Detection of Carcinoembryonic Antigens Using a Surface Plasmon Resonance Biosensor
Abstract
:1. Introduction
2. Results and Discussion
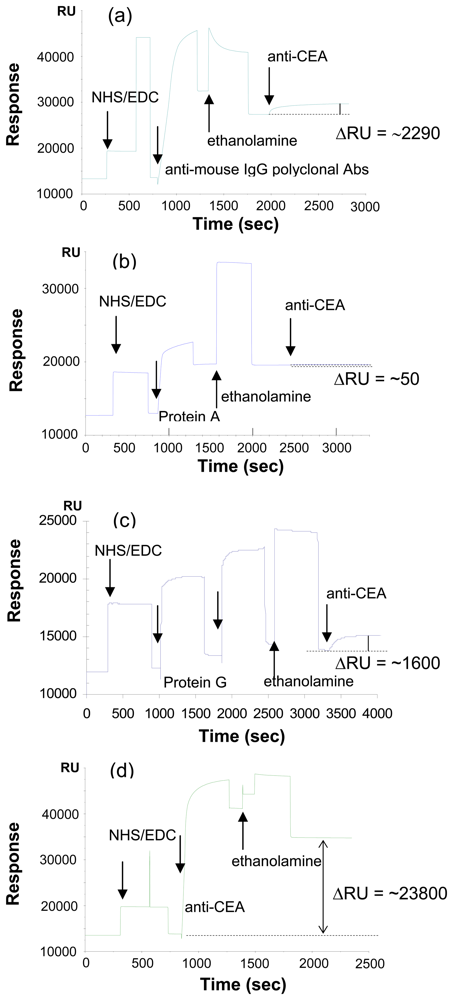
2.1 Effect of immobilization method on the detection of CEA
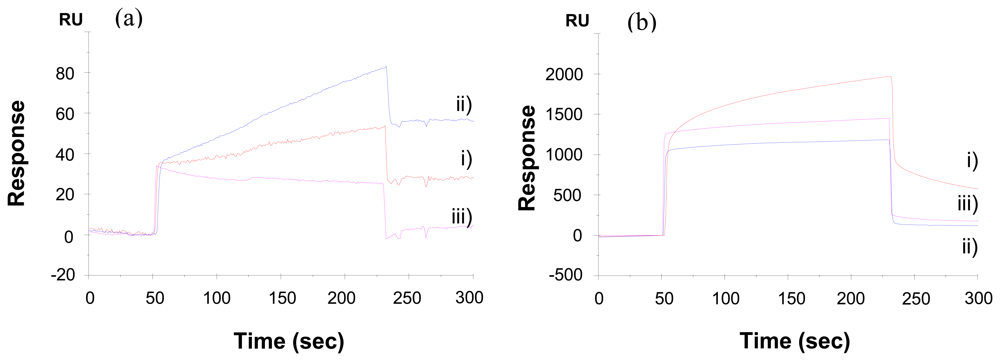
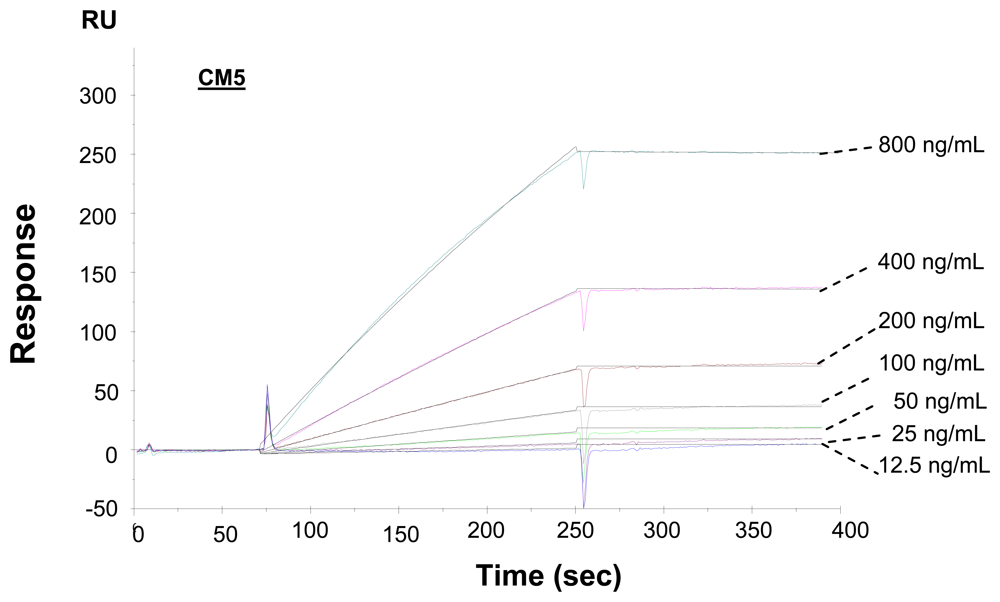
2.2. Kinetic analysis of interactions of carcinoembryonic antigens and antibodies
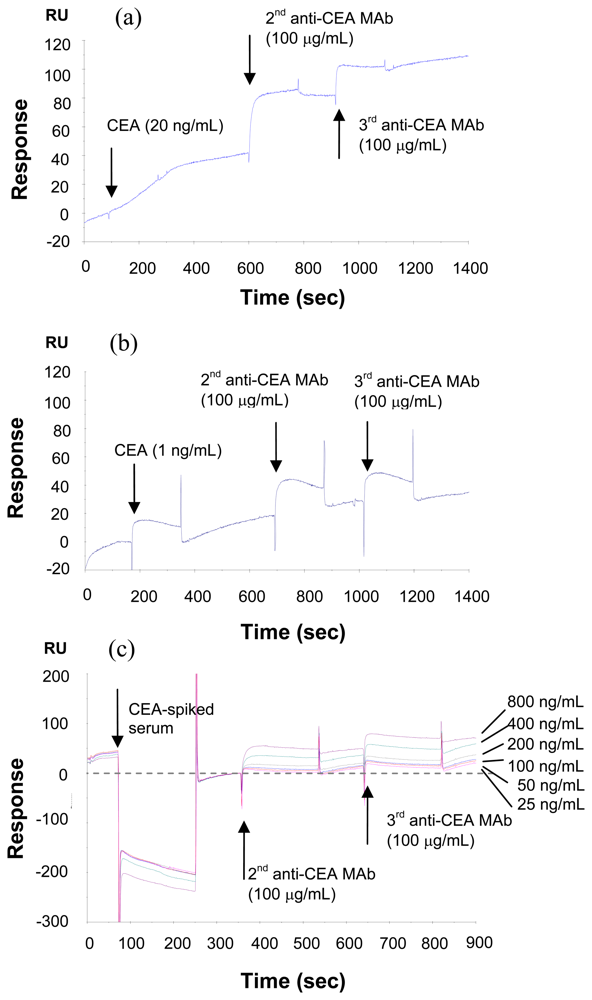
2.3. Method to increase the sensitivity --- sandwich assay
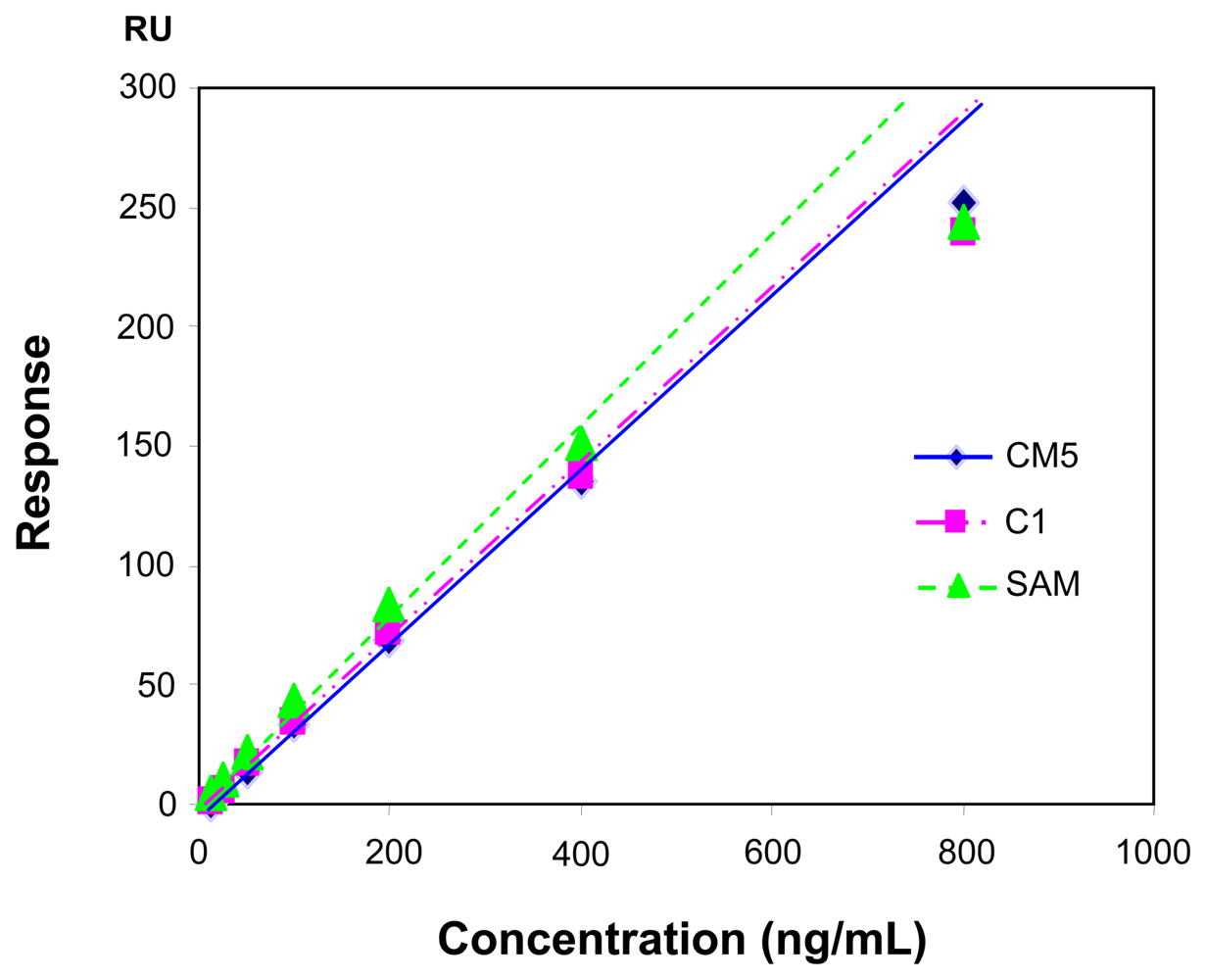
2.4. Influence of the sensor chip
3. Experimental
3.1. Materials
3.2. Instruments
3.3. Immobolization of anti-CEA antibody
3.4. Signal enhancement using second and third antibodies
4. Conclusions
Acknowledgments
References
- Landman, J.; Chang, Y.; Kavaler, E.; Droller, M.J.; Liu, B.C. Sensitivity and specificity of NMP- 22, telomerase and BTA in the detection of human bladder cancer. Urology 1998, 52, 398–402. [Google Scholar]
- Aquino, A.; Formica, V.; Prete, S.P.; Correale, P.P.; Massara, M.C.; Turriziani, M.; De. Vecchis, L.; Bonmassar, E. Drug-induced increase of carcinoembryonic antigen expression in cancer cells. Pharmacological Research 2004, 49, 383–396. [Google Scholar]
- Fernandez, F.G.; Drebin, J.A.; Linehan, D.C.; Dehdashti, F.; Siegel, B.A.; Strasberg, S.M. Five- year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Annals of Surgery 2004, 240, 438–447. [Google Scholar]
