Abstract
A potentiometric sensor device based on screen-printed Nasicon films was investigated. In order to transfer the promising sensor concept of an open sodium titanate reference to thick film technology, “sodium-rich” and “sodium-poor” formulations were compared. While the “sodium-rich” composition was found to react with the ion conducting Nasicon during thermal treatment, the “sodium-poor” reference mixture was identified as an appropriate reference composition. Screen-printed sensor devices were prepared and tested with respect to CO2 response, reproducibility, and cross-interference of oxygen. Excellent agreement with the theory was observed. With the integration of a screen-printed heater, sensor elements were operated actively heated in a cold gas stream.
1. Introduction
Due to its strong impact as a greenhouse gas, monitoring of CO2 emissions has become crucial. Although optical detection of CO2 using infrared radiation is very exact, a more cost-effective method capable of also working in harsh and dirty environments is needed. To meet these requirements, several potentiometric sensor devices based on electrochemical cells with sodium conducting solid electrolytes such as β‘’-Al2O3 or Na1+xZr2P3-xSixO12 (Nasicon, 0 ≤ x ≤ 3) have been investigated (for a detailed review, cf. [1]). Corresponding to a “type III” electrochemical gas sensor [2], these sensor devices rely on the presence of an auxiliary phase such as sodium or barium carbonate, which is deposited at the working electrode and interacts with CO2. As the counter or reference electrode, gold or platinum are often used. Despite their frequent use, it has to be emphasized that such pure metal electrodes are not able to provide a thermodynamically well-defined chemical potential of sodium.
In many of these electrochemical cells, the electrolyte material forms a thin ceramic pellet or tube [3 - 8]. More recently, sensor designs based on screen-printed or dip-coated ion-conducting films have been reported [9 -14].
As a major drawback, the devices relying only on a carbonate auxiliary phase exhibit a pronounced cross-sensitivity towards oxygen, poor reproducibility between single sensor elements, and poor long- term stability. As an alternative approach, sodium titanate (Na2Ti6O13/Na2Ti3O7) or sodium titanate/titania (Na2Ti6O13/TiO2) mixtures were proposed as a reference system [15 - 17]. These two phase mixture systems provided a thermodynamically well-defined signal based on an oxygen-independent overall reaction as shown below.
As derived in detail in [15], the resulting electromotive force emf of these cells is related to the chemical potential difference of the sodium ions at the electrodes according to Eq. 3.
Since both
and
are well-defined within this set-up, the emf of these cells was shown to depend solely on the carbon dioxide partial pressure pCO2 and on the operating temperature T of the device [15]. As a consequence, simultaneous knowledge of the sensor temperature and the geometry-independent parameter emf enables one to precisely determine pCO2. The fact that the emf is defined thermodynamically also implies the absence of long-term drift effects.
Since the cells discussed in [15] were prepared from bulky ceramic pellets, their usefulness in real world applications was limited. In particular, homogeneous heating of such cells can only be achieved in a furnace, and the integration of a temperature sensor to precisely monitor the operating temperature is not straight-forward. It is therefore highly desirable to transfer the present promising sensor concept to thick-film technology, which provides a basis for miniaturization and integration of further functionalities such as heater and temperature sensor with a single sensor chip.
In this contribution, we report results obtained on long-term stable CO2 sensors with a sodium titanate reference prepared entirely via a cost-effective screen-printing technique. In a detailed study, the most appropriate reference system was identified. In addition to the basic sensor characteristics, the devices were tested with respect to cross-interference of oxygen and long-term stability.
2. Experimental
2.1 Precursor preparation
All ceramic precursor powders were prepared by a conventional mixed-oxide route. To obtain the Nasicon composition Na1+xZr2P3-xSixO12 with x = 2.2, Na2CO3 (Merck), NH4H2PO4 (VWR), SiO2 (VWR), and ZrO2 (AlfaAesar) were mixed in stoichiometric amounts in a ball mill and calcined at 1050 °C for 12 h.
In the case of the sodium titanate compositions Na2Ti6O13 and Na2Ti3O7, Na2CO3 (p.a., VWR) and TiO2 (anatase, Sigma Aldrich) served as precursors. The corresponding Na2CO3/TiO2 powder mixtures (molar ratio 1:3 and 1:6, respectively) were mixed for 4 h in a ball mill and then calcined at 900 °C for 6 h.
Figure 1 presents the XRD patterns of the as-prepared Nasicon, Na2Ti3O7, and Na2Ti6O13 powders (Philips PW 3710, Cu-Kα radiation, Bragg-Brentano geometry). While both sodium titanate compositions were found to be phase-pure, some impurity peaks attributed to ZrO2 (symbol ◊) and Na2ZrSi2O7 (symbol □) were observed within the Nasicon.
