The Application of DNA-Biosensors and Differential Scanning Calorimetry to the Study of the DNA-Binding Agent Berenil
Abstract
:1. Introduction
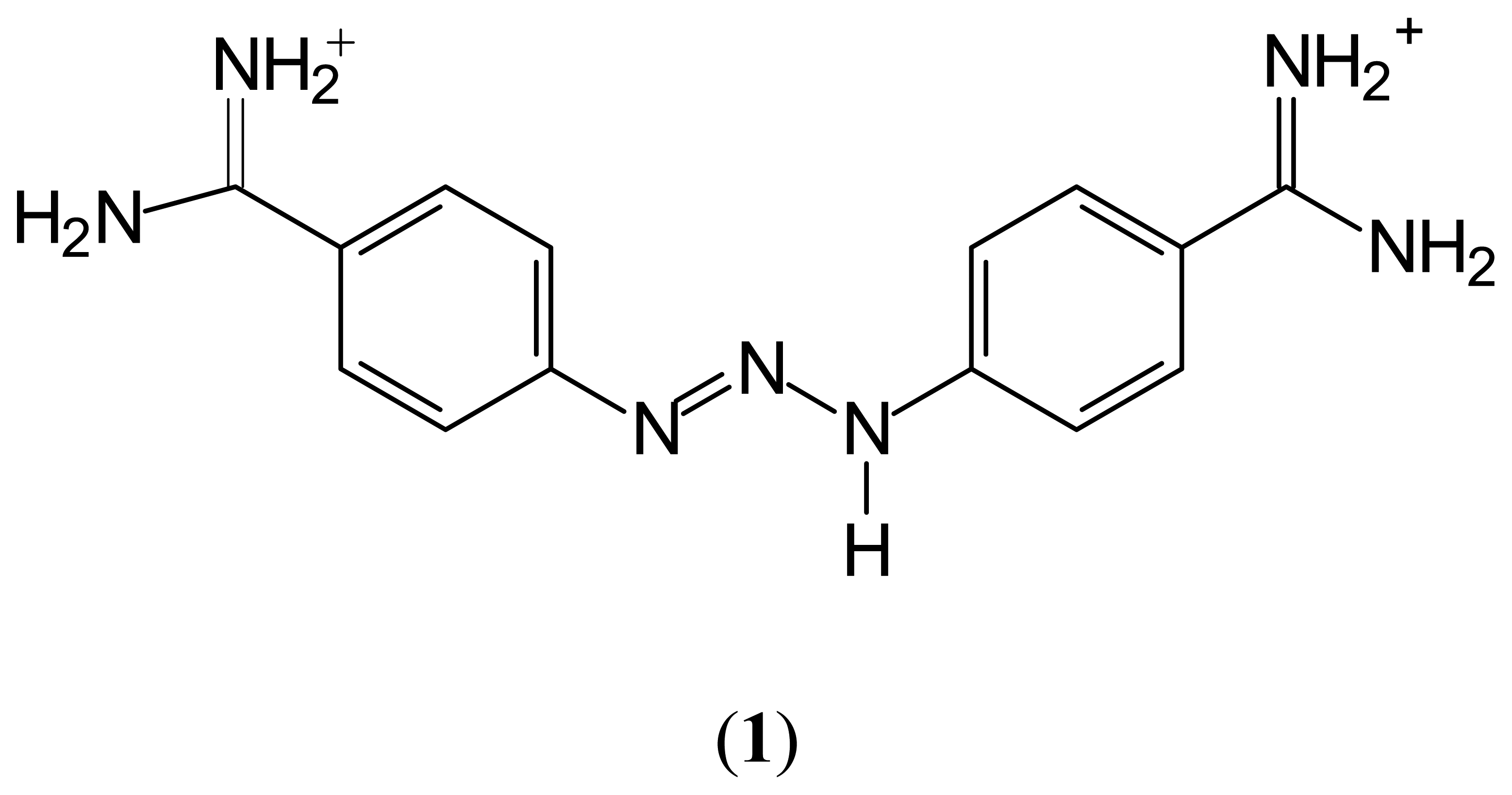
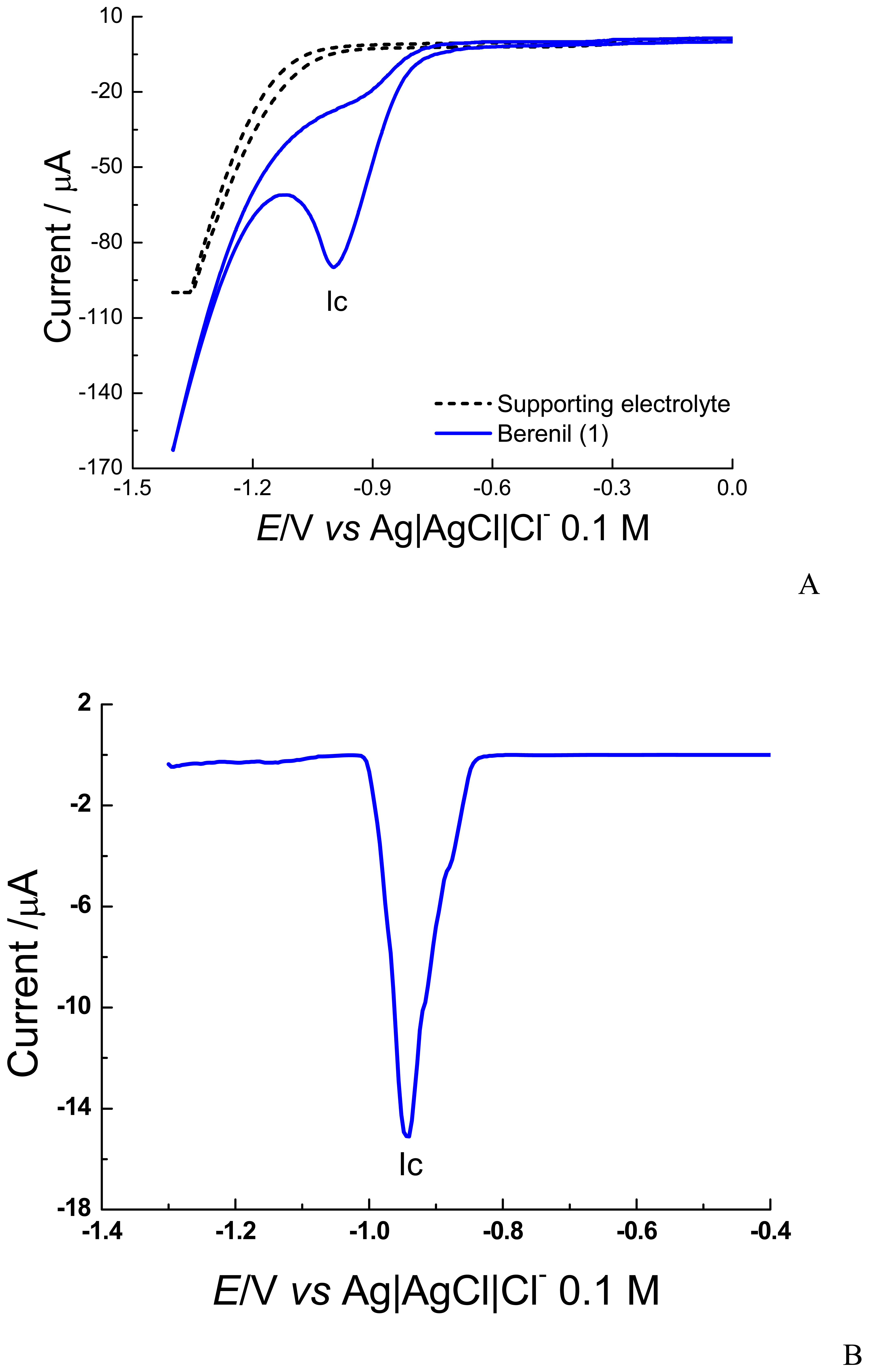
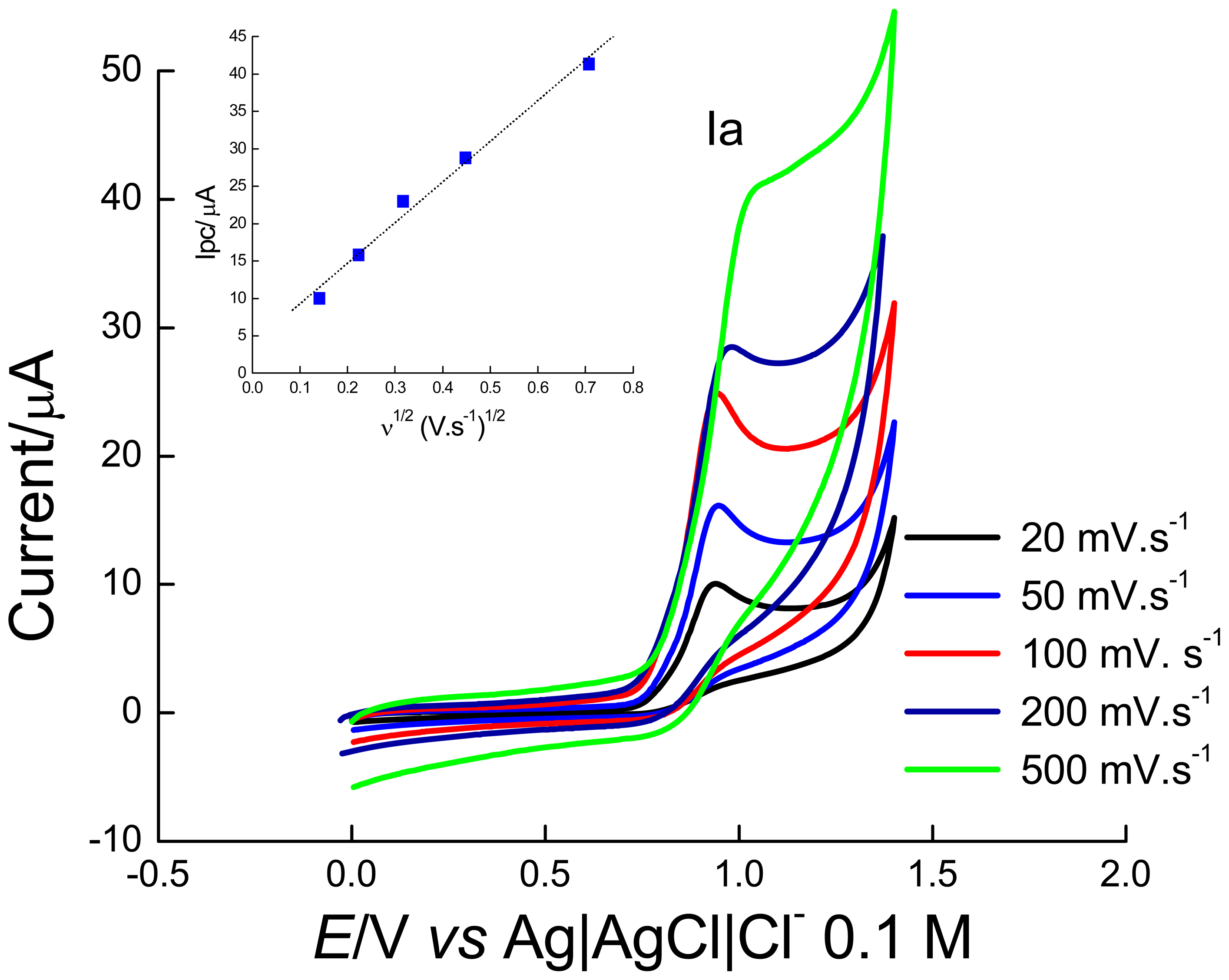
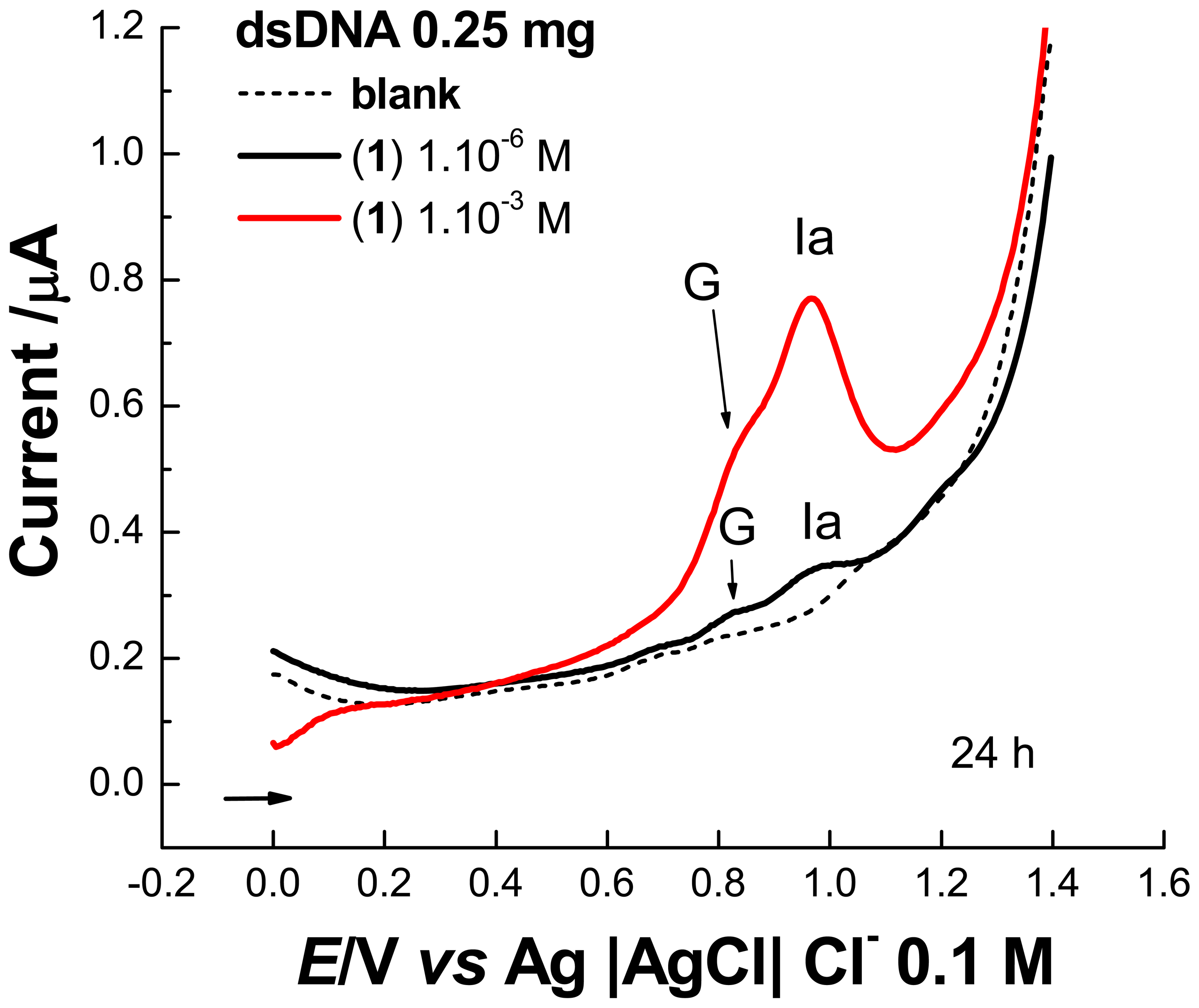
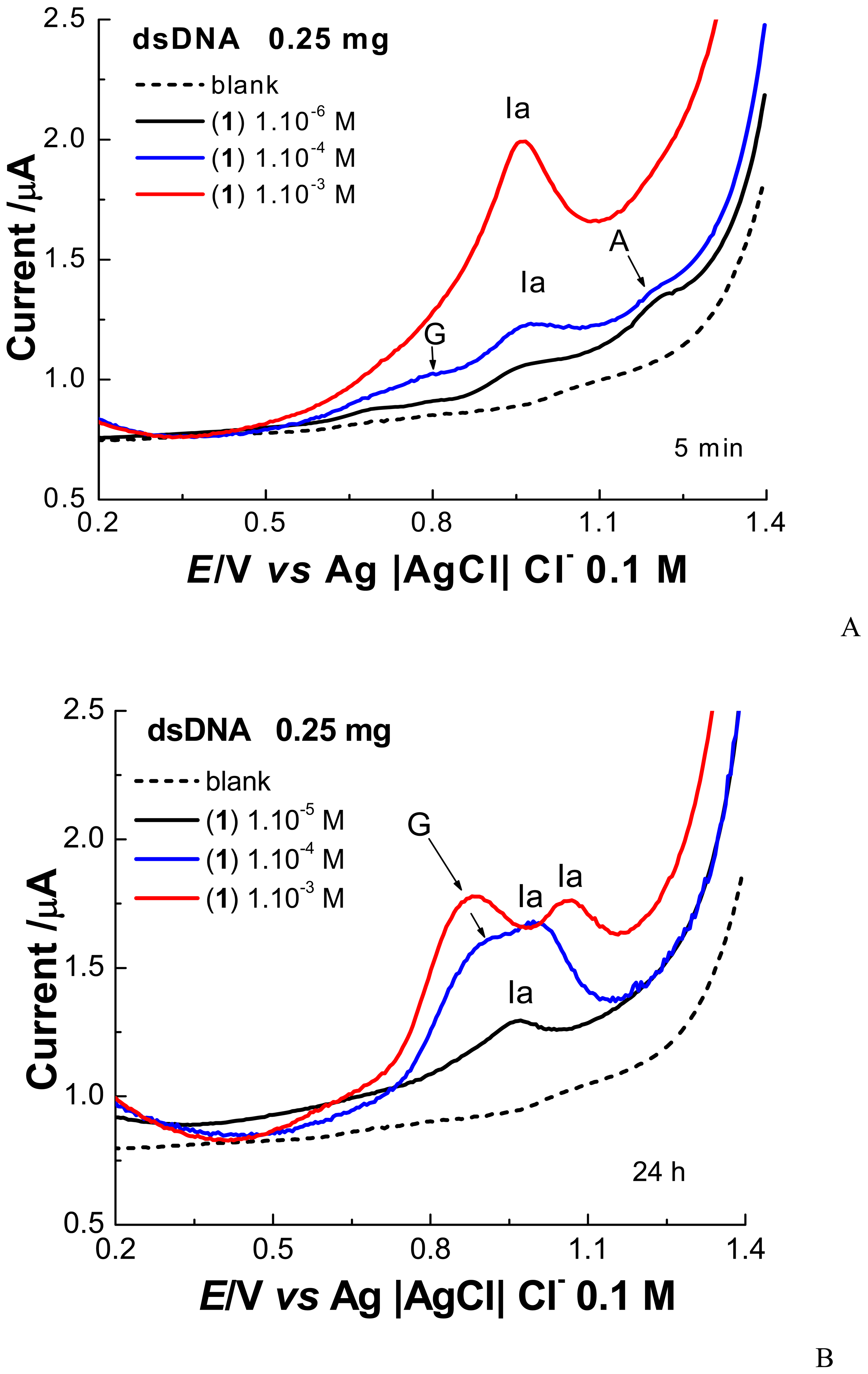
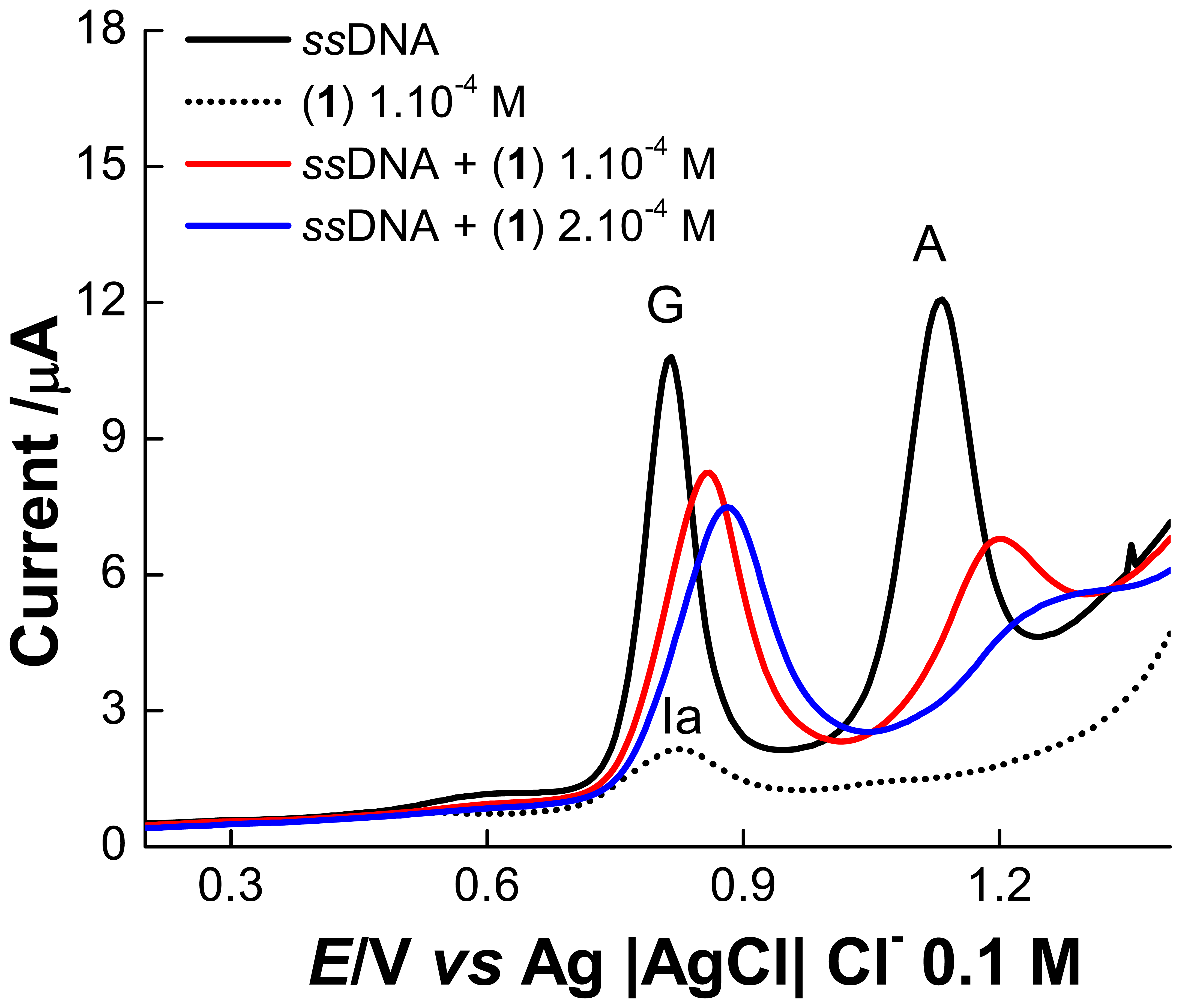
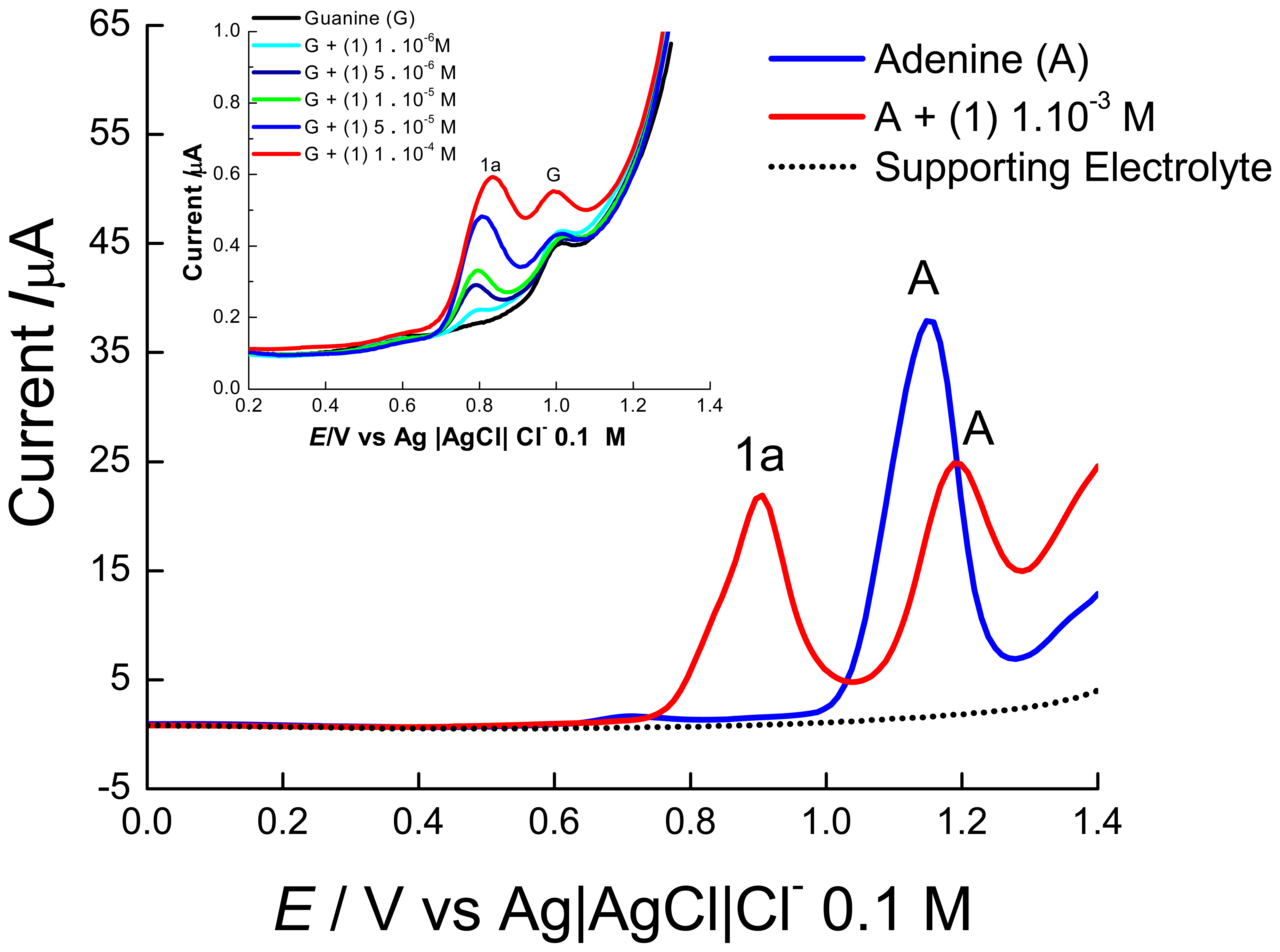
2. Results and Discussion
2.1 Electrochemical experiments
2.2 Differential Scanning Calorimetry
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Electrochemical methods
4.3. Preparation of the dsDNA-GC biosensor
4.4. Interaction of dsDNA with berenil
4.5. Preparation of ssDNA
4.6. Interaction of ssDNA with berenil
4.7. Interaction of polyadenylic and polyguanylic acids with berenil