- Furukawa, J.; Shinohara, Y.; Kuramoto, H.; Miura, Y.; Shimaoka, H.; Kurogochi, M.; Nakano, M.; Nishimura, S.I. Comprehensive Approach to Structural and Functional Glycomics Based on Chemo-selective Glycoblotting and Sequential Tag Conversion. Analytical Chemistry 2008, 80, 1094–1101. [Google Scholar]
- Ohyama, C.; Hosono, M.; Nitta, K.; Oh-eda, M.; Yoshikawa, K.; Habuchi, T.; Arai, Y.; Fukuda, M. Carbohydrate structure and differential binding of prostate specific antigen to Maackia amurensis lectin between prostate cancer and benign prostate hypertrophy. Glycobiology 2004, 14, 671–679. [Google Scholar]
- Cooper, M.A. Label-free screening of bio-molecular interactions. Analytical and Bioanalytical Chemistry 2003, 377, 834–842. [Google Scholar]
- Liedberg, B.; Nylander, C.; Lundstrom, I. Biosensing with surface plasmon resonance --- how it all started. Biosensors & Bioelectronics 1995, 10, i–ix. [Google Scholar]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: review. Sensors and Actuators B 1999, 54, 3–15. [Google Scholar]
- Karlsson, R.; Roos, H.; Fagerstam, L.; Persson, B. Kinetics and concentration analysis using BIA technology. Methods 1994, 9, 99–110. [Google Scholar]
- Rich, R.L.; Myszka, D.G. Advances in surface plasmon resonance biosensor analysis. Current Opinion in Biotechnology 2000, 11, 54–61. [Google Scholar]
- Huang, L.; Reekmans, G.; Saerens, D.; Friedt, J.M.; Frederix, F.; Francis, L.; Muyldermans, S.; Campitelli, A.; Hoof, C.V. Prostate-specific antigen immunosensing based on mixed self- assembled monolayers, camel antibodies and colloidal gold enhanced sandwich assays. Biosensors and Bioelectronics 2005, 21, 483–490. [Google Scholar]