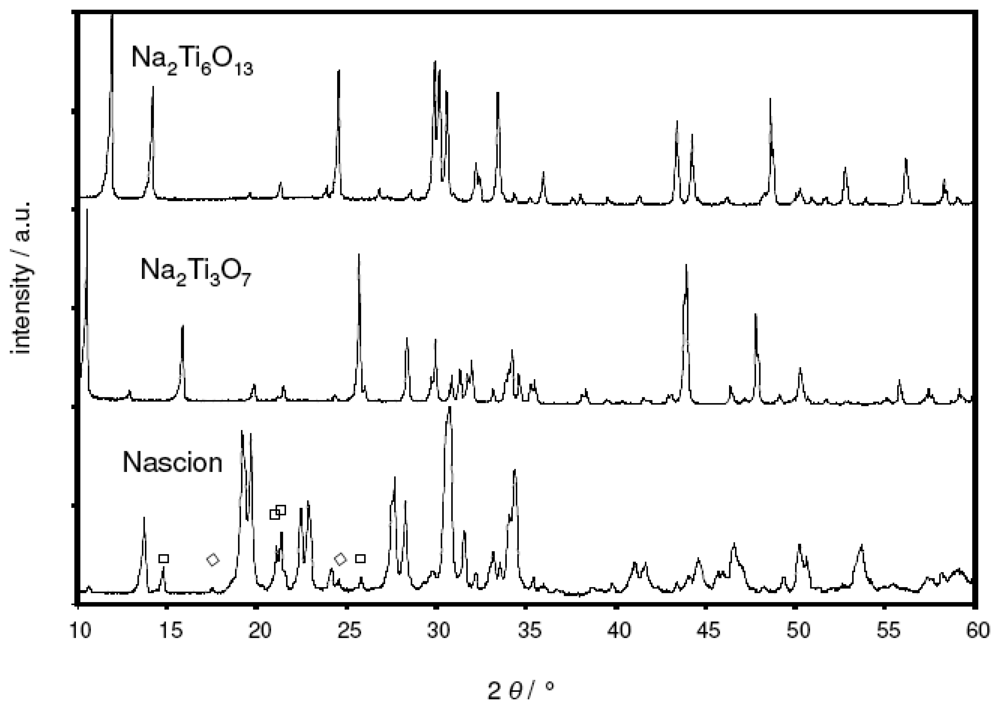
Figure 1.
X-ray diffractograms of the as-prepared Na2Ti3O7, Na2Ti6O13 and Nasicon powder.
Two reference compositions were tested in the present study. The “sodium-rich” reference consisted of a Na2Ti6O13/Na2Ti3O7 (molar ratio: 1:1) mixture, while the “sodium-poor” composition was formed by mixing Na2Ti6O13 and TiO2 powder with a molar ratio of 1:1.
An eutectic mixture of Na2CO3/BaCO3 with a molar ratio of 1.72:1 served as the auxiliary carbonate phase. Prior to mixing, both precursor powders [Na2CO3 (p.a., VWR) and BaCO3 (Selectipur, Merck)] were dried at 450 °C. The carbonate mixture was heated at 5 K/min to 720 °C, a temperature above the melting point. The melt was retrieved from the furnace and quenched onto a brass plate. The as-obtained material was ground in a mortar.
In order to prepare screen-printable pastes, each of the as-prepared powders was sieved (< 200 µm) and mixed with a thixotropic organic binder to form a homogeneous paste.
2.2 Sensor preparation
The sensor set-up is shown diagrammatically in Figure 2. On top of a bare alumina substrate, a Nasicon layer was screen-printed and fired at 1050 °C for 5 h. Then, either the Na2Ti3O7/ Na2Ti6O13 or the Na2Ti6O13/TiO2 reference was printed on one side of the Nasicon film and fired at 950 °C for 5 h. Two gold grid electrodes were printed according to Figure 2 (firing at 850 °C). Finally, the sensitive Na2CO3/BaCO3 mixture was painted on top of one gold grid and heat treated at 600 °C.
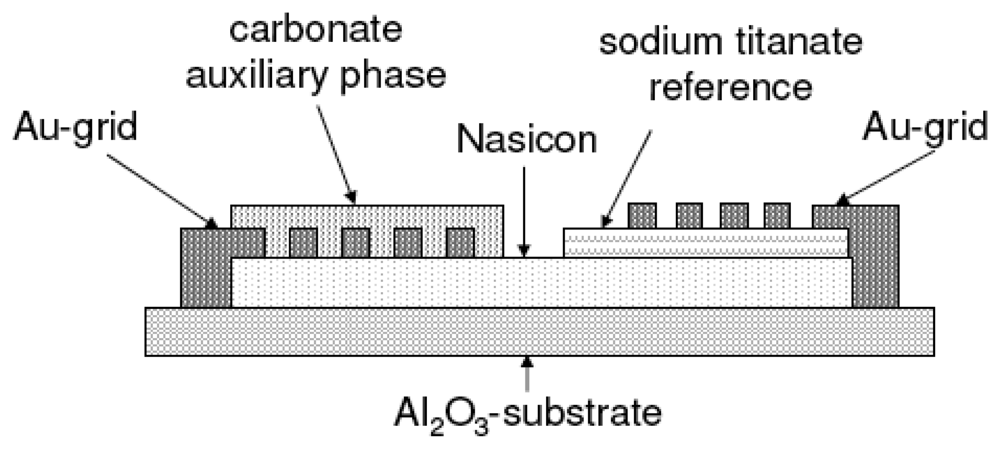
Figure 2.
