Semiconducting Metal Oxide Based Sensors for Selective Gas Pollutant Detection
Abstract
:1. Background
2. Working Principle of SMO Gas Sensors
3. Testing Setup, Film Deposition and Delivery System
4. Applications in Environmental Monitoring and Gas Detection
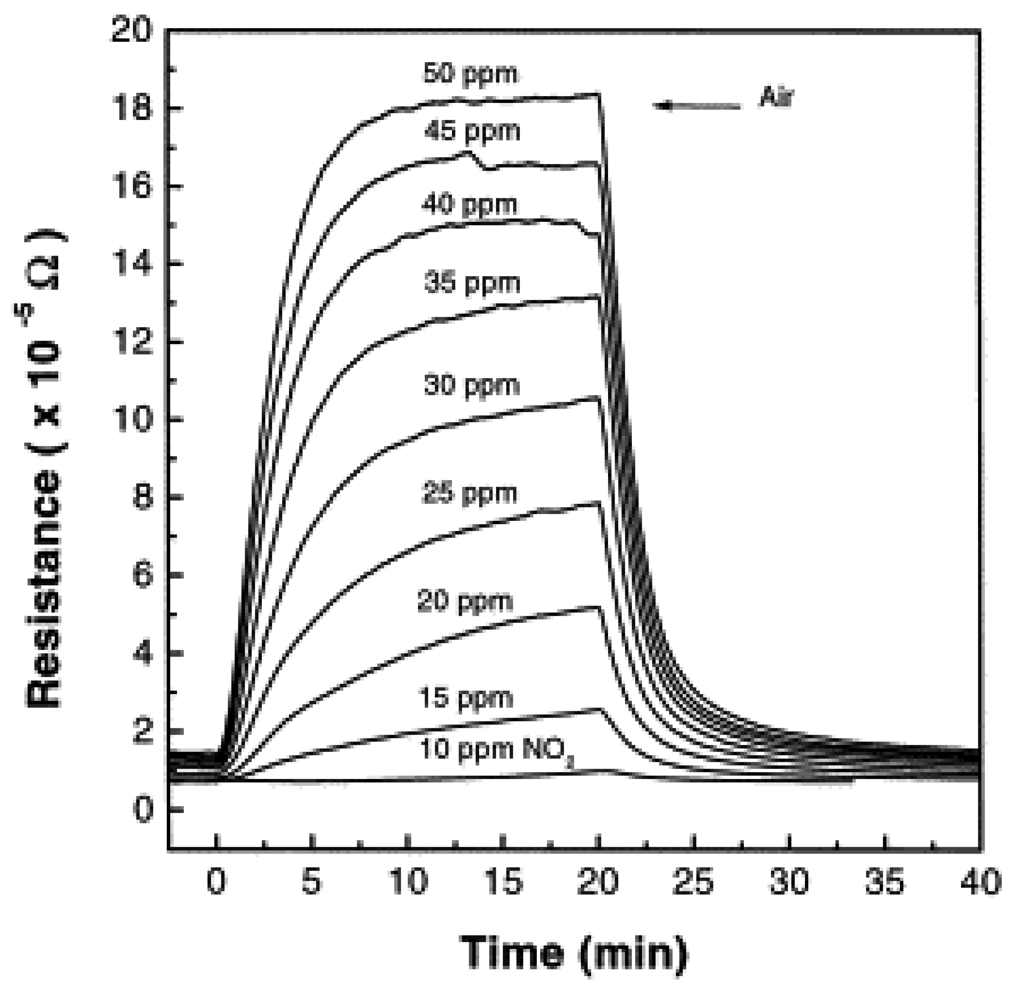
4.1. Nitrogen oxide gases (NOx)
4.2. Sulfur dioxide detection
4.3. H2S detection
4.4. NH3 and amine sensors
4.5. Hydrogen sensors
4.6. Ozone sensors
4.7. Volatile organic compound sensors
4.8. Chemical warfare agents
5. Concluding Remarks
- A Cr2O3-based sensor provided sensitive and selective detection for gaseous ammonia at room temperature [189].
- SnO2 doped silver or platinum provided a unique sensor for H2 detection [192].
- SnO2 doped with basic oxides like La2O3 and CdO provide a unique response toward alcohol in air making it applicable as alcohol breath detectors [210].
- Several SMO based materials have been used to detect sarin-like stimulants (DMMP) in ppb concentration levels but limitations like recovery and selectivity still need to be resolved. Several methods were applied to enhance selectivity including filteration, size selective detection, as well as pattern recognition techniques [13,280,285,293, 300,304].
References
- Brattain, W.H.; Bardeen, J. Surface properties of germanium. Bell. Syst. Tec. J. 1953, 1, 1–41. [Google Scholar]
- Heiland, G. Zum Einfluss von Wasserstoff auf die elektrische leitfähigkeit von ZnO-kristallen. Zeit. Phys. 1954, 138, 459–464. [Google Scholar]
- Bielanski, A.; Deren, J.; Haber, J. Electric conductivity and catalytic activity of semiconducting oxide catalysts. Nature 1957, 179, 668–669. [Google Scholar]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502f. [Google Scholar]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determine our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar]
- Moseley, P.T. Solid state gas sensors. Meas. Sci. Technol. 1997, 8, 223–237. [Google Scholar]
- Sekimoto, S.; Nakagawa, H.; Okazaki, S.; Fukuda, K; Asakura, S.; Shigemori, T.; Takahashi, S.A. Fibre–optic evanescent-wave hydrogen gas sensor using palladium-supported tungsten oxide. Sens. Actuat. B: Chem. 2000, 66, 142–145. [Google Scholar]
- Morazzoni, F.; Scotti, R.; Origoni, L.; D'Arienzo, M; Jimenez, I.; Cornet, A.; Morante, J.R. Mechanism of NH3 interaction with transition metal-added nanosized WO3 for gas sensing: In situ electron paramagnetic resonance study. Catal. Today 2006, 126, 169–176. [Google Scholar]
- Kim, I.J.; Han, S.D; Han, C.H.; Gwak, J.; Hong, D.U.; Jakhar, D; Singh, K.C.; Wang, J.S. Development of micro hydrogen gas sensor with SnO2–Ag2O–PtOx composite using MEMS process. Sens. Actuat. B: Chem. 2007, 127, 441–446. [Google Scholar]
- Albert, K.J.; Lewis, N.S.; Schauer, C.L.; Sotzing, G.A.; Stilzel, S.E.; Vaid, T.P.; Walt, D.R. Cross-reactive chemical sensor arrays. Chem. Rev. 2000, 100, 2595–2626. [Google Scholar]
- Shimizu, Y.; Egashira, M. Basic aspects and challenges of semiconductor gas sensors. MRS Bull. 1999, 24, 18–24. [Google Scholar]
- Martinelli, G.; Carotta, M.C.; Traversa, E.; Ghiotti, G. Thick-film gas sensors based on nano-sized semiconducting oxide powders. MRS Bull. 1999, 24, 30–36. [Google Scholar]
- Tomchenko, A.; Harmer, G.P.; Marquis, B.T. Detection of chemical warfare agents using nanostructured metal oxide sensors. Sens. Actuat. B: Chem. 2005, 108, 41–55. [Google Scholar]
- Tomchenko, A.; Harmer, G.P.; Marquis, B.T.; Allen, J.W. Semiconducting metal oxide sensor array for the selective detection of combustion gases. Sens. Actuat. B: Chem. 2003, 93, 126–134. [Google Scholar]
- Ford, P.C.; Lorkovic, I.M. Mechanistic aspects of the reactions of nitric oxide with Transition-Metal complexes. Chem. Rev. 2002, 102, 993–1018. [Google Scholar]
- Kung, M.C.; Kung, H.H. IR studies of NH3, Pyridine, CO, and NO adsorbed on transition metal oxides. Catal. Rev.-Sci. Eng. 1985, 27, 425–460. [Google Scholar]
- Kutal, C. Spectroscopic and photochemical properties of d10 metal complexes. Coor. Chem. Rev. 1990, 99, 213–252. [Google Scholar]
- Mitchell, M.B.; Sheinker, V.N.; Tesfamichael, A.B.; Gatimu, E.N.; Nunley, M. Decomposition of dimethyl methylphosphonate (DMMP) on supported cerium and iron co-impregnated oxides at room temperature. J. Phys. Chem. B 2003, 107, 580–586. [Google Scholar]
- Mitchell, M.B.; Sheinker, V.N.; Cox, W.W.; Gatimu, E.N.; Tesfamichael, A.B. The room temperature decomposition mechanism of dimethyl methylphosphonate (DMMP) on alumina-supported cerium oxide-participation of nano-sized cerium oxide domains. J. Phys. Chem. B 2004, 108, 1634–1645. [Google Scholar]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar]
- Segal, S.R.; Suib, S.L. Photo-assisted decomposition of dimethyl methylphosphonate over amorphous manganese oxide catalysts. Chem. Mater. 1999, 11, 1687–1695. [Google Scholar]
- Mohamed, A.A.; Mubarak, A.T.; Marestani, Z.M.; Fawy, K.F. Highly sensitive and selective catalytic determination of formaldehyde and acetaldehyde. Talanta 2008, 74, 578–585. [Google Scholar]
- Huang, T.; Lin, X.; Xing, J.; Wang, W.; Shan, Z.; Huang, F. Photocatalytic activities of hetero-junction semiconductors WO3/SrNb2O6. Mater. Sci. Eng. B 2007, 141, 49–54. [Google Scholar]
- Kang, Y.; Wan, B. Gold and iron supported on Y-type zeolite for carbon monoxide oxidation. Catal. Today 1997, 35, 379–392. [Google Scholar]
- Moens, L.; Ruiz, P.; Delmon, B.; Devillers, M. Evaluation of the role played by bismuth molybdates in Bi2Sn2O7–MoO3 catalysts used for partial oxidation of isobutene to methacrolein. Appl. Catal. A: General 1999, 180, 299–315. [Google Scholar]
- Addamo, M.; Augugliaro, V.; Coluccia, S.; Faga, M.; García-López, E.; Loddo, V.; Marcì, G; Martra, G.; Palmisano, L. Photocatalytic oxidation of acetonitrile in gas–solid and liquid–solid regimes. J. Catal. 2005, 235, 209–220. [Google Scholar]
- Kang, M. Methanol conversion on metal-incorporated SAPO-34s (MeAPSO-34s). J. Mol. Catal. A: Chem. 2000, 160, 437–444. [Google Scholar]
- Busca, G. The use of vibrational spectroscopies in studies of heterogeneous catalysis by metal oxides: an introduction. Catal. Today 1996, 27, 323–352. [Google Scholar]
- Dunn, J.P.; Koppula, P.R.; Stenger, H.G.; Wachs, I.E. Oxidation of sulfur dioxide to sulfur trioxide over supported vanadia catalysts. Appl. Catal. B: Environ 1998, 19, 103–117. [Google Scholar]
- Kang, M.; Kim, B; Cho, S.; Chung, C.; Kim, B; Han, G.; Yoon., K. Decomposition of toluene using an atmospheric pressure plasma/TiO2 catalytic system. J. Mol. Catal. A: Chem. 2002, 180, 125–132. [Google Scholar]
- Finlayson, A.P.; Ward, E; Tsaneva, V.N.; Glowacki, B.A. Bi2O3–WO3 compounds for photocatalytic applications by solid state and viscous processing. J. Power Sources 2005, 145, 667–674. [Google Scholar]