- Löfås, S.; Johansson, B. A novel hydrogel marix on gold surfaces in surfaces in surface plasmon resonance resonance sensors for fast and efficient covalent immobilization of ligands. Journal of the Chemical Society, Chemical Communications 1990, 21, 1526–1528. [Google Scholar]
- Löfås, S.; Johansson, B.; Edström, A.; Hansson, A.; Lindquist, G.; Hillgren, R.M.M.; Stigh, L. Methods for site controlled coupling to carboxymethylateddextran surfaces in surface plasmon resonance biosensor. Biosensors & Bioelectronics 1995, 10, 813–822. [Google Scholar]
- Karlsson, R. SPR for molecular interaction analysis: a review of emerging application areas. Journal of Molecular Recognition 2004, 17, 151–161. [Google Scholar]
- Ulman, A. Formation and Structure of Self-Assembled Monolaye. Chemical Reviews 1996, 96, 1533–1554. [Google Scholar]
- Bain, C.; Troughton, B.; Tao, Y.T.; Evall, J.; Whitesides, G.; Nuzzo, R. Formation of monolayer films by the spontaneous assembly of organic thiols from solution to gold. Journal of the American Chemical Society 1989, 9, 321–333. [Google Scholar]
- Frederix, F.; Friedt, J.M.; Choi, K.H.; Laureyn, W.; Campitelli, A.; Mondelaers, D.; Maes, G.; Borghs, G. Biosensing Based on Light Absorption of Nanoscaled Gold and Silver Particles. Analytical Chemistry 2003, 75, 6894–6900. [Google Scholar]
- Tang, D.P.; Yuan, R.; Chai, Y.Q. Novel immunoassay for carcinoembryonic antigen based on protein A-conjugated immunosensor chip by surface plasmon resonance and cyclic voltammetry. Bioprocess and Biosystems Engineering 2006, 28, 315–321. [Google Scholar]