Diagrammatical representation of the sensor cross section.
2.3. XRD study
In order to study compatibility between the reference materials and the solid electrolyte, XRD studies were conducted in the 2θ range from 10 ° to 90 °. Two samples were prepared by mixing either 0.75 g Na2Ti3O7 with 1 g Nasicon or 0.6 g Na2Ti6O13 with 1 g Nasicon, respectively. While one part of these mixtures was studied directly by XRD, one part was heat-treated at 950 °C for 5 h using the sintering profile of the reference thick films.
2.4 Sensor tests
For tests of the sensor performance, a custom-build test bench similar to the one described in [18] for hydrocarbon sensing was used. The sensors were inserted into a tube furnace and heated to their operating temperature either with the furnace or actively via the platinum heater (see below). The total gas flow was adjusted to 200 sccm/min with dry air serving as the carrier gas. The carbon dioxide partial pressure was varied in the range of 0.4 mbar to 45 mbar by diluting pure CO2 gas with dry air using mass flow controllers. The actual CO2 concentration was monitored by an FTIR (Antaris, ThermoElectron) located downstream the sensor chamber. The emf output of the sensor element was monitored using a digital multimeter (Keithley 2700).
3. Results and Discussion
3.1 Sodium-rich reference
In an initial test series, sensor samples with the sodium-rich reference composition Na2Ti3O7/Na2Ti6O13 were measured. In Figure 3, the emf trace at 500 °C upon CO2 exposure is shown exemplarily. The sensor device presented a stable and perfectly reversible response. An additional variation of the oxygen partial pressure from 0.15 bar to 0.27 bar indicated no cross sensitivity of the sensor to this gas as expected from the literature.
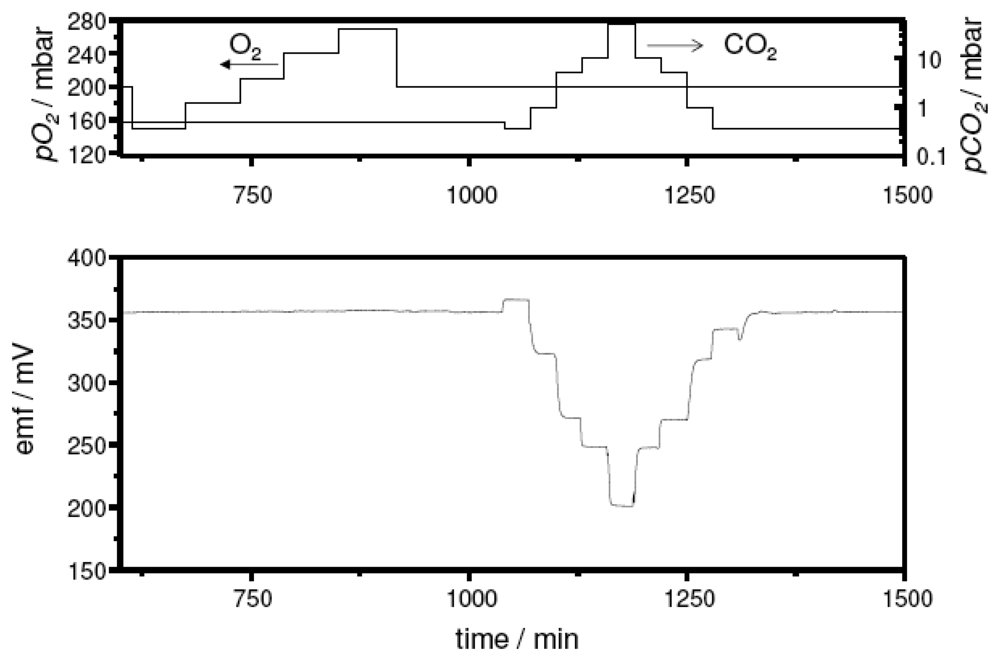
Figure 3.
Sensor response towards pCO2 and oxygen cross-interference test at 500 °C on a sensor device with the sodium-rich reference. Top: partial pressure of the test gases. Bottom: Sensor signal.
