A Label-Free Electronic Biosensor for Detection of Bone Turnover Markers
Abstract
:1. Introduction
2. Experimental Setup
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Gold Electrode Preparation and Antibody Immobilization
3. Results and Discussion
4. Conclusions
Acknowledgments
References and Notes
- Seeman, E.; Delmas, P.D. Bone quality-the material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar]
- Ryouji, M.; Itsuo, Y.; Masahiko, T.; Yasuyo, H.; Itsuaki, Y.; Rikushi, M. Comparison of various biochemical measurements with bone mineral densitometry and quantitative ultrasound for the assessment of vertebral fracture. J. Bone Miner. Metab. 2000, 18, 158–168. [Google Scholar]
- Watts, N.B. Clinical utility of biochemical markers of bone remodeling. Clin. Chem. 1999, 45, 1359–1368. [Google Scholar]
- Review and search engine for osteoporosis. Bone Health and Osteoporosis Center: Southington, CT, USA, 2008. Available at: http://www.ucosteoporosis.com/ (accessed 31 May 2008).
- Burgeson, R.E. Serum cross Laps one step ELISA: first application of monoclonal antibodies for measurement in serum of bone-related degradation products from C-terminal telopeptides of type I collagen. Annu. Rev. Cell. Biol. 1998, 4, 552–577. [Google Scholar]
- Rosenquist, C.; Fledeliu, C.; Christgau, S.; Pedersen, B.J.; Bonde, M.; Qvist, P.; Christiansen, C. First application of monoclonal antibodies for measurement in serum of bone-related degradation products from C-terminal telopdptides of type I collagen. Clin. Chem. 1998, 44, 2281–2289. [Google Scholar]
- Okuno, S.; Inaba, M.; Kitatani, K.; Ishimura, E.; Yamakawa, T.; Nishizawa, Y. Serum levels of C-terminal telopeptide of type I collagen: a useful new marker of cortical bone loss in hemodialysis patient. Osteoporosis Int. 2005, 16, 501–509. [Google Scholar]
- Ross, P.D. Predicting bone loss and fracture risk with biochemical markers: A review. J. Clin. Dent. 1999, 2, 285–294. [Google Scholar]
- Wittich, A.; Casco, C.; Oviedo, A.; Zeni, S.; Nadal, M.; Mautalen, C. Serum determination of C-terminal telopeptide of type 1 collagen (CTx) is a sensitive bone resorption marker in renal osteodystrophy. Bone 2001, 29, 300. [Google Scholar]