4.8. Differential scanning calorimetry
Acknowledgments
References and Notes
- Pindur, U.; Jansen, M.; Lemster, T. Advances in DNA-Ligands with Groove Binding, Intercalating and/or Alkylating Activity: Chemistry, DNA-Binding and Biology. Curr. Med. Chem. 2005, 12, 2805–2847. [Google Scholar]
- Bell, C. A.; Hall, J. E.; Kyle, D. E.; Grogl, M.; Ohemeng, K. A.; Alln, M.A.; Tidwell, R. R. Structure-activity studies of dicationically substituted bis-benzimidazoles against Giardia lamblia: correlation of antigiardial activity with DNA binding affinity and giardial topoisomerase II inhibition. Antimicrob. Agents Chemother. 1993, 37, 2668–2673. [Google Scholar]
- Tidwell, R. R.; Boykin, D.W. Dicationic DNA Minor Groove Binders as Anti-microbial Agents. In DNA and RNA Binders: From Small Molecules to Drugs; Demeunynck, M., Bailly, C., Wilson, W. D., Eds.; Wiley-VCH: Weinheim, 2003; pp. 414–460. [Google Scholar]
- Chaires, J. B. A thermodynamic signature for drug-DNA binding mode. Arch. Biochem. Biophys. 2006, 453, 26–31. [Google Scholar]
- Waring, M. J. DNA modification and cancer. Annu. Rev. Biochem. 1981, 50, 159–192. [Google Scholar]
- Wells, R. D.; Larson, J. E. Studies on the binding of actinomycin D to DNA and DNA model polymers. J. Mol. Biol. 1970, 49, 319–342. [Google Scholar]
- Barton, J. K.; Goldberg, J. M.; Kumar, C. V.; Turro, N. J. Binding modes and base specificity of tris(phenanthroline)ruthenium(II) enantiomers with nucleic acids: tuning the stereoselectivity. J. Am. Chem. Soc. 1986, 108, 2081–2088. [Google Scholar]