To evaluate the sensor performance, the semilogarithmic representation emf = f (log(pCO2)) was used. With the slope of this plot, the electron transfer number n of the electrochemical reaction can be calculated according to the Nernst equation (Eq. 4)
with p0 = 1013 mbar.
As known from the cell reactions (1) and (2), the theoretical electron transfer number equals 2. With the present sensor set up, values of 2.14 ± 0.06 (total of 4 specimens, each measured 3 times at 500 °C) and 2.12 ± 0.06 (total of 4 specimens at 600 °C) were determined.
In spite of the promising sensor characteristics, the thick film devices with the sodium-rich reference presented a much higher emf reading than expected from the thermodynamic calculations and experimental results on the pellet-type sensor discussed in [15]. In Figure 4, this deviation between pellet-type sensor (open symbols) and the corresponding thick-film device measured in the present study (closed symbols) is presented. In this case, the temperature-corrected form of the Nernst equation (Eq. 4) was used to compare the experimental results directly with the values expected from theory.
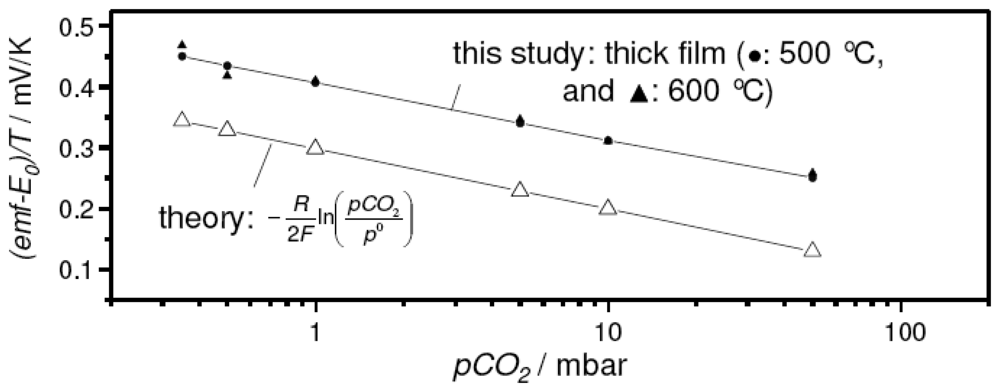
Figure 4.
Comparison between the experimental results of the thick-film sensor with the sodium-rich reference and the results expected from theory. For details see text.
The required temperature-dependent values for E0, i.e. the cell emf at pCO2 = p0 = 1013 mbar, were taken from the literature [15].
In a supplementary XRD study, the deviation from the theoretical emf was attributed to a parasitic reaction between the Nasicon electrolyte and the Na2Ti3O7 phase. Figure 5a compares two XRD diagrams obtained from a Nasicon/Na2Ti3O7 powder mixture prior (bottom) and after (top) heat treatment at 950 °C, i.e., the sintering temperature of the sodium titanate reference film. For the sake of clarity, only the 2 θ range from 10° to 60° is shown. In the X-ray diffractogram of the untreated powder mixtures, the peaks from both the Nasicon and the titanate phase were identified. In addition, some impurity peaks were found, which were attributed to ZrO2 und Na2ZrSi2O7. These impurities were also present in the Nasicon precursor powder (cf. Figure 1).
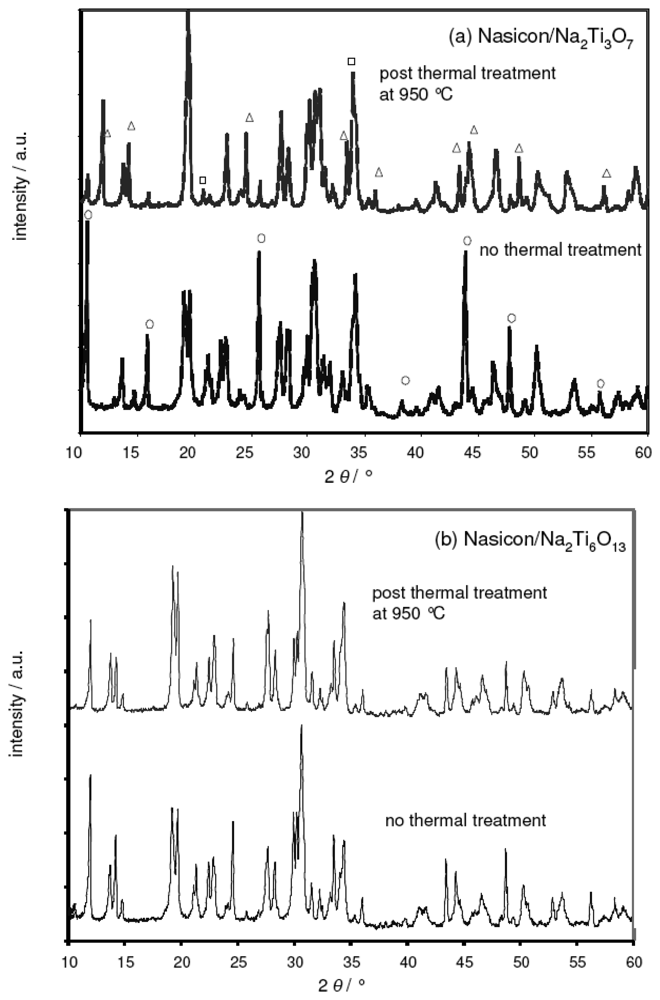
Figure 5.
