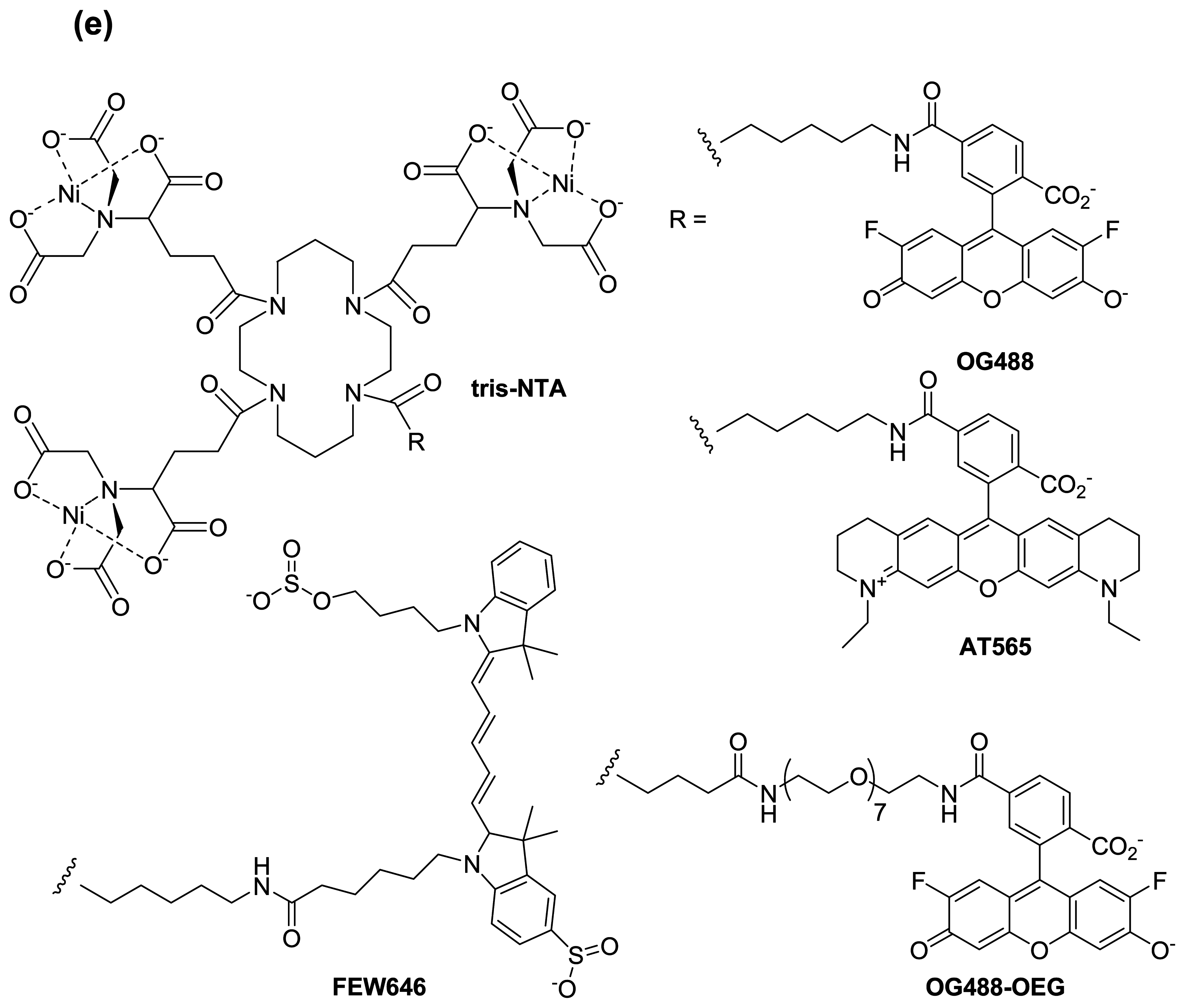
© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Soh, N. Selective Chemical Labeling of Proteins with Small Fluorescent Molecules Based on Metal-Chelation Methodology. Sensors 2008, 8, 1004-1024. https://doi.org/10.3390/s8021004
Soh N. Selective Chemical Labeling of Proteins with Small Fluorescent Molecules Based on Metal-Chelation Methodology. Sensors. 2008; 8(2):1004-1024. https://doi.org/10.3390/s8021004
Chicago/Turabian StyleSoh, Nobuaki. 2008. "Selective Chemical Labeling of Proteins with Small Fluorescent Molecules Based on Metal-Chelation Methodology" Sensors 8, no. 2: 1004-1024. https://doi.org/10.3390/s8021004