- Hannon, R.A.; Eastell, R. Bone markers and current laboratory assays. Cancer Treat. Rev. 2006, 32, 7–14. [Google Scholar]
- Srivastava, A.K.; Macfarlane, G.; Srivastava, V.P.; Mohan, S.; Baylink, D.J. A new monoclonal antibody ELISA for detection and characterization of C-telopeptide fragments of type I collagen in urine. Calcified Tissue Int. 2001, 69, 327–336. [Google Scholar]
- Yun, Y.H.; Bange, A.; Heineman, W.R.; Halsall, H.B.; Shanov, V.N.; Dong, Z.; Pixley, S.; Behbehani, M. A nanotube array immunosensor for direct electrochemical detection of antigen-antibody binding. Sens. Actuat. B 2007, 123, 177–182. [Google Scholar]
- Ma, K.S.; Zhou, H.; Zoval, J.; Madou, M. DNA hybridization detection by label free versus impedance amplifying label with impedance spectroscopy. Sens. Actuat. B 2006, 114, 58–64. [Google Scholar]
- Rodriguez, M.C.; Kawde, A.N.; Wang, J. Aptamer biosensor for label-free impedance spectroscopy detection of proteins based on recognition-induced switching of the surface charge. Chem. Commun. 2005, 4267–4269. [Google Scholar]
- Belle, J.T.; Bhavsar, K.; Fairchild, A.; Das, A.; Sweeney, J.; Alford, T.L.; Wang, J.; Bhavanandan, V.P.; Joshi, L. A cytokine immunosensor for multiple sclerosis detection based upon label-free electrochemical impedance spectroscopy. Biosens. Bioelectron. 2007, 23, 428–431. [Google Scholar]
- Ding, L.; Du, D.; Wu, J.; Ju, H. A disposable impedance sensor for electrochemical study and monitoring of adhesion and proliferation of K562 leukaemia cells. Electrochem. Commun. 2007, 9, 953–958. [Google Scholar]
- Campbell, C.E.; Laane, M.M.; Haugarvoll, E.; Giaever, I. Monitoring viral-induced cell death using electric cell–substrate impedance sensing. Biosens. Bioelectron. 2007, 23, 536–542. [Google Scholar]
- Peichl, P.; Griesmacher, A.; Marteau, R.; Hejc, S.; Kumpan, W.; Muller, M.; Broll, H. Serum crosslaps in comparison to serum osteocalcin and urinary bone resorption markers. Clin. Biochem. 2001, 34, 131–139. [Google Scholar]
- Terpos, E.; Politou, M.; Rahemtulla, A. The role of markers of bone remodeling in multiple myeloma. Blood Rev. 2005, 19, 125–142. [Google Scholar]