XRD pattern of Nasicon/Na2Ti3O7 and Nasicon/Na2Ti6O13 powder mixtures.(a) Nasicon/Na2Ti3O7 mixtures before (bottom) and after (top) thermal treatment at 950 °C, respectively. Only some of the characteristic peaks of the Na2Ti3O7 are highlighted (symbol ○), which are replaced by the sodium-poor Na2Ti6O13 phase (symbol &Delta:) after the thermal treatment. For details see text.(b) Nasicon/Na2Ti6O13 mixtures before (bottom) and after (top) thermal treatment at 950 °C, respectively.
The XRD pattern after thermal treatment indicated a thermally activated reaction between the Nasicon and the titanate. The characteristic peaks of Na2Ti3O7 (symbol ○ in the bottom figure) are almost completely replaced by the sodium-poor Na2Ti6O13 phase (symbol Δ). In addition, changes in the Nasicon pattern are observed, e.g., the double peak at 19° is reduced to one broadened peak. This might be attributed to a compositional and structural change of the sodium ion conductor from Nasicon to Na4Zr3Si3O12. As an additional phase, Na3PO4 is found (symbol □).
While the reactivity between Nasicon and Na2Ti3O7 is irrelevant in the case of the separately sintered pellets discussed in [15], it is detrimental for thick film layers that are heat-treated simultaneously. During sintering of the reference electrode, most of the Na2Ti3O7 phase reacts with the adjacent Nasicon layer. Due to the different phase composition of the reference electrode mixture its sodium ion activity decreases. As a consequence, the emf value of the cell, which is given by the difference of the chemical potentials of sodium ions at each electrode (Eq. 3), is expected to increase.
3.2 Sodium-poor reference
In contrast to Na2Ti3O7, XRD studies on Nasicon/Na2Ti6O13 mixtures yielded no evidence for parasitic reactions at 950 °C. Identical XRD patterns were observed prior and after thermal treatment (Figure 5b; no new peaks, only some minor changes in peak intensities appear). The sodium-poor reference composition Na2Ti6O13/TiO2 was thus identified as a more promising candidate for thick-film devices.
Figure 6 exemplarily presents the sensitivity plots of a corresponding sensor element. The device was measured several times at three temperatures. In contrast to the sensor devices discussed above, the measured emf values (symbols) were found to agree well with the results reported in the literature (lines). Again, Nernstian behavior with an electron transfer number of 1.9 was found. The reproducibility of the measurements is remarkable. In Figure 6, one can hardly distinguish the different runs at 575 °C
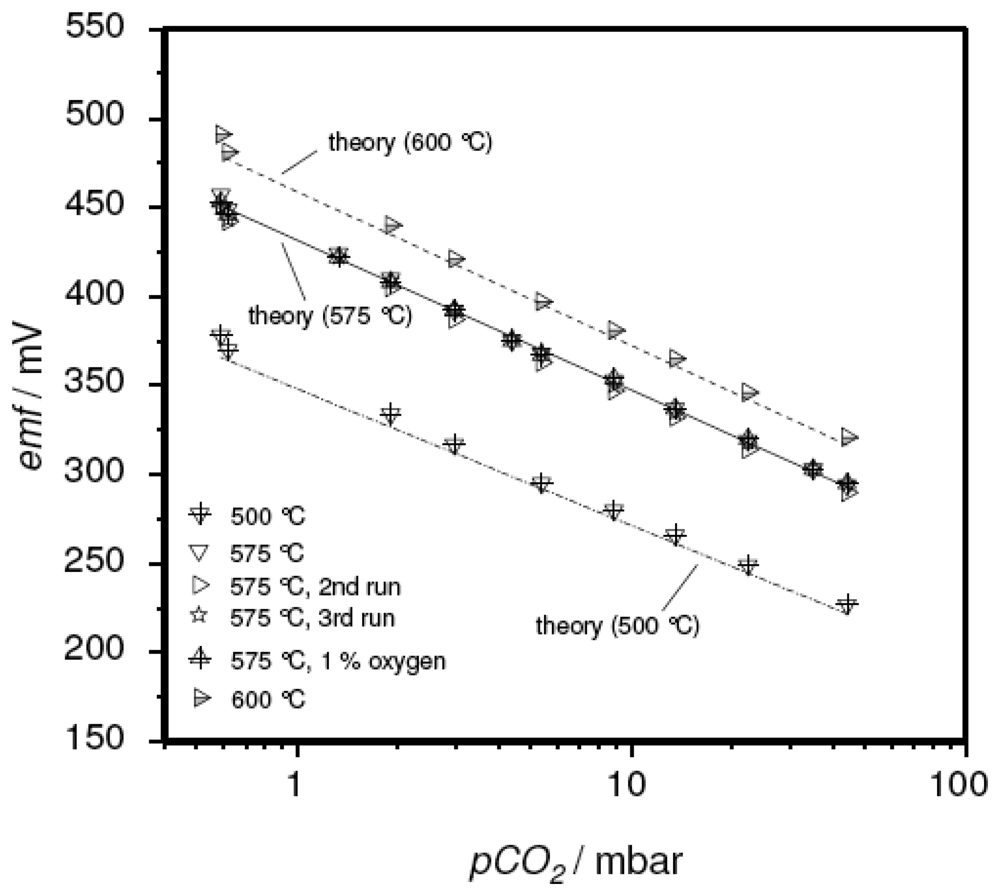
Figure 6.