- Zhou, N.; James, T. L.; Shafer, R. H. Binding of actinomycin D to [d(ATCGAT) ]2: NMR evidence of multiple complexes. Biochemistry 1989, 28, 5231–5239. [Google Scholar]
- Tanious, F. A.; Veal, J. M.; Buczak, H.; Ratmeyer, L. S.; Wilson, W. D. DAPI (4′,6-diamidino-2-phenylindole) binds differently to DNA and RNA: minor-groove binding at AT sites and intercalation at AU sites. Biochemistry 1992, 31, 3103–3112. [Google Scholar]
- Chen, A. Y.; Liu, L. F. DNA Topoisomerases: Essential Enzymes and Lethal Targets. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 191–218. [Google Scholar]
- McKnight, R. E.; Gleason, A. B.; Keyes, J. A.; Sahabi, S. Binding mode and affinity studies of DNA-binding agents using topoisomerase I DNA unwinding assay. Bioorg. Med. Chem. Lett. 2007, 17, 1013–1017. [Google Scholar]
- Diculescu, V. C.; Barbosa, R. M.; Oliveira-Brett, A. M. In situ sensing of the DNA damage by a nitric oxide - releasing compound. Anal. Letters 2005, 38, 2525–2540. [Google Scholar]
- Rauf, S.; Gooding, J. J.; Akhtar, K.; Ghauri, M. A.; Rahman, M.; Anwar, M. A.; Khalid, A. M. Electrochemical approach of anticancer drugs-DNA interaction. J. Pharm. Biomed. Anal. 2005, 37, 205–217. [Google Scholar]
- Oliveira-Brett, A. M.; Serrano, S. H. P.; Piedade, J. A. P. Electrochemistry of DNA. In Comprehensive Chemical Kinetics - Applications of Kinetic Modelling; Compton, R.G., Ed.; Elsevier: Amsterdam, 1999; pp. 91–119. [Google Scholar]
- Oliveira-Brett, A. M.; da Silva, L. A.; Fujii, H.; Mataka, S.; Thiemann, T. Detection of the damage caused to DNA by a thiophene-S-oxide using an electrochemical DNA-biosensor. J. Electroanal. Chem. 2003, 549, 91–99. [Google Scholar]
- Oliveira-Brett, A. M.; Macedo, T. R. A.; Raimundo, D.; Marques, M. H.; Serrano, S. H. P. Voltammetric behaviour of mitoxantrone at a DNA-biosensor. Biosens. Bioelectron. 1998, 13, 861–867. [Google Scholar]
- Oliveira-Brett, A. M.; Goulart, M. O. F.; de Abreu, F. C. Detection of the damage caused to DNA by niclosamide using an electrochemical DNA-biosensor. Biosens. Bioelectron. 2002, 17, 913–919. [Google Scholar]
- Diculescu, V. C.; Paquim, A-M.C.; Brett, A.M.O. Mini-Review: Electrochemical DNA Sensors for Detection of DNA Damage. Sensors 2005, 5, 377–393. [Google Scholar]
- Hillard, E. A.; de Abreu, F. C.; Ferreira, D. C. M.; Jaouen, G.; Goulart, M. O. F.; Amatore, C. Electrochemical parameters and techniques in drug development, with an emphasis on quinones and related compounds. Chem. Comm. in press.