- Huang, Y.; Nie, X.M.; Gan, S.L.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Electrochemical immunosensor of platelet-derived growth factor with aptamer-primed polymerase amplification. Anal. Biochem. 2008, 382, 16–22. [Google Scholar]
- Keighley, S.D.; Estrela, P.; Li, P.; Migliorato, P. Optimization of label-free DNA detection with electrochemical impedance spectroscopy using PNA probes. Biosens. Bioelectron. 2008, 24, 912–917. [Google Scholar]
- Zhang, Y.; Wang, H.; Nie, J.; Zhang, Y.; Shen, G.; Yu, R. Individually addressable microelectrode arrays fabricated with gold-coated pencil graphite particles for multiplexed and high sensitive impedance immunoassays. Biosens. Bioelectron. 2009, 25, 34–40. [Google Scholar]
- Levine, P.M.; Gong, P.; Levicky, R.; Shepard, K.L. Real-time, multiplexed electrochemical DNA detection using an active complementary metal-oxide-semiconductor biosensor array with integrated sensor electronics. Biosens. Bioelectron. 2009, 24, 1995–2001. [Google Scholar]
- Deng, C.; Chen, J.; Nie, Z.; Wang, M.; Chu, X.; Chen, X.; Xiao, X.; Lei, C.; Yao, S. Impedimetric aptasensor with femtomolar sensitivity based on the enlargement of surface-charged gold nanoparticles. Anal. Chem. 2009, 81, 739–745. [Google Scholar]
- Jackson, B.F.; Dyson, P.K.; Lonnell, C.; Verheyen, K.L.; Pfeiffer, D.U.; Price, J.S. Bone biomarkers and risk of fracture in two- and three-year-old Thoroughbreds. Equine Vet. J. 2009, 41, 410–413. [Google Scholar]
- Garnero, P.; Hausherr, E.; Chapuy, M.C.; Marcelli, C.; Grandjean, H.; Muller, C.; Cormier, C.; Bréart, G.; Meunier, P.J.; Delmas, P.D. Markers of bone resorption predict hip fracture in elderly women: The EPIDOS prospective study. J. Bone Miner. Res. 1996, 11, 1531–1538. [Google Scholar]
- Watts, N.B.; Jenkins, D.K.; Visor, J.M.; Casal, D.C.; Geusens, P. Comparison of bone and total alkaline phosphatase and bone mineral density in postmenopausal osteoporotic women treated with alendronate. Osteoporos Int. 2001, 12, 279–288. [Google Scholar]
- Greenspan, S.L.; Parker, R.A.; Ferguson, L.; Rosen, H.N.; Maitland-Ramsey, L.; Karpf, D.B. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J. Bone Miner. Res. 1998, 13, 1431–1438. [Google Scholar]
- Eastell, R.; Barton, I.; Hannon, R.A.; Chines, A.; Garnero, P.; Delmas, P.D. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J. Bone Miner. Res. 2003, 18, 1051–1056. [Google Scholar]