emf Sensor characteristics of a thick-film device with the sodium-poor reference composition. Operating temperature as indicated. For comparison, data reported in the literature for a pellet-like sensor [15] were included.
To ensure the reproducibility of the sensor concept, three sensor devices were prepared and measured at various temperatures. The results are summarized in Figure 7. As before (cf. Figure 4), the very sensitive representation:
was used, in this case to emphasize the excellent agreement of the thick-film device with theory and the data on pellet-type sensors [15].
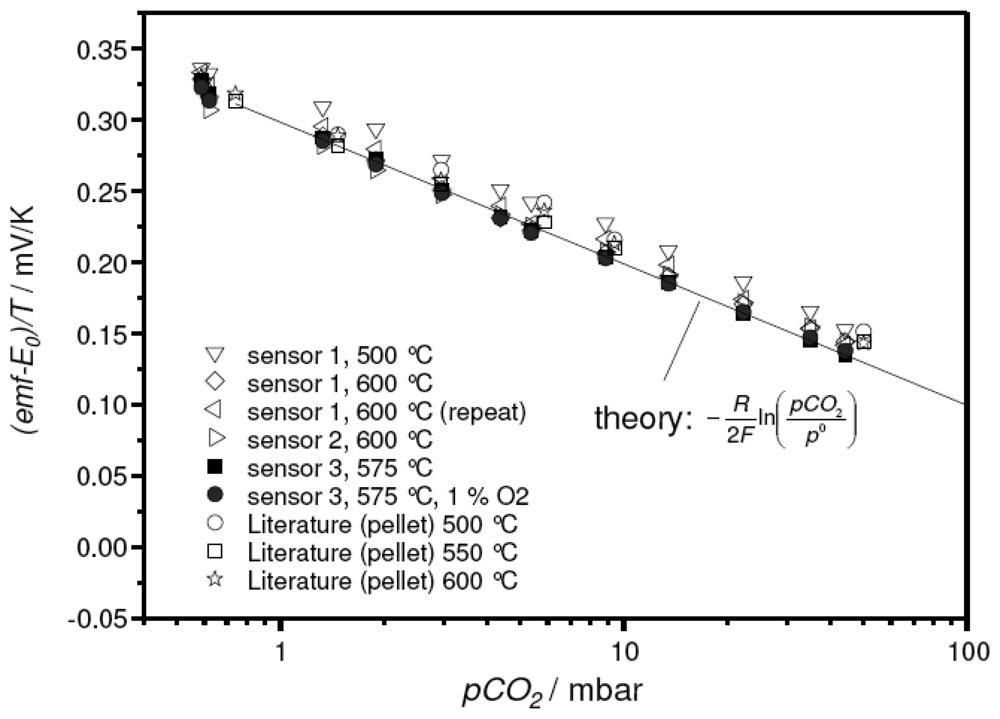
Figure 7.
Reproducibility of the thick-film sensor concept. For comparison, data reported in the literature for a pellet-like sensor [15] were included, as well as the values expected from theory (Eq. 4a, solid line).
Based on these promising results, actively heated devices with the sodium-poor reference material were prepared. For this purpose, a sensor chip was glued on top of a screen-printed platinum heater using ceramic paste. Silver served as a shielding layer to prevent voltage interferences from the heater. The sensor was then mounted into a corresponding sample holder attached to a voltage source. By monitoring the resistance of the platinum heater after previous calibration, its temperature could be controlled in the range from 100 °C to 700 °C. Again, operating temperatures of 500 °C and 600 °C were chosen.