- Gesheva, K.; Szekeres, A.; Ivanova, T. Optical properties of chemical vapor deposited thin films of molybdenum and tungsten based metal oxides. Sol. Energy Mater. Sol. Cells 2003, 76, 563–576. [Google Scholar]
- Zhou, J.; Xia, Q.H.; Shen, S.C.; Kawi, S.; Hidajat, K. Catalytic oxidation of pyridine on the supported copper catalysts in the presence of excess oxygen. J. Catal. 2004, 225, 128–137. [Google Scholar]
- Kamegawa, T.; Takeuchi, R; Matsuoka, M.; Anpo, M. Photocatalytic oxidation of CO with various oxidants by Mo oxide species highly dispersed on SiO2 at 293 K. Catal. Today 2006, 111, 248–253. [Google Scholar]
- Kozlova, E.A.; Vorontsov, A.V. Noble metal and sulfuric acid modified TiO2 photocatalysts: Mineralization of organophosphorous compounds. Appl. Catal. B: Environ. 2006, 63, 114–123. [Google Scholar]
- Boronat, M.; Concepcion, P.; Corma, A.; Renz, M. Pecuiarities of Sn-beta and potential industrial applications. Catal. Today 2007, 121, 39–44. [Google Scholar]
- Larrubia, M.A.; Ramis, G.; Busca, G. An FT-IR study of the adsorption and oxidation of N-containing compounds over Fe2O3-TiO2 SCR catalysts. Appl. Catal. B: Environ. 2001, 30, 101–110. [Google Scholar]
- Battisha, I.K. Visible up-conversion photoluminescence from IR diode-pumped SiO2–TiO2 nano-composite films heavily doped with Er3+-Yb3+ and Nd3+-Yb3+. J. Non-Cryst. Solids 2007, 353, 1748–1754. [Google Scholar]
- Cao, L.; Segal, S.R.; Suib, S.L.; Tang, X.; Satyapal, S. Thermocatalytic oxidation of dimethyl methylphosphonate on supported metal oxides. J. Catal. 2000, 194, 61–70. [Google Scholar]
- Segal, S.R.; Cao, L.; Suib, S.L.; Tang, X.; Satyapal, S. Thermal decomposition of dimethyl methylphosphonate over manganese oxide catalysts. J. Catal. 2001, 198, 66–76. [Google Scholar]
- Kanan, S.M.; Abu-Yousef, I,A.; Abdo, N.M. The Photodecomposition of phosmet over UV irradiated silver nanoclusters doped in mordenite zeolite. Appl. Catal. B: Environ. 2007, 74, 130–136. [Google Scholar]
- Waghe, A.; Kanan, S.M.; Abu-Yousef, I.A.; Jensen, B.; Tripp, C.P. Infrared study of UV irradiated tungsten trioxide powders containing adsorbed dimethyl methyl phosphonate and trimethyl phosphate. Res. Chem. Int. 2006, 32, 613–623. [Google Scholar]
- Kanan, S.M.; Kanan, M.C.; Patterson, H.H. Silver nanoclusters doped in X and mordenite zeolites as heterogeneous catalysts for the decomposition of carbamate pesticides in solution. Res. Chem. Int. 2006, 32, 871–885. [Google Scholar]
- Kanan, M.C.; Kanan, S.M.; Patterson, H.H. Luminescence properties of silver(I)-exchanged zeolite Y and its use as a catalyst to photodecompose carbaryl in the presence of natural organic matter. Res. Chem. Intermed. 2003, 29, 691–704. [Google Scholar]
- Kanan, M.C.; Kanan, S.M.; Austin, R.N.; Patterson, H.H. The photodecomposition of carbaryl in the presence of silver-doped zeolite Y and Suwannee River natural organic matter. Environ. Sci. Technol. 2003, 37, 2280–2285. [Google Scholar]
- Kanan, S.M.; Kanan, M.C.; Patterson, H.H. Photophysical properties of Ag(I)-exchanged zeolite A and the photoassisted degradation of malathion. J. Phys. Chem. B 2001, 105, 7508–7516. [Google Scholar]
- Gamburg, Y.D.; Grosheva, M.; Biallozor, S.; Hass, M. The electrochemical deposition of nickel from electrolytes containing malonic acid. Surf. Coat. Technol. 2002, 150, 95–100. [Google Scholar]
- Hammond, J.; Marquis, B.; Michaels, R.; Oickle, B.; Segee, B.; Vetelino, J.; Camire, M.E.; Davis-Dentici, K. A semiconducting metal-oxide array for monitoring fish freshiness. Sens. Actuat. B: Chem. 2002, 84, 113–122. [Google Scholar]
- Lei, H.; Zhu, X.; Sun, Y.; Song, W. Preparation of SrMoO4 thin films on Si substrates by chemical solution deposition. J. Cryst. Growth 2008, 310, 789–793. [Google Scholar]
- Suominen, T.; Raittila, J.; Paturi, P. Pure and fully texturized Sr2FeMoO6 thin films prepared by pulsed laser deposition from target made with citrate-gel method. Thin Solid Films 2009, 517, 5793–5797. [Google Scholar]
- Cui, C.; Bi, J.; Gao, D. A simple chemical method for the deposition of highly crystallized SrMoO4 films. J. Alloys Comp. 2009, 470, L21–L24. [Google Scholar]
- Dai, H.Y.; Wang, B.; Zhang, M.; Wang, R.Z.; Song, X.M.; Du, Y.S.; Yan, H. Growth of La0.7Sr0.3MnO3 films on Si(001) using SrMnO3 template layer. Vacuum 2006, 80, 914–917. [Google Scholar]
- Sugiyama, K.; Hayashi, K; Sasaki, J.; Ichiko, O.; Hashiguchi, Y. Microstructure and wear behaviour of chromium nitride films formed by ion-beam-enhanced deposition. Surf. Coat. Technol. 1994, 66, 505–508. [Google Scholar]
- Willmott, P.R. Deposition of complex multielemental thin films. Prog. Surf. Sci. 2004, 76, 163–217. [Google Scholar]
- Pereira, A.; Cultrera, L.; Dima, A.; Susu, M.; Perrone, A.; Du, H.L.; Volkov, A.O.; Cutting, R.; Datta, P.K. Pulsed laser deposition and characterization of textured Pd-doped-SnO2 thin films for gas sensing applications. Thin Solid Films 2006, 497, 142–148. [Google Scholar]
- Zhao, H.; Levi, C.G.; Wadley, H.N. Vapor deposited samarium zirconate thermal barrier coatings. Surf. Coat. Technol. 2009, 203, 3157–3167. [Google Scholar]
- Bonnet, G.; Lachkar, M.; Colson, J.C.; Larpin, J.P. Characterization of thin solid films of rare earth oxides formed by the metallo-organic chemical vapour deposition technique, for high temperature corrosion applications. Thin Solid Films 1995, 261, 31–36. [Google Scholar]
- Bernhardt, G.; Silvestre, C.; LeCursi, N.; Moulzolf, S.C.; Frankel, D.J.; Lad, R.J. Performance of Zr and Ti adhesion layers for bonding of platinum metallization to sapphire substrates. Sens. Actuat. B: Chem. 2001, 77, 368–374. [Google Scholar]
- Atanasov, P.A.; Tomov, R.I.; Serbezov, V.S. Plasma assisted in situ laser deposition of Y1Ba2Cu3O7−x superconducting thin films with laser heating and annealing. Vacuum 1994, 45, 1215–1219. [Google Scholar]
- Archer, N.J. Vapour deposition of wear-resistant surfaces. Tribol. Int. 1978, 11, 135–138. [Google Scholar]
- Moseley, P.T.; Norris, J.O.; Williams, D.E. (Eds.) Techniques and Mechanisms in Gas Sensing; Adam Hilger: Bristol, UK, 1991.
- Madou, M.J.; Morrison, S.R. Chemical Sensing with Solid State Devices; Academic Press, Inc./Harcourt Brace Jovanovich Publ.: Boston, MA, USA, 1987. [Google Scholar]
- Gas Sensors-Principles Operation and Developmens; Sberveglieri, G. (Ed.) Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992.
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: state of the art and approaches. Sens. Actuat. B: Chem. 2005, 107, 209–232. [Google Scholar]
- Lu, Z.; Kanan, S.M.; Tripp, C.P. Synthesis of high surface area monoclinic WO3 particles using organic ligands and emulsion based methods. J. Mater. Chem. 2002, 12, 983–989. [Google Scholar]
- Williams, D. Semiconducting oxides as gas-sensitive resistors. Sens. Actuat. B: Chem. 1999, 57, 1–16. [Google Scholar]
- Kupriyanov, L.Y. (Ed.) Semiconductor Sensors in Physico-Chemical Studies; Elsevier: Amsterdam, The Netherlands, 1996.