- Delmas, P.D.; Vrijens, B.; Eastell, R.; Roux, C.; Pols, H.A.; Ringe, J.D.; Grauer, A.; Cahall, D.; Watts, N.B. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 2007, 92, 1296–1304. [Google Scholar]
- Looker, A.C.; Bauer, D.C.; Chesnut, C.H.; Gundberg, C.M.; Hochberg, M.C.; Klee, G.; Kleerekoper, M.; Watts, N.B.; Bell, N.H. Clinical use of biochemical markers of bone remodeling: current status and future directions. Osteoporos Int. 2000, 11, 467–480. [Google Scholar]
- Watts, N.B. Clinical utility of biochemical markers of bone remodeling. Clin. Chem. 1999, 45, 359–1368. [Google Scholar]
- Kleerekoper, M.; Camacho, P. Monitoring osteoporosis therapy. Clin. Chem. 2005, 51, 2227–2228. [Google Scholar]
- Camacho, P.M.; Lopez, N.A. Use of biochamical markers of bone turnover in the management of postmenopausal osteoporosis. Clin. Chem. Lab. Med. 2008, 46, 1345–1357. [Google Scholar]


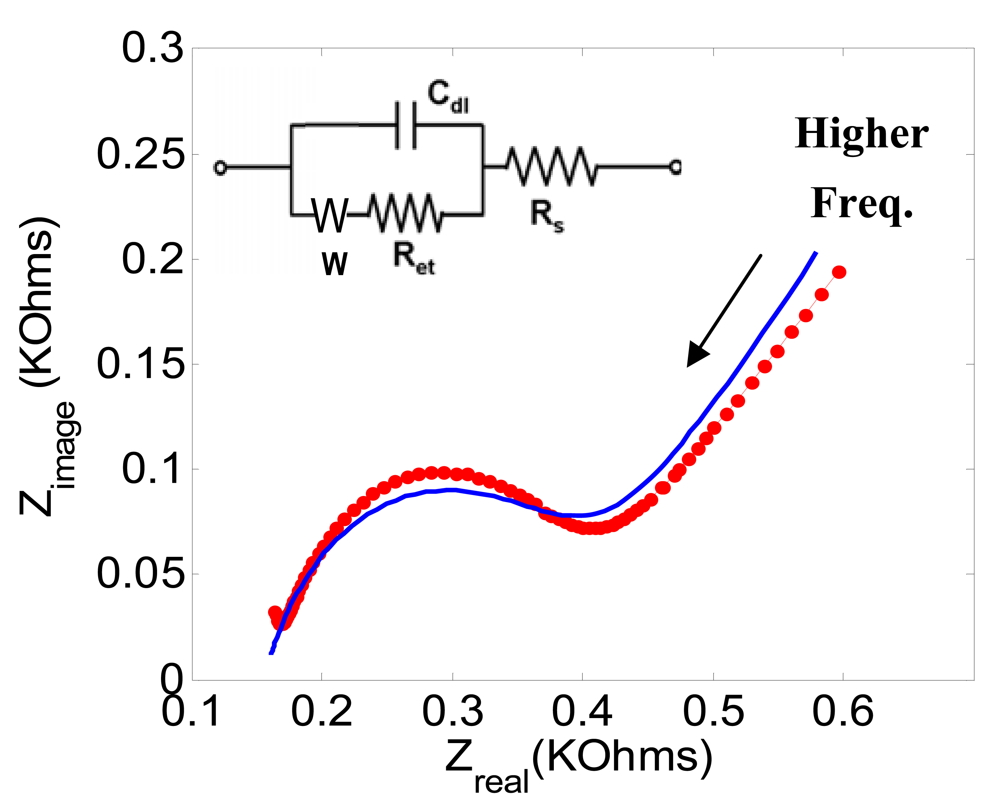


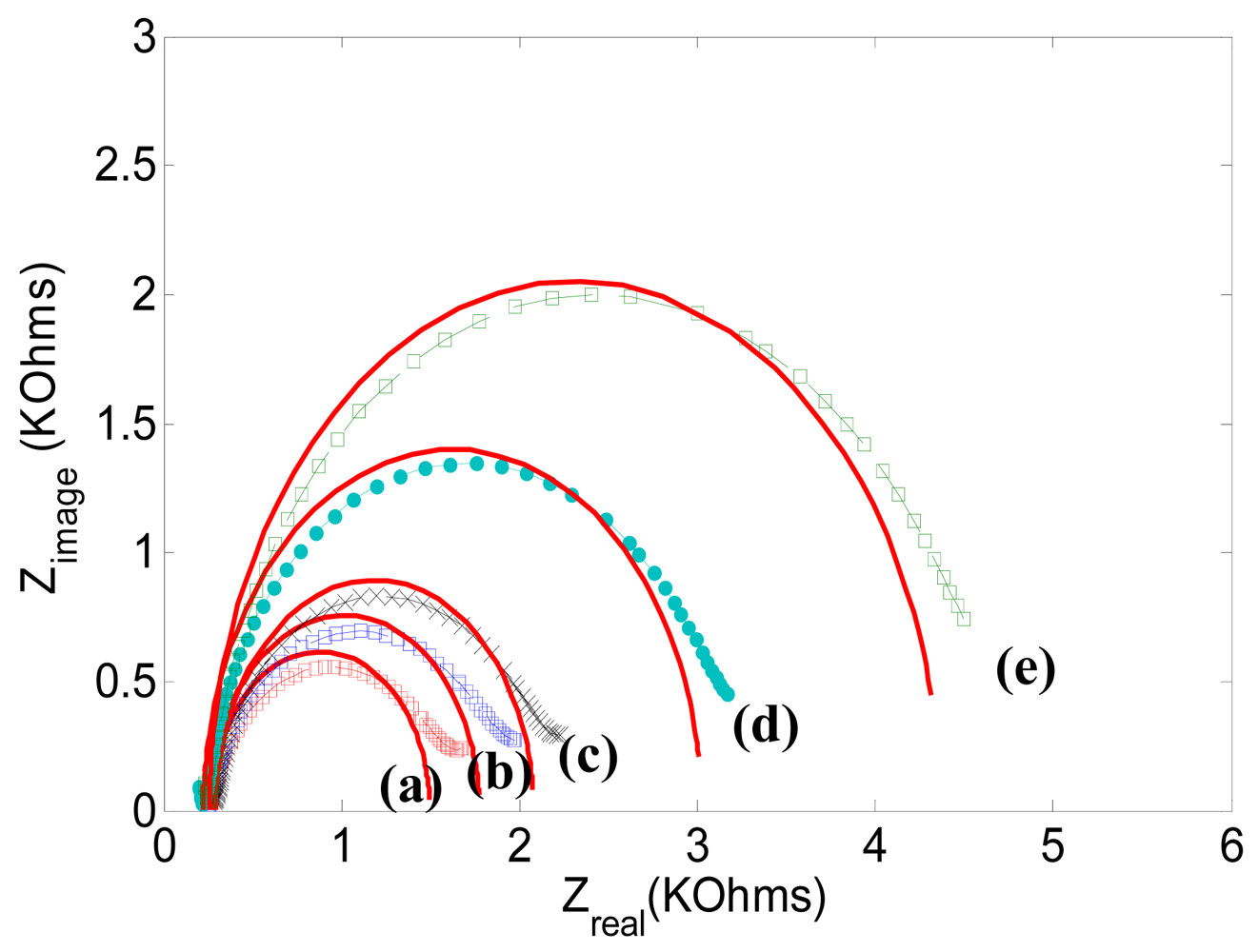



| Ret [KΩ] | Cdl [μF] | Rs [Ω] | |
|---|---|---|---|
| Figure 3 (a) | 0.23 | 0.415 | 170 |
| Figure 6 (a) | 1.25 | 0.443 | 172 |
| Figure 6 (b) | 1.51 | 0.441 | 173 |
| Figure 6 (c) | 1.78 | 0.434 | 173 |
| Figure 6 (d) | 2.78 | 0.445 | 174 |
| Figure 6 (e) | 4.10 | 0.452 | 173 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yun, Y.-H.; Bhattacharya, A.; Watts, N.B.; Schulz, M.J. A Label-Free Electronic Biosensor for Detection of Bone Turnover Markers. Sensors 2009, 9, 7957-7969. https://doi.org/10.3390/s91007957
Yun Y-H, Bhattacharya A, Watts NB, Schulz MJ. A Label-Free Electronic Biosensor for Detection of Bone Turnover Markers. Sensors. 2009; 9(10):7957-7969. https://doi.org/10.3390/s91007957
Chicago/Turabian StyleYun, Yeo-Heung, Amit Bhattacharya, Nelson B. Watts, and Mark J. Schulz. 2009. "A Label-Free Electronic Biosensor for Detection of Bone Turnover Markers" Sensors 9, no. 10: 7957-7969. https://doi.org/10.3390/s91007957
APA StyleYun, Y.-H., Bhattacharya, A., Watts, N. B., & Schulz, M. J. (2009). A Label-Free Electronic Biosensor for Detection of Bone Turnover Markers. Sensors, 9(10), 7957-7969. https://doi.org/10.3390/s91007957