Figure 8 presents the results of four consecutive measurement cycles conducted on a thick-film sensor with the sodium-poor reference. The temperature of the device was adjusted to 575 °C and exposed to a cold gas stream of 200 sccm/min. The carbon dioxide partial pressure pCO2 was varied stepwise between 0.4 mbar and 45 mbar. The spikes that occur consistently between the 13.5 mbar and the 9 mbar step are an artifact related to the gas dosage system of the test bench. In order to access low pCO2 values, the gas dosage is switched to a dilution line and back again to achieve high pCO2. This switching is accompanied by a pressure surge leading to the observed emf spikes. The actively heated sensor presented a stable and reproducible response, which was insensitive to a variation in the oxygen partial pressure from 200 mbar (20 % O2) to 10 mbar (1 % O2). As shown in the top part of Figure 8, the sensor characteristics of each measurement cycle coincide. The electron transfer number calculated from the slope of this semilogarithmic plot equaled 1.94. For comparison, the values expected from theory were included in Figure 8 as a solid line. They were calculated using the Nernst equation (Eq. 4) with the E0 value estimated from Ref. [15].
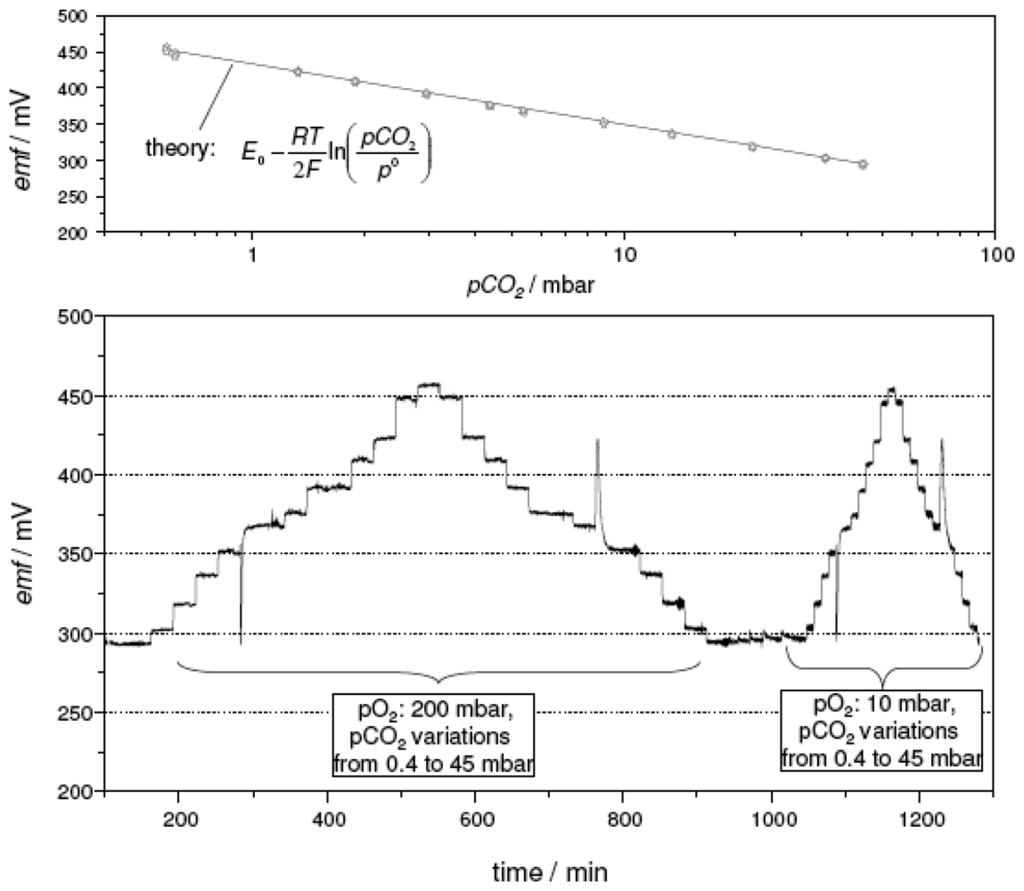
Figure 8.
emf response of a thick-film device with the sodium-poor reference composition, heated to 575 °C by a screen-printed platinum heater. Bottom part: emf trace. Top part: semilogarithmic plot as a function of pCO2 (values expected from theory (Eq. 4) were included for comparison as solid line). Total gas flow: 200 sccm/min. For details see text.
4. Conclusions
The potentiometric sensor concept with an open sodium titanate reference, which had formerly been investigated in a ceramic pellet set-up, was successfully transferred to thick-film technology. After identifying the appropriate reference composition, screen-printed sensor devices were prepared and tested with respect to CO2 response, reproducibility, and cross-interference of oxygen. For the thick- film sensors using a sodium-poor reference formulation, excellent agreement with the theory was observed. After attaching a screen-printed heater, sensor elements were operated actively in a cold gas stream.