- Morrison, S.R. Mechanism of semiconductor gas sensor operation. Sens. Actuat. 1987, 11, 283–287. [Google Scholar]
- Van de Krol, R.; Tuller, H.L. Electroceramics-the role of interfaces. Solid State Ionics 2002, 150, 167–179. [Google Scholar]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia. 2003, 7, 63–75. [Google Scholar]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuat. B: Chem. 2005, 108, 2–14. [Google Scholar]
- Henrich, V.E.; Cox, P.A. The Surface Science of Metal Oxides; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Cox, P.A. Transition Metal Oxides: An Introduction to their Electronic Structure and Properties; Clarendon Press: Oxford, UK, 1992. [Google Scholar]
- Hamnett, A.; Goodenough, J.B.O. Semiconductors, Group III: Crystal and Solid State Physics; Springer-Verlag: Berlin, Germany, 1984; Volume 17. [Google Scholar]
- Krilov, O.V.; Kisilev, V.F. Adsorption and Catalysis on the Transition Metals and their Oxides; Springer-Verlag: Berlin: Heidelberg, 1989. [Google Scholar]
- Samsonov, G.V. The Oxide Handbook; IFI/Plenum: New York, NY, USA, 1973. [Google Scholar]
- Boakye, F.; Nusenu, D. The energy band gap of cadmium sulphide. Solis State Commun. 1997, 102, 323–326. [Google Scholar]
- Ueda, M.; Ohtsuka, T. Luminescence from band-gap photo-excitation of titanium anodic oxide films. Corr. Sci. 2002, 44, 1633–1638. [Google Scholar]
- Dorenbos, P. The Eu3+ charge transfer energy and the relation with the band gap of compounds. J. Luminescence 2005, 111, 89–104. [Google Scholar]
- Kumar, V.; Sharma, S.K.; Sharma, T.P. ; Singh. V. Band gap determination in thick films from reflectance measurements. Opt. Mater. 1999, 12, 115–119. [Google Scholar]
- Gupta, L.; Mansingh, A.; Srivastava, P.K. Band gap narrowing and the band structure of tin-doped indium oxide films. Thin Solid Films 1989, 176, 33–44. [Google Scholar]
- Palankovski, V.; Kaiblinger-Grujin, G.; Selberherr, S. Study of dopant-dependent band gap narrowing in compound semiconductor devices. Mater. Sci. Eng. B 1999, 66, 46–49. [Google Scholar]
- Yang, J.; Kim, W.S.; Park, H. Chemical bonding states and energy band gap of SiO2-incorporated La2O3 films on n-GaAs (001). Thin Solid Films 2006, 494, 311–314. [Google Scholar]
- Siddiqi, S.A.; Masih, M.; Mateen, A. Optical band gap in Cd-Mn-phosphate glasses. Mater. Chem. Phys. 1995, 40, 69–72. [Google Scholar]
- Moseley, P.T.; Tofield, B.C. (Eds.) Solid State Gas Sensors; Adam Hilger: Bristol, U.K. and Philadelphia, PA, USA, 1987.
- Mandelis, A.; Christofides, C. (Eds.) Physics, Chemistry and Technology of Solid State Gas Sensor Devices; Wiley: Hoboken, NJ, USA, 1993.
- Schierbaum, K.D. Engineering of oxide surfaces and metal/oxide interfaces for chemical sensors: recent trends. Sens. Actuat. B: Chem. 1995, 24, 239–247. [Google Scholar]
- Kappler, J.; Tomescu, A.; Barsan, N.; Weimar, U. CO consumption of Pd doped SnO2 based sensors. Thin Solid Films 2001, 391, 186–191. [Google Scholar]
- Sayago, I.; Santos, H.; Horrillo, M.C.; Aleixandere, M.; Fernandez, M.J.; Terrrado, E.; Tacchini, I.; Aroz, R.; Maser, W.K.; Benito, A.M.; Martinez, M.T.; Gutierrez, J.; Munoz, E. Carbon nanotube networks as gas sensors for NO2 detection. Talanta 2008, 77, 758–764. [Google Scholar]
- Ueda, T.; Katsuki, K.; Narges, H.A.; Ikegami, T.; Mitsugi, F. Fabrication and characterization of carbon nanotube based high sensitive gas sensors operable at room temperature. Diamond Rel. Mater. 2008, 17, 1586–1589. [Google Scholar]
- Penza, M.; Rossi, R.; Alvisi, M.; Cassano, G.; Signore, M.A.; Serra, E.; Giorgi, R. Pt- and Pd-nanoclusters functionalized carbon nanotubes networked films for sub-ppm gas sensors. Sens. Actuat. B: Chem. 2008, 135, 289–297. [Google Scholar]
- Balázsi, C.; Sedlácková, K.; Llobet, E.; Ionescu, R. Novel hexagonal WO3 nanopowder with metal decorated carbon nanotubes as NO2 gas sensor. Sens. Actuat. B: Chem. 2008, 133, 151–155. [Google Scholar]
- Pnozoni, A.; Comini, E.; Ferroni, M.; Sberveglieri, G. Nanostructured WO3 deposited by modified thermal evaporation for gas-sensing applications. Thin Solid Films 2005, 490, 81–85. [Google Scholar]
- Boulova, M.; Gaskov, A.; Lucazeau, G. Tungsten oxide reactivity versus CH4, CO and NO2 molecules studied by Raman spectroscopy. Sens. Actuat. B: Chem. 2001, 81, 99–106. [Google Scholar]
- Tamaki, J.; Miyaji, A.; Makinodan, J.; Ogura, S.; Konishi, S. Effect of micro-gap electrode on detection of dilute NO2 using WO3 thin film microsensors. Sens. Actuat. B: Chem. 2005, 108, 202–206. [Google Scholar]
- Vallejos, S.; Khatko, V.; Calderer, J.; Gracia, I.; Cané, C.; Llobet, E.; Correig, X. Micro-machined WO3-based sensors selective to oxidizing gases. Sens. Actuat. B: Chem. 2008, 132, 209–215. [Google Scholar]
- Siciliano, T.; Tepore, A.; Micocci, G.; Serra, A.; Manno, D.; Filippo, E. WO3 gas sensors prepared by thermal oxidization of tungsten. Sens. Actuat. B: Chem. 2008, 133, 321–326. [Google Scholar]
- Jiun-Chan, Y.; Prabir, K.D. Solution-based synthesis of efficient WO3 sensing electrodes for high temperature potentiometric NOx sensors. Sens. Actuat. B: Chem. 2009, 136, 523–529. [Google Scholar]
- Ferroni, M.; Boscarino, D.; Comini, E.; Gnani, D.; Guidi, V.; Martinelli, G.; Nelli, P.; Rigato, V.; Sberveglieri, G. Nanosized thin films of tungsten-titanium mixed oxides as gas sensors. Sens. Actuat. B: Chem. 1999, 58, 289–294. [Google Scholar]
- Guidi, V.; Boscarino, D.; Comini, E.; Faglia, G.; Ferroni, M.; Malagù, C.; Martinelli, G.; Rigato, V.; Sberveglieri, G. Preparation and characterisation of titanium–tungsten sensors. Sens. Actuat. B: Chem. 2000, 65, 264–266. [Google Scholar]
- Comini, E.; Ferroni, M.; Guidi, V.; Faglia, G.; Martinelli, G.; Sberveglieri, G. Nanostructured mixed oxides compounds for gas sensing applications. Sens. Actuat. B: Chem. 2002, 84, 26–32. [Google Scholar]
- Penza, M.; Martucci, C.; Cassano, G. NOx gas sensing characteristics of WO3 thin films activated by noble metals (Pd, Pt, Au) layers. Sens. Actuat. B: Chem. 1998, 50, 52–59. [Google Scholar]
- Marquis, B.T.; Vetelino, J.F. A semiconducting metal oxide sensor array for the detection of NOx and NH3. Sens. Actuat. B: Chem. 2001, 65, 100–110. [Google Scholar]
- Kawasaki, H.; Ueda, T.; Suda, Y.; Ohshima, T. Properties of metal doped tungsten oxide thin films for NOx gas sensors grown by PLD method combined with sputtering process. Sens. Actuat. B: Chem. 2004, 100, 266–269. [Google Scholar]
- Reyes, L.F.; Hoel, A.; Saukko, S.; Heszler, P.; Lantto, V.; Granqvist, C.G. Gas sensor response of pure and activated WO3 nanoparticle films made by advanced reactive gas deposition. Sens. Actuat. B: Chem. 2006, 117, 128–134. [Google Scholar]
- Xia, H.J.; Wang, Y.; Kong, F.H.; Wang, S.R.; Zhu, B.L.; Guo, X.Z.; Zhang, J.; Wang, Y.M.; Wu, S.H. Au-doped WO3-based sensor for NO2 detection at low operating temperature. Sens. Actuat. B: Chem. 2008, 134, 133–139. [Google Scholar]
- Filippini, D.; Fraigi, L.; Aragón, R.; Weimar, U. Thick film Au-gate field-effect devices sensitive to NO2. Sens. Actuat. B: Chem. 2002, 81, 296–300. [Google Scholar]
- Yang, J.; Dutta, P.K. Promoting selectivity and sensitivity for a high temperature YSZ-based electrochemical total NOx sensor by using a Pt-loaded zeolite Y filter. Sens. Actuat. B: Chem. 2007, 125, 30–39. [Google Scholar]
- Tomchenko, A.A. Structure and gas-sensitive properties of WO3–Bi2O3 mixed thick films. Sens. Actuat. B: Chem. 2000, 68, 48–52. [Google Scholar]
- Khatko, V.; Llobet, E.; Vilanova, X.; Brezmes, J.; Hubalek, J.; Malysz, K.; Correig, X. Gas sensing properties of nanoparticle indium-doped WO3 thick films. Sens. Actuat. B: Chem. 2005. [Google Scholar]
- Xu, P.C.; Cheng, Z,X.; Pan, Q.Y.; Xu, J.Q.; Xiang, Q.; Yu, W.J.; Chu, Y.L. High aspect ratio In2O3 nanowires: Synthesis, mechanism and NO2 gas-sensing properties. Sens. Actuat. B: Chem. 2008, 130, 802–808. [Google Scholar]
- Ali, M.; Wang, C.Y.; Röhlig, C.C.; Cimalla, V.; Stauden, Th.; Ambacher, O. NOx sensing properties of In2O3 thin films grown by MOCVD. Sens. Actuat. B: Chem. 2008, 129, 467–472. [Google Scholar]
- Steffes, H.; Imawan, C.; Solzbacher, F.; Obermeier, E. Enhancement of NO2 sensing properties of In2O3-based thin films using an Au or Ti surface modification. Sens. Actuat. B: Chem. 2001, 78, 106–112. [Google Scholar]
- Cantalini, C.; Wlodarski, W.; Sun, H.T.; Atashbar, M.Z.; Passacantando, M.; Santucci, S. NO2 response of In2O3 thin film gas sensors prepared by sol–gel and vacuum thermal evaporation techniques. Sens. Actuat. B: Chem. 2000, 65, 101–104. [Google Scholar]
- Sber veglieri, G.; Benussi, P.; Coccoli, G.; Groppelli, S.; Nelli, P. Reactivity sputtered indium tin oxide polycrystalline thin films as NO and NO2 gas sensors. Thin Solid Films 1990, 186, 349–360. [Google Scholar]
- Sayago, I.; Gutiérrez, J.; Arés, L.; Robla, J.I.; Horillo, M.C.; Getino, J.; Rino, J.; Agapito, J.A. The effect of additives in tin oxide on the sensitivity and selectivity to NOx and CO. Sens. Actuat. B. Chem. 1995, 26/27, 19–23. [Google Scholar]
- Barbi, G.B.; Blanco, J.S. Structure of tin oxide layers and operating temperature as factors determining the sensitivity to NOx. Sens. Actuat. B 1993, 15/16, 372–378. [Google Scholar]
- Tanaka, K.; Morimoto, S.; Sonoda, S.; Matsuura, S.; Moriya, K.; Egashira, M. Combustion monitoring sensor using tin dioxide semiconductor. Sens. Actuat. B. Chem. 1991, 3, 247–253. [Google Scholar]
- Williams, G.; Coles, G.S. NOx response of tin dioxide based gas sensors. Sens. Actuat. B. Chem. 1993, 16, 349–353. [Google Scholar]
- Gutierrez, F.J.; Arés, L.; Robla, J.I.; Horillo, M.C.; Sayago, I.; Getino, J.M.; Agapito, J.A. NOx tin dioxide sensor activities as a function of doped materials and temperature. Sens. Actuat. B. Chem. 1993, 15/16, 354–356. [Google Scholar]
- Sberveglieri, S.; Faglia, G.; Groppelli, S.; Nelli, P. Methods for the preparation of NO, NO2 and H2 sensors based on tine oxide thin films grown by means of RF magnetron sputtering techniques. Sens. Actuat. B. Chem. 1992, 8, 79–88. [Google Scholar]
- Satake, K.; Kobayashi, A.; Inoue, T.; Nakahara, T.; Takeuchi, T. NOx sensors for exhaust monitoring. Proceedings of the Third International Meeting on Chem. Sensors, Cleveland, OH, USA, September 24–26, 1990; pp. 334–337.