- Burri, C.; Bodley, A. L.; Shapiro, T. A. Topoisomerases in kinetoplastids. Parasitol. Today 1996, 12, 226–231. [Google Scholar]
- Clausen, P. H.; Waiswa, C.; Katunguka-Rwakishaya, E.; Scahres, G.; Steuber, S.; Mehlitz, D. Polymerase chain reaction and DNA probe hybridization to assess the efficacy of diminazene treatment in Trypanosoma brucei□ - infected cattle. Parasitol. Res. 1999, 85, 206–211. [Google Scholar]
- Gresh, N.; Pullman, B. A theoretical study of the non-intercalative binding of berenil and stilbamidine to double-stranded (dA-dT)n oligomers. Mol. Pharmacol. 1984, 25, 452–458. [Google Scholar]
- Portugal, J.; Waring, M. J. Comparison of binding sites in DNA for berenil, netropsin and dislamycin. A footprinling study. Eur. J. Biochem. 1987, 167, 281–289. [Google Scholar]
- Brown, D. G.; Sanderson, M. R.; Garman, E.; Neidle, S. Crystal structure of a berenil-d(CGCAAATTTGCG) complex: An example of drug-DNA recognition based on sequence-dependent structural features. J. Mol. Biol. 1992, 226, 481–490. [Google Scholar]
- Reinert, K. E. DNA multimode interaction with berenil and pentamidine: double helix stiffening, unbending and bending. J. Biomol. Struct. Dyn. 1999, 17, 311–321. [Google Scholar]
- Barceló, F.; Ortiz-Lombardia, M.; Portugal, J. Heterogeneous DNA binding modes of berenil. Biochim. Biophys. Acta 2001, 1519, 175–184. [Google Scholar]
- Coates, L.; Ikpeazu, E. V.; Chen, Y.; Valenzuela, M. S. Inhibition of DNA Replication by Berenil in Plasmids Containing Poly(dA)poly(dT) Sequences. Plasmid 2002, 47, 120–128. [Google Scholar]
- Durand, M.; Seche, E.; Maurizot, J. C. Effect of strand orientation on the interaction of berenil with DNA triple helices. Eur. Biophys. J. 2002, 30, 625–630. [Google Scholar]
- Burr, S. J.; Mseslati, A.; Thomas, E. W. Photochemical DNA cleavage by a berenil analog. Tetrahedron Lett. 2003, 44, 7307–7309. [Google Scholar]
- Nguyen, B.; Hamelberg, D.; Bailly, C.; Colson, P.; Stanek, J.; Brun, R.; Neidle, S.; Wilson, W. D. Characterization of a novel DNA minor-Groove complex. Biophys. J. 2004, 86, 1028–1041. [Google Scholar]
- Roig, A.; Pena, M. P.; Vicente, F.; Stockert, J. C. Voltammetric behavior of berenil. J. Pharm. Sci. 1993, 82, 251–253. [Google Scholar]
- Wei, X.; Speiser, B. Electrochemistry of triazenes—Part 4. Reaction of diazonium ions generated electrochemically from 1-aryl-3,3-dimethyltriazenes in acetonitrile. Electrochim. Acta. 1995, 40, 2477–2482. [Google Scholar]
- Cooper, A.; Nutley, M. A.; Wadood, A. Differential scanning calorimetry. In Protein-ligand Interactions: Hydrodynamics and Calorimetry; Harding, S.E., Chowdhry, B.Z., Eds.; Oxford University Press: New York, 2001; pp. 287–319. [Google Scholar]
- Poland, D.; Scheraga, H. A. Theory of Helix-coil Transitions in Biopolymers; Academic Press: New York, 1970. [Google Scholar]
- Marky, L. A.; Breslauer, K. J. Calculating thermodynamic data for transitions of any molecularity from equilibrium melting curves. Biopolymers 1987, 26, 1601–1620. [Google Scholar]
- Tsong, T. Y.; Hearn, R. P.; Wrathall, D. P.; Sturtevant, J. M. A calorimetric study of thermally induced conformational transitions of ribonuclease A and certain of its derivatives. Biochemistry 1970, 9, 2666–2677. [Google Scholar]