Future work is directed to further miniaturizing the thick film sensor, i.e., in a hot-plate set-up as shown in [19] and [20]. In particular, the direct integration of a heating element on one single chip is envisioned.
References
- Holzinger, M.; Maier, J.; Sitte, W. Potentiometric detection of complex gases: application to CO2. Solid State Ionics 1997, 94, 217–225. [Google Scholar]
- Weppner, W. Solid-state electrochemical gas sensors. Sens.Actuators 1987, 12, 107–119. [Google Scholar]
- Kaneyasu, K.; Otsuka, K.; Setoguchi, Y.; Sonoda, S.; Nakahara, T.; Aso, I.; Nakagaichi, N. A carbon dioxide gas sensor based on solid electrolyte for air quality control. Sens.Actuators B: Chemical 2000, 66, 56–58. [Google Scholar]
- Mason, L.W.; Oh, S.; Joseph, J.P. Development and testing of a solid-state CO2gas sensor for use in reduced-pressure environments. Sens. Actuators B: Chemical 1995, 24-25, 407–411. [Google Scholar]
- Kida, T.; Shimanoe, K.; Miura, N.; Yamazoe, N. Stability of NASICON-based CO2 sensor under humid conditions at low temperature. Sens.Actuators B: Chemical 2001, 75, 179–187. [Google Scholar]
- Näfe, H.; Aldinger, F. CO2 sensor based on a solid state oxygen concentration cell. Sens.Actuators B: Chemical 2000, 69, 46–50. [Google Scholar]
- Alonso-Porta, M.; Kumar, R.V. Use of NASICON/Na2CO3 system for measuring CO2. Sens.Actuators B: Chemical 2000, 71, 173–178. [Google Scholar]
- Obata, K.; Shimanoe, K.; Miura, N.; Yamazoe, N. NASICON devices attached with Li2CO3- BaCO3 auxiliary phase for CO2 sensing under ambient conditions. J. Mater. Sci. 2003, 38, 4283– 4288. [Google Scholar]
- Quan, Y.H.B.; Wang, B.; Zhang, C.; Liu, F. Investigation of miniature CO2 gas sensor based on NASICON. Russ. J. Electrochem. 2007, 43, 1289–1293. [Google Scholar]
- Wang, L.; Kumar, R.V. Thick film CO2 sensors based on Nasicon solid electrolyte. Solid State Ionics 2003, 158, 309–315. [Google Scholar]
- Kida, T.; Kishi, S.; Yuasa, M.; Shimanoe, K.; Yamazoe, N. Planar NASICON-based CO2 sensor using BiCuVOx/perovskite-type oxide as a solid-reference electrode. J. Electrochem. Soc. 2008, 155, J117–J121. [Google Scholar]
- Shim, H.B.; Kang, J.H.; Choi, J.W.; Yoo, K.S. Characteristics of thick-film CO2 sensors based on NASICON with Na2CO3-CaCO3 auxiliary phases. J.Electroceramics 2006, 17, 971–974. [Google Scholar]
- Miyachi, Y.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Fabrication of CO2 sensor using NASICON thick film. Sens.Actuators B: Chemical 2003, 93, 250–256. [Google Scholar]
- Kida, T.; Miyachi, Y.; Shimanoe, K.; Yamazoe, N. NASICON thick film-based CO2 sensor prepared by a sol-gel method. Sens.Actuators B: Chemical 2001, 80, 28–32. [Google Scholar]
- Holzinger, M.; Maier, J.; Sitte, W. Fast CO2-selective potentiometric sensor with open reference electrode. Solid State Ionics 1996, 86-88, 1055–1062. [Google Scholar]
- Maier, J.; Holzinger, M.; Sitte, W. Fast potentiometric CO2 sensors with open reference electrodes. Solid State lonics 1994, 74, 5–9. [Google Scholar]
- Maier, J. Electrochemical sensor principles for redox–active and acid-base–active gases. Sens.Actuators B: Chemical 2000, 65, 199–203. [Google Scholar]
- Sahner, K.; Schönauer, D.; Moos, R.; Matam, M.; Post, M.L. Effect of electrodes and zeolite cover layer on hydrocarbon sensing with p-type perovskite SrTi0.8Fe0.2O3-δ thick and thin films. J. Mater. Sci. 2006, 41, 5828–5835. [Google Scholar]
- Rettig, F.; Moos, R. Ceramic meso hot-plates for gas sensors. Sens.Actuators B: Chemical 2004, 103, 91–97. [Google Scholar]
- Kita, J.; Rettig, F.; Moos, R.; Drue, K.; Thust, H. Hot plate gas sensors - Are ceramics better? Int. J. Appl. Ceram. Technol. 2005, 2, 383–389. [Google Scholar]
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).