- Wiegleb, G.; Heitbaum, J. Semiconductor gas sensor for detecting NO and CO traces in ambient air of road traffic. Sens. Actuat. B. Chem. 1994, 17, 93–99. [Google Scholar]
- DiNatale, C.; D'Amico, A.; Davide, F.A.; Faglia, G.; Nelli, P.; Sberveglieri, G. Performance evalation of an SnO2-based sensor array for the quantitative measurement of mixtures of H2S and NO2. Sens. Actuat. B. Chem. 1994, 20, 217–224. [Google Scholar]
- Sayago, I.; Gutiérrez, J.; Arés, L.; Robla, J.I.; Horillo, M.C.; Getino, J.; Rino, J.; Agapito, J.A. Long-term reliability of sensors for detection of nitrogen dioxides. Sens. Actuat. B. Chem. 1995, 26/27, 56–58. [Google Scholar]
- Kaur, J.; Roy, S.C.; Bhatnagar, M.C. Highly sensitive SnO2 thin film NO2 gas sensor operating at low temperature. Sens. Actuat. B: Chem. 2007, 123, 1090–1095. [Google Scholar]
- Yongki, M.; Harry, L.,T.; Stefan, P.; Jürgen, W.; Harald, B. Gas response of reactively sputtered ZnO films on Si-based micro-array. Sens. Actuat. B: Chem. 2003, 93, 435–441. [Google Scholar]
- Shalaka, N.; Ravi, V.; Srinivas, D.; Mulla, I.S.; Gosavi, S.W.; Kulkarni, S.K. EPR and DRS evidence for NO2 sensing in Al-doped ZnO. Sens. Actuat. B: Chem. 2008, 130, 668–673. [Google Scholar]
- Nanto, H.; Minami, T.; Takata, S. Ammonia gas sensor using sputtered zinc oxide thin film. Proceedings of the 5th Sensor Symposium; Institute of Electrical Engineering of Japan: Tokyo, Japan, 1985; pp. 191–194. [Google Scholar]
- Arya, S.P.; D'Amico, A.; Verona, E. Study of sputtered ZnO–Pd thin films as solid state H2 and NH3 gas sensors. Thin Solid Films 1988, 157, 169–174. [Google Scholar]
- Nanto, H.; Sokooshi, H.; Usuda, T. Smell sensor using aluminum-doped zinc oxide thin film prepared by sputtering technique. Sens. Actuat. B. Chem. 1993, 10, 79–83. [Google Scholar]
- Sberveglieri, G.; Groppelli, S.; Nelli, P. A novel method for the preparation of ZnO–In thin films for selective NH3 detection. Proceedings of the 5th International Meeting on Chemical Sensors, Rome, Italy; 1994; pp. 748–751. [Google Scholar]
- Nanto, H.; Sokooshi, H.; Kawai, T.; Usuda, T. Zinc-oxide thin-film trimethylamine sensor with high sensitivity and excellent selectivity. J. Mater. Sci. Lett. 1992, 11, 235–237. [Google Scholar]
- Nanto, H.; Minami, T.; Takata, S. Zinc-oxide thin-film ammonia gas sensors with high sensititivity and excellent selectivity. J. Appl. Phys. 1986, 60, 482–484. [Google Scholar]
- Siciliano, T.; Di Giulio, M.; Tepore, M.; Filippo, E.; Micocci, G.; Tepore, A. Tellurium sputtered thin films as NO2 gas sensors. Sens. Actuat. B: Chem. 2008, 135, 250–254. [Google Scholar]
- Siciliano, T.; Giulio, M.D.; Tepore, M.; Filippo, E.; Micocci, G.; Tepore, A. Room temperature NO2 sensing properties of reactivity sputtered TeO2 thin films. Sens. Actuat. B: Chem. 2009, 137, 644–648. [Google Scholar]
- Barazzouk, S.; Tandon, R.P.; Hotchandani, S. MoO3-based sensor for NO, NO2 and CH4 detection. Sens. Actuat. B: Chem. 2006, 119, 691–694. [Google Scholar]
- Brunet, J.; Parra Garcia, V.; Pauly, A.; Varenne, C.; Lauron, B. An optimised gas sensor microsystem for accurate and real-time measurement of nitrogen dioxide at ppb level. Sens. Actuat. B: Chem. 2008, 134, 632–639. [Google Scholar]
- Leo, G.; Rella, R.; Siciliano, P.; Capone, S.; Alonso, J.C.; Pankov, V.; Ortiz, A. Sprayed SnO2 thin films for NO2 sensors. Sens. Actuat. B: Chem. 1999, 58, 370–374. [Google Scholar]
- Girardin, D.; Berger, F.; Chambaudet, A.; Planade, R. Modelling of SO2 detection by tin dioxide gas sensors. Sens. Actuat. B: Chem. 1997, 43, 147–153. [Google Scholar]
- Do, J.S.; Chen, P.J. Amperometric sensor array for NOx, CO, O2 and SO2 detection. Sens. Actuat. B: Chem. 2007, 122, 165–173. [Google Scholar]
- Wang, L.; Kumar, R.V. A new SO2 gas sensor based on an Mg2+ conducting solid electrolyte. J. Electroanal. Chem. 2003, 543, 109–114. [Google Scholar]
- Li, H.; Wang, Q.; Xu, J.; Zhang, W.; Jin, L. A novel nano-Au-assembled amperometric SO2 gas sensor: preparation, characterization and sensing behavior. Sens. Actuat. B. Chem. 2002, 87, 18–24. [Google Scholar]
- Min, B.; Choi, S. SO2-sensing characteristics of Nasicon sensors with Na2SO4–BaSO4 sensing electrolytes. Sens. Actuat. B. Chem. 2003, 93, 209–213. [Google Scholar]
- Wang, L.; Kumar, R.V. A SO2 gas sensor based upon composite Nasicon/Sr-β-Al2O3 bielectrolyte. Mater. Res. Bull. 2005, 40, 1802–1815. [Google Scholar]
- Liang, X.H.; Zhong, T.G.; Quan, B.F.; Wang, B.; Guan, H.S. Solid-state potentiometric SO2 sensor combining NASICON with V2O5-doped TiO2 electrode. Sens. Actuat. B: Chem. 2008, 134, 25–30. [Google Scholar]
- Matsuguchi, M.; Tamai, K.; Sakai, Y. SO2 gas sensors using polymers with different amino groups. Sens. Actuat. B: Chem. 2001, 77, 363–367. [Google Scholar]
- Dultsev, F.N.; Sveshnikova, L.L. The use of the substituted imidazoline radical as a receptor for sulphur dioxide gas sensor. Sens. Actuat. B: Chem. 2007, 120, 434–438. [Google Scholar]
- Endres, H.E.; Drost, S.; Hutter, F. Impedance spectroscopy on dielectric gas sensors. Sens. Actuat. B. Chem. 1994, 22, 7–11. [Google Scholar]
- Pribil, R.; Bilkova, E. The use of a piezoelectric crystal to determine sulphur dioxide in gases. Talanta 1992, 39, 361–366. [Google Scholar]
- Agbor, N.E.; Petty, M.C.; Monkman, A.P. Polyaniline thin films for gas sensing. Sens. Actuat. B. Chem. 1995, 28, 173–179. [Google Scholar]
- Ranucci, E.; Putelli, L.; Ferruti, P.; Ferrari, V.; Marioli, D.; Taroni, A. Use of poly(amidoamines) as CO2- and SO2-sensitive material for gravimetric sensors. Mikrochim. Acta 1995, 120, 257–270. [Google Scholar]
- Benmarkroha, F.; Boudjerda, T.; Boufenar, R.; Allag, H.; Djerboua, F.; McCallum, J.J. Monitoring of sulfur dioxide using a piezoelectric crystal based controller. Analyst 1993, 118, 401–406. [Google Scholar]
- Berger, F.; Fromm, M.; Chambaudet, A.; Planade, R. Tin dioxide-based gas sensors for SO2 detection: a chemical interpretation of the increase in sensitivity obtained after a primary detection. Sens. Actuat. B: Chem. 1997, 45, 175–181. [Google Scholar]
- Torvela, H.; Huusko, J.; Lantto, V. Reduction of the interference caused by NO and SO2 in the CO response of Pd-catalysed SnO2 combustion gas sensors. Sens. Actuat. B: Chem. 1991, 4, 479–484. [Google Scholar]
- Shimizu, Y.; Matsunaga, N.; Hyodo, T.; Egashira, M. Improvement of SO2 sensing properties of WO3 by noble metal loading. Sens. Actuat. B: Chem. 2001, 77, 35–40. [Google Scholar]
- Stankova, M.; Vilanova, X.; Calderer, J.; Llobet, E.; Ivanov, P.; Gràcia, I.; Cané, C.; Correig, X. Detection of SO2 and H2S in CO2 stream by means of WO3-based micro-hotplate sensors. Sens. Actuat. B: Chem. 2004, 102, 219–225. [Google Scholar]
- Penza, M.; Cassano, G.; Tortorella, F. Gas recognition by activated WO3 thin-film sensors array. Sens. Actuat. B: Chem. 2001, 81, 115–121. [Google Scholar]
- Lin, H.; Hsu, C.; Yang, H.; Lee, P.; Yang, C. Nanocrystalline WO3-based H2S sensors. Sens. Actuat. B: Chem. 1994, 22, 63–68. [Google Scholar]