- Oh, B.K.; Kim, Y.K.; Lee, W.C.; Bae, Y.M.; Lee, W.H.; Choi, J.W. Immunosensor for detection of Legionella pneumophila using surface plasmon resonance. Biosensors and Bioelectronics 2003, 18, 605–611. [Google Scholar]
- Garcia, M.; Seigner, C.; Bastid, C.; Choux, R.; Payan, M.J.; Reggio, H. Carcinoembryonic Antigen Has a Different Molecular Weight in Normal Colon and in Cancer Cells due to N- Glycosylation Differences. Cancer Research 1991, 51, 5679–5686. [Google Scholar]
- Taya, M. Electronic Composites.; Cambridge University Press: Oxford, 2005; pp. 257–270. [Google Scholar]


© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Su, F.; Xu, C.; Taya, M.; Murayama, K.; Shinohara, Y.; Nishimura, S.-I. Detection of Carcinoembryonic Antigens Using a Surface Plasmon Resonance Biosensor. Sensors 2008, 8, 4282-4295. https://doi.org/10.3390/s8074282
Su F, Xu C, Taya M, Murayama K, Shinohara Y, Nishimura S-I. Detection of Carcinoembryonic Antigens Using a Surface Plasmon Resonance Biosensor. Sensors. 2008; 8(7):4282-4295. https://doi.org/10.3390/s8074282
Chicago/Turabian StyleSu, Fengyu, Chunye Xu, Minoru Taya, Kimie Murayama, Yasuro Shinohara, and Shin-Ichiro Nishimura. 2008. "Detection of Carcinoembryonic Antigens Using a Surface Plasmon Resonance Biosensor" Sensors 8, no. 7: 4282-4295. https://doi.org/10.3390/s8074282
APA StyleSu, F., Xu, C., Taya, M., Murayama, K., Shinohara, Y., & Nishimura, S.-I. (2008). Detection of Carcinoembryonic Antigens Using a Surface Plasmon Resonance Biosensor. Sensors, 8(7), 4282-4295. https://doi.org/10.3390/s8074282