- Colson, P.; Houssier, C.; Bailly, C. Use of electric linear dichroism and competition experiments with intercalating drugs to investigate the mode of binding of Hoechst 32258, berenil and DAPI to GC sequences. J. Biomol. Struct. Dyn. 1995, 13, 351–366. [Google Scholar]
- Colson, P.; Bailly, C.; Houssier, C. Electric linear dichroism as a new tool to study sequence preference in drug binding to DNA. Biophys. Chem. 1996, 58, 125–140. [Google Scholar]
- Pilch, D. S.; Kirolos, M. A.; Liu, X.; Plum, G. E.; Breslauer, K. J. Berenil [1,3-bis(4′-amidinophenyl)triazene] binding to DNA duplexes and to a RNA duplex: evidence for both intercalative and minor groove binding properties. Biochemistry 1995, 34, 9962–9976. [Google Scholar]
- Krautbauer, R.; Pope, L. H.; Schrader, T. E.; Allen, S.; Gaub, H. E. Discriminating small molecule DNA binding modes by single molecule force spectroscopy. FEBS Lett. 2002, 510, 154–158. [Google Scholar]
- Meric, B.; Kerman, K.; Ozkan, D.; Kara, P.; Erdem, A.; Kucukogu, O.; Erciyas, E.; Ozsoz, M. Electrochemical biosensor for the interaction of DNA with the alkylating agent 4,4′-dihydroxy chalcone based on guanine and adenine signals. J. Pharm. Biomed. Anal. 2002, 30, 1329–1346. [Google Scholar]
- Rich, A.; Davies, D. R.; Crick, F. H. C.; Watson, J. D. The molecular structure of polyadenylic acid. J. Mol. Biol. 1961, 3, 71–86. [Google Scholar]
- Pilch, D. S. Calorimetry of nucleic acids. In Current Protocols in Nucleic Acid Chemistry; John Wiley and Sons, Inc.: New York, 2000; Chapter 7, Unit 7.4. [Google Scholar]
- Freire, F.; Biltonen, R. L. Statistical mechanical deconvolution of thermal transitions in macromolecules. I. Theory and application to homogeneous systems. Biopolymers. 1978, 17, 436–479. [Google Scholar]
- Freire, F.; Biltonen, R. L. Thermodynamic characterization of conformational states of biological macromolecules using differential scanning calorimetry. Crit. Rev. Biochem. 1978, 5, 85–124. [Google Scholar]
- Relkin, P. Differential scanning calorimetry: A useful tool for studying protein denaturation. Thermochim. Acta 1994, 246, 371–386. [Google Scholar]

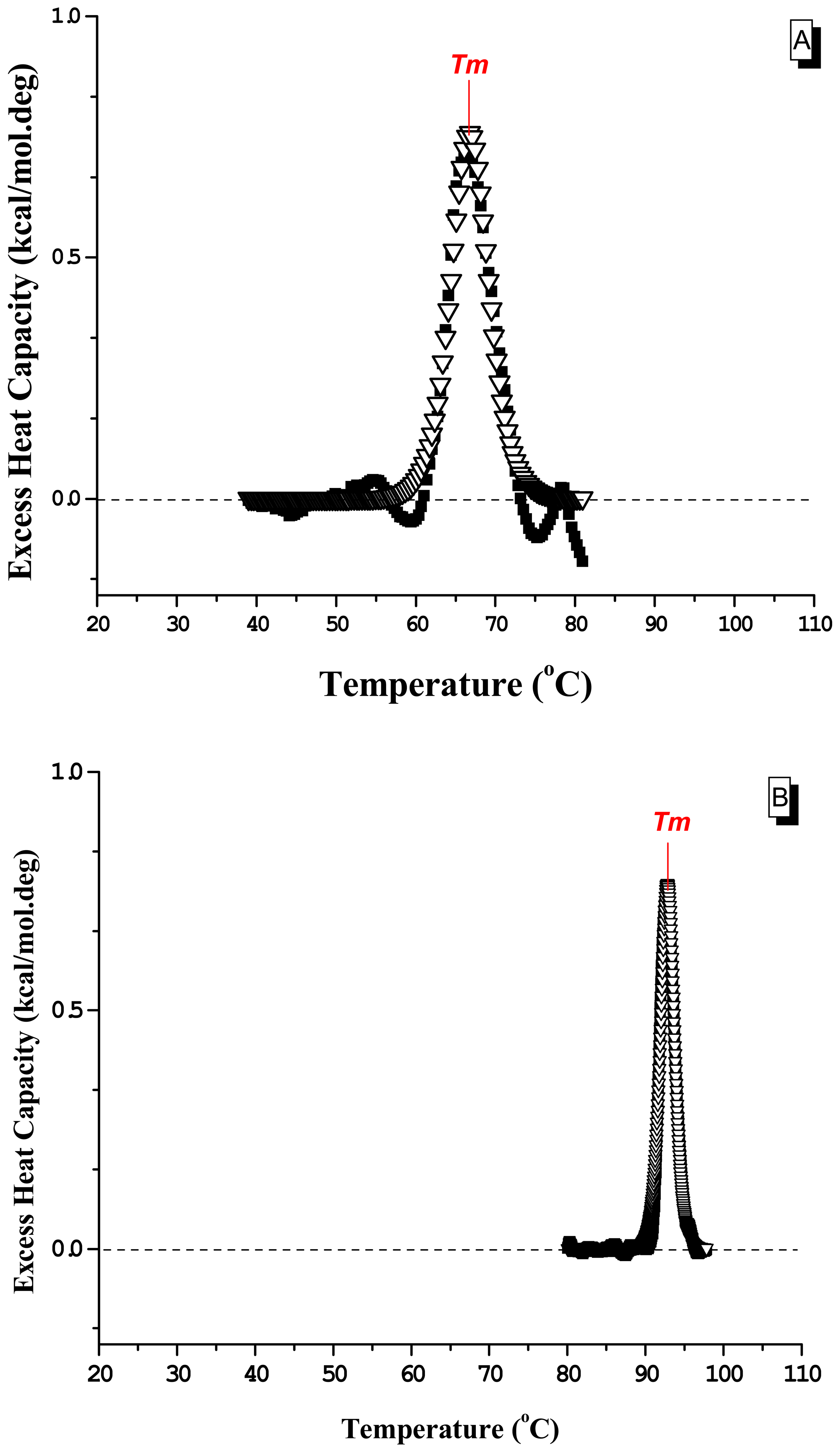

| ΔHcala | ΔHvHb | ΔSTmc | Tmd | <ηmelt>e | RCIf | |
|---|---|---|---|---|---|---|
| dsDNA | 4.76 | 146 | 14.0 | 66.8 | 31 | 6.5 |
| dsDNA + berenil | 1.80 | 452 | 4.9 | 92.8 | 251 | 1.9 |
© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
De Abreu, F.C.; De Paula, F.S.; Ferreira, D.C.M.; Nascimento, V.B.; Lopes, J.C.D.; Santos, A.M.C.; Santoro, M.M.; Salas, C.E.; Goulart, M.O.F. The Application of DNA-Biosensors and Differential Scanning Calorimetry to the Study of the DNA-Binding Agent Berenil. Sensors 2008, 8, 1519-1538. https://doi.org/10.3390/s8031519
De Abreu FC, De Paula FS, Ferreira DCM, Nascimento VB, Lopes JCD, Santos AMC, Santoro MM, Salas CE, Goulart MOF. The Application of DNA-Biosensors and Differential Scanning Calorimetry to the Study of the DNA-Binding Agent Berenil. Sensors. 2008; 8(3):1519-1538. https://doi.org/10.3390/s8031519
Chicago/Turabian StyleDe Abreu, Fabiane C., Francine S. De Paula, Danielle C. M. Ferreira, Valberes B. Nascimento, Julio C. D. Lopes, Alexandre M. C. Santos, Marcelo M. Santoro, Carlos E. Salas, and Marília O. F. Goulart. 2008. "The Application of DNA-Biosensors and Differential Scanning Calorimetry to the Study of the DNA-Binding Agent Berenil" Sensors 8, no. 3: 1519-1538. https://doi.org/10.3390/s8031519