- Frühberger, B.; Grunze, M.; Dwyer, D.J. Surface chemistry of H2S-sensetive tungsten oxide films. Sens. Actuat. B: Chem. 1996, 31, 167–174. [Google Scholar]
- Ruokamo, I.; Kärkkäinen, T.; Huusko, J.; Ruokanen, T.; Blomberg, M.; Torvela, H.; Lantto, V. H2S response of WO3 thin-film sensors manufactured bysilicon processing technology. Sens. Actuat. B: Chem. 1994, 18–19, 486–488. [Google Scholar]
- Vangrunderbeek, J.; Vandecruys, F.; Kumar, R.V. Sensing mechanism of high temperature hydrogen sulphide sensor based on sodium β-alumina. Sens. Actuat. B: Chem. 1999, 56, 129–135. [Google Scholar]
- Solis, J.L.; Saukko, S.; Kish, L.B.; Granqvist, C.G.; Lantto, V. Nanocrystalline tungsten oxide thick-films with high sensitivity to H2S at room temperature. Sens. Actuat. B: Chem. 2001, 77, 316–321. [Google Scholar]
- Tao, W.H.; Tsai, C.H. H2S sensing properties of noble metal doped WO3 thin film sensor fabricated by micromachining. Sens. Actuat. B: Chem. 2002, 81, 237–247. [Google Scholar]
- Sekhar, C.R.; Manu, H.; Rao, C.N. H2S sensors based on tungsten oxide nanostructures. Sens. Actuat. B: Chem. 2008, 128, 488–493. [Google Scholar]
- Vuong, D.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Hydrogen sulfide gas sensing properties of thin films derived from SnO2 sols different in grain size. Sens. Actuat. B: Chem. 2005, 105, 437–442. [Google Scholar]
- Kim, S; Park, M.; Kim, H. Systematic approach for the evaluation of the optimal fabrication conditions of a H2S gas sensor with Taguchi method. Sens. Actuat. B: Chem. 2004, 102, 253–260. [Google Scholar]
- Gong, J; Chen, Q.; Lian, M.; Liu, N.; Stevenson, R.G.; Adami, F. Micromachined nanocrystalline silver doped SnO2 H2S sensor. Sens. Actuat. B: Chem. 2006, 114, 32–39. [Google Scholar]
- Liang, X.; He, Y.; Liu, F.; Wang, B.; Zhong, T.; Quan, B.; Lu, G. Solid-state potentiometric H2S sensor combining NASICON with Pr6O11- doped SnO2 electrode. Sens. Actuat. B: Chem. 2007, 125, 544–549. [Google Scholar]
- Ghimbeu, C.M.; Lumbreras, M.; Siadat., M.; Van Landschoot, R.C.; Schoonman, J. Electrostatic sprayed SnO2 and Cu-doped SnO2 films for H2S detection. Sens. Actuat. B: Chem. 2008, 133, 694–698. [Google Scholar]
- Gong, S.; Jing, X.; Jianqiao, L.; Dongxiang, Z. Highly sensitive SnO2 thin film with low operating temperature prepared by sol-gel technique. Sens. Actuat. B: Chem. 2008, 134, 57–61. [Google Scholar]
- Wong, C.; Chu, X.; Wu, M. Detection of H2S down to ppb levels at room temperature using sensors based on ZnO nanorods. Sens. Actuat. B: Chem. 2006, 113, 320–323. [Google Scholar]
- Liu, Z.; Fan, T.; Zhang, D.; Gong, X.; Xu, J. Hierachically porous ZnO with high sensitivity and selectivity to H2S derived from biotemplates. Sens. Actuat. B: Chem. 2009, 136, 499–509. [Google Scholar]
- Wallace, K.J.; Cordero, S.R.; Tan, C.P.; Lynch, V.M.; Anslyn, E.V. A colorimetric response to hydrogen sulphide. Sens. Actuat. B: Chem. 2007, 120, 362–367. [Google Scholar]
- Jain, G.H.; Patil, L.A. CuO-doped BSST thick film resistors for ppb level H2S gas sensing at room temperature. Sens. Actuat. B: Chem. 2007, 123, 246–253. [Google Scholar]
- Wang, Y.; Wang, S.; Zhao, Y.; Zhu, B.; Kong, F.; Wang, D.; Wu, S.; Huang, W.; Zhang, S. H2S sensing characterisitic of Pt-doped α-Fe2O3 thick film sensors. Sens. Actuat. B: Chem. 2007, 125, 79–84. [Google Scholar]
- Kapse, V.D.; Ghosh, S.A.; Raghuwanshi, F.C.; Kapse, S.D. Enhanced H2S sensing characteristics of La- doped In2O3: Effect of Pd sensitization. Sens. Actuat. B: Chem. 2009, 137, 681–686. [Google Scholar]
- Kaur, M.; Jain, N.; Sharma, K.; Bhattacharya, S.; Roy, M.; Tyagi, A.K.; Gupta, S.K.; Yakhmi, J.V. Room-temperature H2S gas sensing at ppb level by single crystal In2O3 whiskers. Sens. Actuat. B: Chem. 2008, 133, 456–461. [Google Scholar]
- Yang, W.; Wang, Y.; Cao, J.; Kong, F.; Xia, H.; Zhang, J.; Zhu, B.; Wang, S.; Wu, S. Low-Temperature H2S sensors based on Ag-doped α- Fe2O3 nanoparticles. Sens. Actuat. B: Chem. 2008, 131, 183–189. [Google Scholar]
- Fam, D.; Tok, A.I.; Palaniappan, A.; Nopphawan, P.; Anup, L.; Mhaisalkar, S.G. Selective sensing of hydrogen sulphide using silver nanoparticle decorated carbon nanotubes. Sens. Actuat. B: Chem. 2009, 138, 189–192. [Google Scholar]
- Jain, G.H.; Patil, L.A.; Wagh, M.S.; Patil, D.R.; Patil, S.A.; Amalnerkar, D.P. Surface modified BaTiO3 thick film resistors as H2S gas sensors. Sens. Actuat. B: Chem. 2006, 117, 159–165. [Google Scholar]
- Sarma, T.; Tao, S. An active core fiber optic sensor for detecting trace H2S at high temperature using a cadmium oxide doped porous silica optical fiber as a transducer. Sens. Actuat. B: Chem. 2007, 127, 471–479. [Google Scholar]
- Sen, S.; Bhandarkar, V.; Muthe, K.P.; Roy, M.; Deshpande, S.K.; Aiyer, R.C.; Gupta, S.K.; Yakhmi, J.V.; Sahni, V.C. Highly sensitive hydrogen sulphide sensors operable at room temperature. Sens. Actuat. B: Chem. 2006, 115, 270–275. [Google Scholar]
- Tong, M.; Dai, G.; Gao, D. WO3 thin film sensor prepared by sol-gel technique and its low-temperature sensing properties to trimethlyamine. Mater. Chem. Phys. 2001, 69, 176–179. [Google Scholar]
- Bendahan, M.; Lauque, P.; Seguin, J.; Aguir, K.; Knauth, P. Development of an ammonia gas sensor. Sens. Actuat. B: Chem. 2003, 95, 170–176. [Google Scholar]
- Wagh, M.S.; Jain, G.H.; Patil, D.R.; Patil, S.A.; Patil, L.A. Modified zinc oxide thick film resistors as NH3 gas sensor. Sens. Actuat. B: Chem. 2006, 115, 128–133. [Google Scholar]
- Wang, Y.D.; Wu, X.; Su, Q.; Li, Y.; Zhou, Z. Ammonia-sensing characteristics of Pt and SiO2 doped SnO2 materials. Solid-State Electron. 2001, 45, 347–350. [Google Scholar]
- Suryawanshi, D.N.; Patil, D.R.; Patil, L.A. Fe2O3-activated Cr2O3 thick films as temperature dependent gas sensors. Sens. Actuat. B: Chem. 2008, 134, 579–584. [Google Scholar]
- Patil, D.R.; Patil, L.A.; Patil, P.P. Cr2O3-activated ZnO thick film resistors for ammonia gas sensing operable at room temperature. Sens. Actuat. B: Chem. 2007, 126, 368–374. [Google Scholar]
- Petrov, V.V.; Nazarova, T.N.; Korolev, A.N.; Kopilova, N.F. Thin sol-gel SiO2-SnOx-AgOy films for low temperature ammonia gas sensor. Sens. Actuat. B: Chem. 2008, 133, 291–295. [Google Scholar]
- Gong, J.W.; Chen, Q.F.; Fei, W.F.; Seal, S. Micromachined nanocrystalline SnO2 chemical gas sensors for electronic nose. Sens. Actuat. B: Chem. 2004, 102, 117–125. [Google Scholar]
- Li, J.G.; Kawi, S. Synthesis, characterization and sensing application of novel semiconductor oxides. Talanta 1998, 45, 759–766. [Google Scholar]
- Gong, J.W.; Sun, J.R.; Chen, Q.F. Micromachined sol-gel carbon nanotube/SnO2 nanocomposite hydrogen sensor. Sens. Actuat. B: Chem. 2008, 130, 829–835. [Google Scholar]
- Sekimoto, S.; Nakagawa, H.; Okazaki, S.; Fukuda, K.; Asakura, S.; Shigemori, T.; Takahashi, S. A fibre–optic evanescent-wave hydrogen gas sensor using palladium-supported tungsten oxide. Sens. Actuat. B: Chem. 2000, 66, 142–145. [Google Scholar]
- Scientific Facts on Air Pollution Ozone. Available online: http://www.greenfacts.org/en/ozone-o3/index.htm (accessed September 20, 2009).
- Aguir, K.; Lemire, C.; Lollman, D.B. Electrical properties of reactively sputtered WO3 thin films as ozone gas sensor. Sens. Actuat. B: Chem. 2002, 84, 1–5. [Google Scholar]
- Guerin, J.; Aguir, K.; Bendahan, M.; Lambert-Mauriat, C. Thermal modelling of a WO3 ozone sensor response. Sens. Actuat. B: Chem. 2005, 104, 289–293. [Google Scholar]
- Utembe, S.R.; Hansford, G.M.; Sanderson, M.G.; Freshwater, R.A.; Pratt, K.F.; Williams, D.E.; Cox, R.A.; Jones, R.L. An ozone monitoring instrument based on the tungsten trioxide (WO3) semiconductor. Sens. Actuat. B: Chem. 2006, 114, 507–512. [Google Scholar]
- Labidi, A.; Gillet, E.; Delamare, R.; Maaref, M.; Aguir, K. Ethanol and ozone sensing characteristics of WO3 based sensors activated by Au and Pd. Sens. Actuat. B: Chem. 2006, 120, 338–345. [Google Scholar]
- Boulmani, R.; Bendahan, M.; Lambert-Mauriate, C.; Gillet, M.; Aguir, K. Correlation between rf-sputtering parameters and WO3 sensor response towards ozone. Sens. Actuat. B: Chem. 2007, 125, 622–627. [Google Scholar]
- Guerin, J.; Bendahan, M.; Aguir, K. A dynamic response model for the WO3-based ozone sensors. Sens. Actuat. B: Chem. 2008, 128, 462–467. [Google Scholar]
- Vallejos, S.; Khatko, V.; Aguir, K.; Ngo, K.A.; Calderer, J.; Gracia, I.; Cane, C.; Llobet, E.; Correig, X. Ozone monitoring by micro-machined sensors with WO3 sensing films. Sens. Actuat. B: Chem. 2007, 126, 573–578. [Google Scholar]
- Belkacem, W.; Labidi, A.; Guerin, J.; Mliki, N.; Aguir, K. Cobalt nanograins effect on the ozone detection by WO3 sensors. Sens. Actuat. B: Chem. 2008, 132, 196–201. [Google Scholar]
- Sauter, D.; Weimar, U.; Noetzel, G.; Mitrovics, J.; Gopel, W. Development of Modular Ozone Sensor System for application in practical use. Sens. Actuat. B: Chem. 2000, 69, 1–9. [Google Scholar]
- Korotcenkov, G.; Blinov, I.; Ivanov, M.; Stetter, J.R. Ozone sensors on the base of SnO2 films deposited by spray pyrolysis. Sens. Actuat. B: Chem. 2007, 120, 679–686. [Google Scholar]
- Bendahan, M.; Boulmani, R.; Seguin, J.L.; Aguir, K. Characterisation of ozone sensors based on WO3 reactively sputtered films: influence of O2 concentration in the sputtering gas and working temperature. Sens. Actuat. B. Chem. 2004, 100, 320–324. [Google Scholar]
- Hellegouarc'h, F.; Arefi-Khonsari, F.; Planade1, R.; Amouroux, J. PECVD prepared SnO2 thin Films for ethanol sensors. Sens. Actuat. B: Chem. 2001, 73, 27–34. [Google Scholar]
- Jinkawa, T.; Sakai, G.; Tamaki, J.; Miura, N.; Yamazoe, N. Relationship between ethanol gas sensitivity and surface catalytic property of tin oxide sensors modified with acidic or basic oxides. J. Mol. Catal. A: Chem. 2000, 155, 193–200. [Google Scholar]
- Chu, D.; Zeng, Y.; Jiang, D.; Masuda, Y. In2O3–SnO2 nano-toasts and nanorods: Precipitation preparation, formation mechanism, and gas sensitive properties. Sens. Actuat. B: Chem. 2009, 137, 630–636. [Google Scholar]
- Zhang, T.S.; Hing, P.; Li, Y.; Zhang, J.C. Selective detection of ethanol vapor and hydrogen using Cd-doped SnO –based sensors. Sens. Actuat. B: Chem. 1999, 60, 208–215. [Google Scholar]
- Hieu, N.; Kim, H.; Ju, B.; Lee, J. Enhanced performance of SnO2 nanowires ethanol sensor by functionalizing with La2O3. Sens. Actuat. B: Chem. 2008, 133, 228–234. [Google Scholar]
- Ivanov, P.; Llobet, E.; Vilanova, X.; Brezmes, J.; Hubalek, J.; Correig, X. Development of high sensitivity ethanol gas sensors based on Pt-doped SnO2 surfaces. Sens. Actuat. B: Chem. 2004, 99, 201–206. [Google Scholar]
- Li, F.; Xu, J.; Yu, X.; Chen, L.; Zhu, J.; Yang, Z.; Xin, X. One-step solid-state reaction synthesis and gas sensing property of tin oxide nanoparticles. Sens. Actuat. B: Chem. 2002, 81, 165–169. [Google Scholar]
- de Lacy Costello, B.P.; Ewen, R.J.; Guernion, N.; Ratcliffe, N.M. Highly sensitive mixed oxide sensors for the detection of ethanol. Sens. Actuat. B: Chem. 2002, 87, 207–210. [Google Scholar]
- Pin, L.V.; Tang, Z.A.; Yu, J.; Zhang, F.T.; Wei, G.F.; Huang, Z.X.; Hu, Y. Study on a micro-gas sensor with SnO2–NiO sensitive film for indoor formaldehyde detection. Sens. Actuat. B: Chem. 2008, 132, 74–80. [Google Scholar]
- Qi, Q.; Zhang, T.; Zheng, X.; Fan, H.; Liu, L.; Wang, R.; Zeng, Y. Electrical response of Sm2O3-doped SnO2 to C2H2 and effect of humidity interference. Sens. Actuat. B: Chem. 2008, 134, 36–42. [Google Scholar]
- Haridasa, D.; Sreenivasa, K.; Gupta, V. Improved response characteristics of SnO2 thin film loaded with nanoscale catalysts for LPG detection. Sens. Actuat. B: Chem. 2008, 133, 270–275. [Google Scholar]
- Wagh, M.S.; Jain, G.H.; Patil, D.R.; Patil, S.A.; Patil, L.A. Surface customization of SnO2 thick films using RuO2 as a surfactant for the LPG response. Sens. Actuat. B: Chem. 2007, 122, 357–364. [Google Scholar]
- Jain, K.; Pant, R.P.; Lakshmikumar, S.T. Effect of Ni doping on thick film SnO2 gas sensor. Sens. Actuat. B: Chem. 2006, 113, 823–829. [Google Scholar]
- Pourfayaz, F.; Khodadadi, A.; Mortazavi, Y.; Mohajerzadeh, S.S. CeO2 doped SnO2 sensor selective to ethanol in presence of CO, LPG and CH4. Sens. Actuat. B: Chem. 2005, 108, 172–176. [Google Scholar]
- Ionescu, R.; Hoel, A.; Granqvist, C.G.; Llobet, E.; Heszler, P. Ethanol and H2S gas detection in air and in reducing and oxidising ambience: application of pattern recognition to analyse the output from temperature-modulated nanoparticulate WO3 gas sensors. Sens. Actuat. B: Chem. 2005, 104, 124–131. [Google Scholar]
- Garzella, C.; Bontempi, E.; Depero, L.E.; Vomiero, A.; Della Mea, G.; Sberveglieri, G. Novel selective ethanol sensors: W/TiO2 thin films by sol–gel spin-coating. Sens. Actuat. B: Chem. 2003, 93, 495–502. [Google Scholar]
- Khadayate, R.S.; Sali, J.V.; Patil, P.P. Acetone vapor sensing properties of screen printed WO3 thick films. Talanta 2007, 72, 1077–1081. [Google Scholar]
- Cao, X.; Wu, W.; Chen, N.; Peng, Y.; Liu, Y. An ether sensor utilizing cataluminescence on nanosized ZnWO4. Sens. Actuat. B: Chem. 2009, 137, 83–87. [Google Scholar]
- Nguyen, V.H.; Nguyen, V.D.; Pham Thanh, H.; Nguyen, D.C. Inclusion of SWCNTs in Nb/Pt co-doped TiO2 thin film sensor for ethanol vapor detection. Phys. E. 2008, 40, 2950–2958. [Google Scholar]
- Pokhrel, S.; Huo, L.; Zhao, H.; Gao, S. Thick film of LaCr1−xTixO3 (x ≤ 0.4) perovskites prepared by combustion technique for alcohol sensing application. Sens. Actuat. B: Chem. 2007, 122, 321–327. [Google Scholar]
- Pokhrel, S.; Yang, M.; Huo, L.; Zhao, H.; Gao, S. Cr2−xTixO3 (x ≤ 0.5) as CH3COCH3 sensitive resistors. Sens. Actuat. B: Chem. 2007, 125, 550–555. [Google Scholar]
- Xu, J.; Hanb, J.; Zhang, Y.; Sun, Y.; Xie, B. Studies on alcohol sensing mechanism of ZnO based gas sensors. Sens. Actuat. B: Chem. 2008, 132, 334–339. [Google Scholar]
- Zhao, S.; Sin, J.; Xu, B.; Zhao, M.; Peng, Z.; Cai, H. A high performance ethanol senor based on field-effect transistor using a LaFeO3 nano-crystalline thin-film as a gate electrode. Sens. Actuat. B: Chem. 2000, 64, 83–87. [Google Scholar]
- Liu, X.; Cheng, B.; Hu, J.; Qin, H.; Jiang, M. Preparation, structure, resistance and methane-gas sensing properties of nominal La1−xMgxFeO3. Sens. Actuat. B: Chem. 2008, 133, 340–344. [Google Scholar]
- Ajamia, S.; Mortazavia, Y.; Khodadadia, A.; Pourfayaza, F.; Mohajerzadehb, S. Highly selective sensor to CH4 in presence of CO and ethanol using LaCoO3 perovskite filter with Pt/SnO2. Sens. Actuat. B: Chem. 2006, 117, 420–425. [Google Scholar]
- Xuana, Y.; Hua, J.; Xua, K.; Houa, X.; Lva, Y. Development of sensitive carbon disulfide sensor by using its cataluminescence on nanosized-CeO2. Sens. Actuat. B: Chem. 2009, 136, 218–223. [Google Scholar]
- Kida, T.; Minami, T.; Kishib, S.; Yuasa, M.; Shimanoea, K.; Yamazoe, N. Planar-type BiCuVOx solid electrolyte sensor for the detection of volatile organic compounds. Sens. Actuat. B: Chem. 2009, 137, 147–153. [Google Scholar]
- Daza, L.; Dassy, S.; Delmon, B. Chemical sensors based on SnO2 and WO3 for the detection of formaldehyde: cooperative effects. Sens. Actuat. B: Chem. 1993, 10, 99–105. [Google Scholar]
- Sberveglieri, G.; Atashbar, M.Z.; Li, Y.; Wlodarski, W.; Comini, E.; Faglia, G.; Ghantasala, M.K. Nanocrystalline TiO2 thin films prepared by the sol–gel process for alcohol sensing. Proceedings of the 10th International Conference on Solid-State Sensors and Actuators (Transducers 1999), Sendai, Japan, June 7–10, 1999; pp. 1690–1693.
- Beaudry, W.T.; Wagner, G.W.; Ward, J.R. Characterization of the molecular exchange process observed for dimethyl methylphosphonate adsorbed on a sorptive/reactive resin mixture by 31P magnetization-transfer and 2-D exchange MAS NMR. J. Mol. Catal. 1993, 83, 183–195. [Google Scholar]
- Beaudry, W.T.; Wagner, G.W.; Ward, J.R. Solid-state 31P MAS NMR study of the distribution and reaction of organophosphorus esters adsorbed on synthetic resin catalysts. J. Mol. Catal. 1992, 73, 77–90. [Google Scholar]
- Lien, Y.H.; Zhou, H.Z.; Job, C.; Barry, J.A.; Gillies, R.J. In vivo31P NMR study of early cellular responses to hyperosmotic shock in cultured glioma cells. Biochimie 1992, 74, 931–939. [Google Scholar]
- Zhang, J.; Giotto, M.V.; Wen, W.; Jones, A. An NMR study of the state of ions and diffusion in perfluorosulfonate ionomer. J. Memb. Sci. 2006, 269, 118–125. [Google Scholar]
- Canet, D.; Delpuech, J.A.; Khaddar, M.R.; Rubini, P. Carbon-13 NMR of solvation shells: Aluminium cation in aqueous organic solvents. J. Magn. Reson. 1974, 15, 325–338. [Google Scholar]
- Beaudry, W.T.; Wagner, G.W.; Ward, J.R. CuII—diamine complex catalyzed hydrolysis of phosphate triesters adsorbed on strong-base ion exchange resins. 31P NMR relaxation measurements. J. Mol. Catal. 1994, 93, 221–231. [Google Scholar]
- DeWolf, M.Y. The NMR spectra of dimethyl methyl phosphonate. J. Mol. Spect. 1965, 18, 59–61. [Google Scholar]
- Kirk, K.; Kuchel, P.W. Equilibrium exchange of dimethyl methylphosphonate across the human red cell membrane measured using NMR spin transfer. J. Magn. Reson. 1986, 68, 311–318. [Google Scholar]
- Delpuech, J.J.; Peguy, A.; Khaddar, M.R. An NMR study of solvation shells of diamagnetic cations in aqueous mixtures of organophosphorus solvents. J. Magn. Reson. 1972, 6, 325–335. [Google Scholar]
- Kirk, K.; Raftos, J.E.; Kuchel, P.W. Triethyl phosphate as an internal 31P NMR reference in biological samples. J. Magn. Reson. 1986, 70, 484–487. [Google Scholar]
- Harris, R.K.; Thompson, T.V.; Norman, P.R.; Pottage, C. Phosphorus-31 NMR studies of adsorption onto activated carbon. Carbon 1999, 37, 1425–1430. [Google Scholar]
- Hsu, C.; Dulcey, C.S.; Horwitz, J.S.; Lin, M.C. Mass spectrometric characterization of performance of a low-temperature oxidation catalyst. J. Mol. Catal. 1990, 60, 389–398. [Google Scholar]
- Werner, J.H.; Cool, T.A. Flame sampling photoionization mass spectrometry of CH3PO2 and CH3OPO2. Chem. Phys. Let. 1997, 275, 278–282. [Google Scholar]
- Cai, L.; Koziel, J.A.; O'Neal, M.E. Determination of characteristic odorants from Harmonia axyridis beetles using in vivo solid-phase microextraction and multidimensional gas chromatography–mass spectrometry–olfactometry. J. Chrom. A 2007, 1147, 66–78. [Google Scholar]
- Yang, M.; Kim, T.; Hwang, H.; Yi, S.; Kim, D. Development of a Palm Portable Mass Spectrometer. J. Am. Soc. Mass Spect. 2008, 19, 1442–1448. [Google Scholar]
- Kettrup, A.; Ohrbach, K.; Matuschek, G.; Joachim, A. Thermal analysis-mass spectrometry and thermogravimetric adsorption on fire retardants. Thermochim. Acta 1990, 166, 41–52. [Google Scholar]
- Pilling, R.S.; Bernhardt, G.; Kim, C.S.; Duncan, J.; Crothers, C.B.; Kleinschmidt, D.; Frankel, D.J.; Lad, R.J.; Frederick, B.G. Quantifying gas sensor and delivery system response time using GC/MS. Sens. Actuat. B: Chem. 2003, 96, 200–214. [Google Scholar]
- Chou, J.S.; Sumida, D.; Wittig, C. Two-frequency two-photon ionization of nascent PO(X2II) from the collision-free IR photolysis of dimethyl methylphosphonate. Chem. Phys. Lett. 1983, 100, 397–402. [Google Scholar]
- Kantcheva, M.; Cayirtepe, I. Routes of formation and composition of NOx complexes adsorbed on palladium-promoted tungstated zirconia. J. Mol. Catal. A: Chem. 2006, 247, 88–98. [Google Scholar]
- Solymosi, F.; Zakar, T.S. FT-IR study on the interaction of CO2 with H2 and hydrocarbons over supported Re. J. Mol. Catal. A: Chem. 2005, 235, 260–266. [Google Scholar]
- Datka, J.; Kozyra, P. TPD–IR studies of CO desorption from zeolites CuY and CuX. J. Mol. Struct. 2005, 744–747, 991–996. [Google Scholar]
- Akcay, M. FT-IR spectroscopic investigation of the adsorption pyridine on the raw sepiolite and Fe-pillared sepiolite from Anatolia. J. Mol. Struct. 2004, 694, 21–26. [Google Scholar]
- Taranenko, N.; Pierre, J.; Stokes, D.; Vo-Dinh, T. Surface-Enhanced Raman Detection of Nerve Agent Simulant (DMMP and DIMP) Vapor on Electrochemically Prepared Silver Oxide Substrates. J. Raman Spectrosc. 1996, 27, 379–384. [Google Scholar]
- Tevault, D.E.; Pellenbarg, R.E. Measurement of atmospheric pollutants by Raman spectroscopy. Sci. Tot. Environ. 1988, 73, 65–69. [Google Scholar]
- Creasy, W.R.; Rodríguez, A.A.; Stuff, J.R.; Warren, R.W. Atomic emission detection for the quantitation of trimethylsilyl derivatives of chemical-warfare-agent related compounds in environmental samples. J. Chromatogr. A 1995, 709, 333–344. [Google Scholar]
- Eiceman, G.A.; Nazarov, E.G.; Stone, J.A. Chemical standards in ion mobility spectrometry. Anal. Chim. Acta 2003, 493, 185–194. [Google Scholar]
- Tabrizchi, M. Temperature effects on resolution in ion mobility spectrometry. Talanta 2004, 62, 65–70. [Google Scholar]
- Dworzanski, J.P.; Kim, M.; Snyder, A.P.; Arnold, N.S.; Meuzelaar, H.L. Performance advances in ion mobility spectrometry through combination with high speed vapor sampling, preconcentration and separation techniques. Anal. Chim. Acta 1994, 293, 219–235. [Google Scholar]
- Urbasand, A.A.; Harrington, P.B. Two-dimensional wavelet compression of ion mobility spectra. Anal. Chim. Acta 2001, 446, 391–410. [Google Scholar]
- Karpas, Z.; Pollevoy, Y. Ion mobility spectrometric studies of organophosphorus compounds. Anal. Chim. Acta 1992, 259, 333–338. [Google Scholar]
- Kanu, A.B.; Haigh, P.E.; Hill, H. Surface detection of chemical warfare agent simulants and degradation products. Anal. Chim. Acta 2005, 553, 148–159. [Google Scholar]
- Suenram, R.D.; Lovas, F.J.; Plusquellic, D.F.; Lesarri, A.; Kawashima, Y.; Jensen, J.O.; Samuels, A.C. Fourier transform microwave spectrum and ab initio study of dimethyl methylphosphonate. J. Mol. Spec. 2002, 211, 110–118. [Google Scholar]
- Du, X.; Ying, Z.; Jiang, Y.; Liu, Z.; Yang, T.; Xie, G. Synthesis and evaluation of a new polysiloxane as SAW sensor coatings for DMMP detection. Sens. Actuat. B: Chem. 2008, 134, 409–413. [Google Scholar]
- Nimal, A.T.; Mohan, S.; Mittal, U.; Yadava, R.D. A comparative analysis of one-port Colpitt and two-port Pierce SAW oscillators for DMMP vapour sensing. Sens. Actuat. B: Chem. 2006, 114, 316–325. [Google Scholar]
- Mascaro, D.J.; Baxter, J.C.; Halvorsen, A.; White, K.; Scholz, B.; Schulz, D.L. ChemiBlock transducers. Sens. Actuat. B: Chem. 2007, 120, 353–361. [Google Scholar]
- Ying, Z.; Jiang, Y.; Du, X.; Xie, G.; Yu, J.; Wang, G. PVDF coated quartz crystal microbalance sensor for DMMP vapour detection. Sens. Actuat. B: Chem. 2007, 125, 167–172. [Google Scholar]
- Zuo, G.; Li, X.; Li, P.; Yang, T.; Wang, Y.; Cheng, Z.; Feng, S. Detection of trace organophosphorous vapour with a self-assembled bilayer functionalized SiO2 microcantilever piezoresistive sensor. Anal. Chim. Acta 2006, 580, 123–127. [Google Scholar]
- Riebel, S.; Stier, A.; Voigt, M.; Rapp, M. Influence of phase position on the performance of chemical sensors based on SAW device oscillators. Anal. Chem. 1998, 70, 5190–5197. [Google Scholar]
- Levit, N.; Pestov, D.; Tepper, G. High surface area polymer coatings for SAW-based chemical sensor applications. Sens. Actuat. B: Chem. 2002, 82, 241–249. [Google Scholar]
- Lewis, N.S. Comparisons between mammalian and artificial olfaction based on arrays of carbon black-polymer composite vapour detectors. Acc. Chem. Res. 2004, 37, 663–672. [Google Scholar]
- Dai, L.M.; Soundarrajan, P.; Kim, T. Sensors and sensor arrays based on conjugated polymers and carbon nanotubes. Pure Appl. Chem. 2002, 74, 1753–1772. [Google Scholar]
- Gao, T.; Tillman, E.S.; Lewis, N.S. Detection and classification of volatile organic amines and carboxylic acids using arrays of carbon black-dendrimer composite vapour detectors. Chem. Mater. 2005, 17, 2904–2911. [Google Scholar]
- Zee, F.; Judy, J.W. Micromachined polymer-based chemical gas sensor array. Sens. Actuat. B: Chem. 2001, 72, 120–128. [Google Scholar]
- Sberveglieri, G.; Baratto, C.; Comini, E.; Faglia, G.; Ferroni, M.; Pardo, M.; Ponzoni, A.; Vomiero, A. Semiconducting tin oxide nanowires and thin films for chemical warfare agents detection. Thin Solid Films 2009, 517, 6156–6160. [Google Scholar]
- Lee, S.C.; Choi, H.Y.; Lee, S.J.; Lee, W.S.; Huh, J.S.; Lee, D.D.; Kim, J.C. The development of SnO2- based recoverable gas sensors for the detection of DMMP. Sens. Actuat. B: Chem. 2009, 137, 239–245. [Google Scholar]
- Lee, W.S.; Lee, S.C.; Lee, S.J.; Lee, D.D.; Huh, J.S.; Jun, H.K.; Kim, J.C. The sensing behaviour of SnO2-based thick-film gas sensors at a low concentration of chemical agent stimulants. Sens. Actuat. B: Chem. 2005, 108, 148–153. [Google Scholar]
- Oh, S.W.; Kim, Y.H.; Yoo, D.J.; Oh, S.M.; Park, S.J. Sensing behaviour of semiconducting metal oxides for the detection of organophosphorus compounds. Sens. Actuat. B: Chem. 1993, 13–14, 400–403. [Google Scholar]
- Brunol, E.; Berger, F.; Fromm, M.; Planade, R. Detection of dimethyl methylphosphonate (DMMP) by tin dioxide-based gas sensor: response curve and understanding of the reactional mechanism. Sens. Actuat. B: Chem. 2006, 120, 35–41. [Google Scholar]
- Berger, F.; Planade, R.; Chambaudet, A. Detection of DEMP vapors usion SnO2-based gas sensors: understanding of the chemical reactional mechanism. Thin Solid Films 2003, 436, 1–8. [Google Scholar]
- Kanan, S.M.; Waghe, A.; Jensen, B.L.; Tripp, C.P. Dual WO3 based sensors toselectively detect DMMP in the presence of alcohols. Talanta 2007, 72, 401–407. [Google Scholar]
- Kanan, S.M.; Tripp, C.P. Synthesis, FTIR studies and sensor properties of WO3 powders. Curr. Opin. Solid State Mater. Sci. 2007, 11, 19–27. [Google Scholar]
- Yang, Y.C.; Baker, J.A.; Ward, R.A. Decontamination of Chemical Warfare Agnents. Chem. Rev. 1992, 92, 1729–1743. [Google Scholar]
- Obee, T.N.; Satyapal, S. Photocatalytic decomposition of DMMP on titania. J. Photochem. Photobiol. A: Chem. 1998, 118, 45–51. [Google Scholar]
- Atiqur Rahman, M.; Muneer, M.; Bahnemann, D. Phtocatalytic degradation of dimethyl terephthalate in aqueous suspensions of titanium dioxide. Res. Chem. Intermed. 2003, 29, 35–50. [Google Scholar]
- Bender, F.; Kim, C.; Mlsna, T.; Vetelino, J.F. Characterization of a WO3 thin film chlorine sensor. Sens. Actuat. B: Chem. 2001, 77, 281–286. [Google Scholar]
- Kim, C.S.; Lad, R.J.; Tripp, C.P. Interaction of organophosphorous compounds with TiO2 and WO3 surfaces probed by vibrational spectroscopy. Sens. Actuat. B: Chem. 2001, 76, 442–448. [Google Scholar]
- Sun, L.; Qiu, F.; Quan, B. Investigation of a new catalytic combustion-type CH4 gas sensor with low power consumption. Sens. Actuat. B: Chem. 2000, 66, 289–292. [Google Scholar]
- Niranjan, R.S.; Sainkar, S.R.; Vijayamohanan, K.; Mulla, I.S. Ruthenium: tin oxide thin film as a highly selective hydrocarbon sensor. Sens. Actuat. B: Chem. 2002, 82, 82–88. [Google Scholar]
- Chaudthary, V.A.; Mull, L.S.; Vijayamohanan, K. Synergistic sensitivity effects in surface-modified tin oxide hydrogen sensors using ruthenium and palladium oxides. J. Mater. Sci. Lett. 1997, 16, 1819–1821. [Google Scholar]
- Chaudhary, V.A.; Mulla, I.S.; Sainkar, S.R.; Belhekar, A.A.; Vijayamohanan, K. Surface-ruthenated tin oxide as a novel hydrocarbon sensor. Sens. Actuat. A 1998, 65, 197–202. [Google Scholar]
- Tamaki, J.; Maekawa, T.; Miura, N.; Yamazoe, N. CuO-SnO2 element for highly sensitive and selective detection of H2S. Sens. Actuat. B: Chem. 1992, 9, 197–203. [Google Scholar]
- Yamaura, H.; Jinkawa, T.; Tamaki, J.; Morjiya, K.; Miura, N.; Yamazoe, N. Indium oxide-based gas sensor for selective detection of CO. Sens. Actuat. B: Chem. 1996, 35–36, 325–332. [Google Scholar]
- Fruhberger, B.; Stirling, N.; Grillo, F.; Ma, G.; Ruthven, S.D.; Lad, R.J.; Frederick, B.G. Detection and quantification of nitric oxide in human breath using a semiconducting oxide based chemiresistive microsensor. Sens. Actuat. B: Chem. 2001, 76, 226–234. [Google Scholar]
- Choi, N.J.; Kwak, J.H.; Lim, Y.T.; Bahn, T.H.; Yun, K.Y.; Kim, J.C.; Huh, J.S.; Lee, D.D. Classification of chemical warfare agents using thick film gas sensor array. Sens. Actuat. B: Chem. 2005, 108, 298–304. [Google Scholar]
- Kanan, S.M.; Tripp, C.P. Prefiltering strategies for metal oxide based sensors: Use of chemical displacers to selectively cleave adsorbed organophosphonates from silica surfaces. Langmuir 2002, 18, 722–728. [Google Scholar]
- Semancik, S.; Cavicchi, R.; Wheeler, M.C.; Tiffany, J.E.; Poirier, G.E.; Walton, R.M.; Suehle, J.; Panchapakesan, S.B.; DeVoe, D.L. Microhotplate platforms for chemical sensor research. Sens. Actuat. B: Chem. 2001, 77, 579–591. [Google Scholar]
- Menzel, R.; Goschnick, J. Gradient gas sensor microarrays of on- line process controla new dynamic classification model for fast and reliable air quality assessment. Sens. Actuat. B: Chem. 2000, 43, 235–238. [Google Scholar]
- Kanan, S.M.; Lu, Z.; Tripp, C.P. A comparative study of the adsorption of chloro and non-chloro conatianing organophosphonates on WO3. J. Phys. Chem. B 2002, 106, 9576–9580. [Google Scholar]
- Kanan, S.M.; Tripp, C.P. An Infrared study of adsorbed organophosphonates on silica: a prefiltering strategy for the detection of nerve agents on metal oxide Sensors. Langmuir 2001, 17, 2213–2218. [Google Scholar]
- Cox, D.F.; Fryberger, T.B.; Semancik, S. Surface rexonstructions of oxygen deficient SnO2 (110). Surf. Sci. 1989, 224, 121–142. [Google Scholar]
- Cox, D.F.; Fryberger, T.B.; Semancik, S. Summary abstract: Oxygen-Vacancy-derived defect electronic states on the SnO2(110) surface. J. Vac. Sci. Technol. A 1988, 6, 828–829. [Google Scholar]
- Semancik, S.; Cox, D.F. Fundamental characterization of clean and gas-dosed tin oxide. Sens. Actuat. B: Chem. 1987, 12, 101–106. [Google Scholar]
- Fryberger, T.B.; Semancik, S. Conductance response of Pd/SnO2 (110) model gas sensors to H2 and O2. Sens. Actuat. B: Chem. 1990, 2, 305–309. [Google Scholar]
- Cox, D.F.; Gryberger, T.B.; Semancik, S. Oxygen vacancies and defect electronic states on the SnO2(110)-1×1 surface. Phys. Rev. B 1988, 38, 2072–2082. [Google Scholar]
- Cavicchi, R.E.; Suehle, J.S.; Chaparala, P.; Kreider, K.G.; Gaitan, M.; Semancik, S. Microhotplate gas sensor. Proceedings of the 1994 Solid State Sensor and Actuator Workshop, Hilton Head, SC, USA; 1994; pp. 53–56. [Google Scholar]




© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kanan, S.M.; El-Kadri, O.M.; Abu-Yousef, I.A.; Kanan, M.C. Semiconducting Metal Oxide Based Sensors for Selective Gas Pollutant Detection. Sensors 2009, 9, 8158-8196. https://doi.org/10.3390/s91008158
Kanan SM, El-Kadri OM, Abu-Yousef IA, Kanan MC. Semiconducting Metal Oxide Based Sensors for Selective Gas Pollutant Detection. Sensors. 2009; 9(10):8158-8196. https://doi.org/10.3390/s91008158
Chicago/Turabian StyleKanan, Sofian M., Oussama M. El-Kadri, Imad A. Abu-Yousef, and Marsha C. Kanan. 2009. "Semiconducting Metal Oxide Based Sensors for Selective Gas Pollutant Detection" Sensors 9, no. 10: 8158-8196. https://doi.org/10.3390/s91008158
APA StyleKanan, S. M., El-Kadri, O. M., Abu-Yousef, I. A., & Kanan, M. C. (2009). Semiconducting Metal Oxide Based Sensors for Selective Gas Pollutant Detection. Sensors, 9(10), 8158-8196. https://doi.org/10.3390/s91008158




