Applications of Nanomaterials in Electrochemical Enzyme Biosensors
Abstract
:1. Introduction
2. Nanomaterials as Biosensor Modifiers of Glucose Oxidase (GOD) and Horseradish Peroxidase (HRP)
2.1. GOD on ZrO2 Nanoparticles
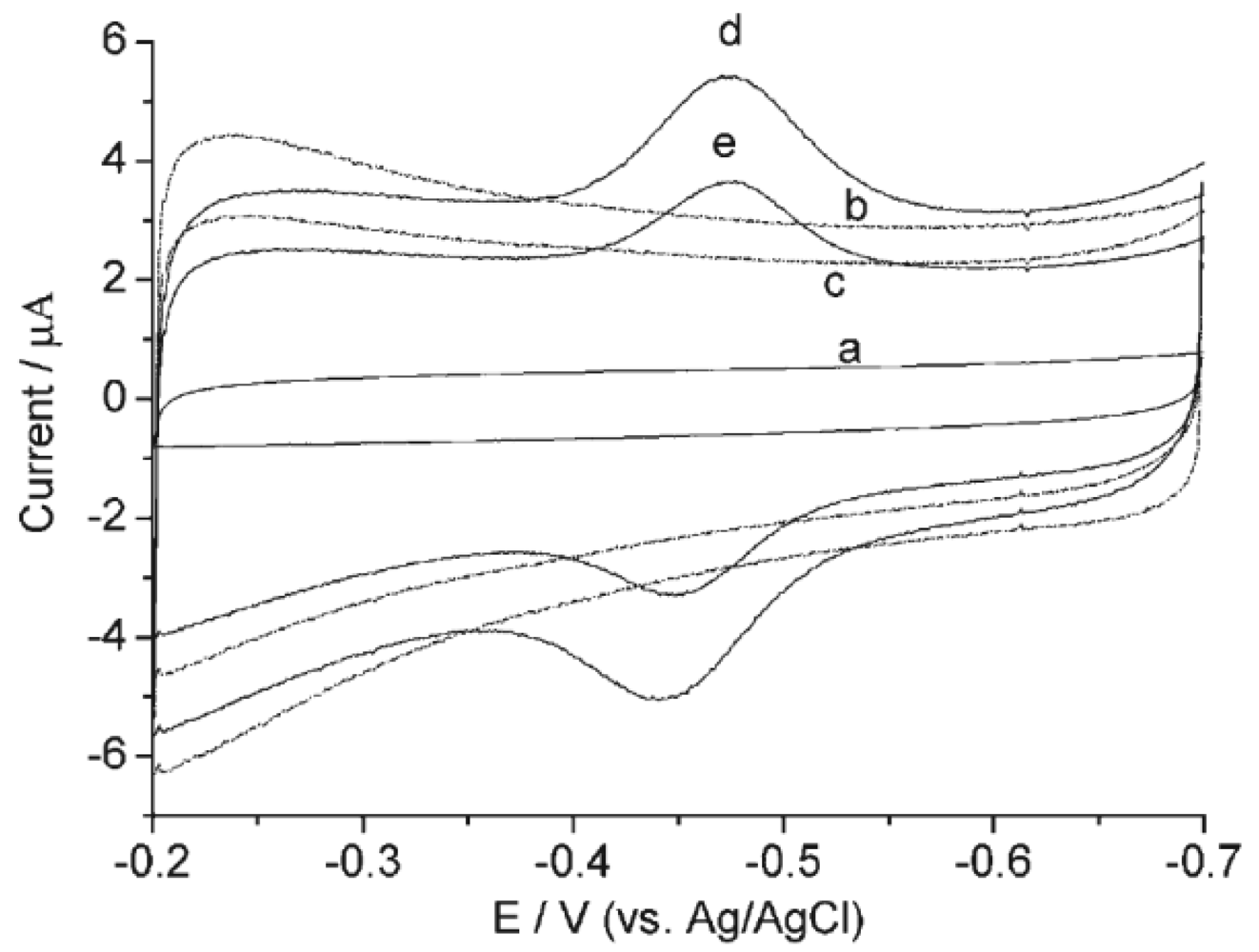
2.2. HRP on TPSP-ZnO Nanostructure
2.3. A GOD-HRP Bienzyme Channeling Glucose Sensor in SBA-15 Mesopores
3. Nanomaterials as Modification Biosensors of Cytochrome P450 (CYP2B6) and Hemoglobin (Hb)
3.1. CYP2B6 on ZrO2 Nanoparticles
3.2. CYP2B6 on Au–chitosan/GCE Film
3.3. Hb on PUE/MWNT/PG Electrode
4. Evaluation the Effect of Nanomaterials on the Enzyme Activity
5. Concluding Remarks
Acknowledgments
References
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar]
- Wang, J. Nanoparticle-based electrochemical DNA detection. Anal. Chim. Acta 2003, 500, 247–257. [Google Scholar]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar]
- Willner, I.; Patolsky, P.; Wasserman, J. Photoelectrochemistry with controlled DNA-cross-linked CdS nanoparticle Arrays. Angew. Chem. Int. Ed. 2001, 40, 1861–1864. [Google Scholar]
- Qi, H.; Peng, Y.; Gao, Q.; Zhang, C. Applications of Nanomaterials in Electrogenerated Chemiluminescence Biosensors. Sensors 2009, 9, 674–695. [Google Scholar]
- Wu, L.; Zhang, X.J.; Ju, H.X. Detection of NADH and Ethanol based on catalytical activity of soluble carbon nanofiber with low overpotential. Anal. Chem. 2007, 79, 453–458. [Google Scholar]
- Wu, L.; McIntosh, M.; Zhang, X.J.; Ju, H.X. Amperometric sensor for ethanol based on one-step electropolymerizationof thionine–carbon nanofiber nanocomposite containing alcohol oxidase. Talanta 2007, 74, 387–392. [Google Scholar]
- Wu, L.; Zhang, X.J.; Ju, H.X. Amperometric glucose sensor based on catalytic reduction of dissolved oxygen at soluble carbon nanofiber. Biosen. Bioelectron. 2007, 23, 479–484. [Google Scholar]
- Guo, S.; Wang, E. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar]
- Wang, J. Nanomaterial-based electrochemical biosensors. Analyst (Cambridge, UK) 2005, 130, 421–426. [Google Scholar]
- Baron, R.; Willner, B.; Willner, I. Biomolecule-nanoparticle hybrids as functional units for nanobiotechnology. Chem. Commun. 2007, 323–332. [Google Scholar]
- Katz, E.; Willner, I.; Wang, J. Electroanalytical and bioelectroanalytical systems based on metal and semiconductor nanoparticles. Electroanalysis (NY) 2004, 16, 19–44. [Google Scholar]
- Yanez-Sedeno, P.; Pingarron, J.M. Gold nanoparticle-based electrochemical biosensors. Anal. Bioanal. Chem. 2005, 382, 884–886. [Google Scholar]
- Hernandez-Santos, D.; Gonzalez-Garcia, M.B.; Garcia, A.C. Metal-nanoparticles based electroanalysis. Electroanalysis (NY) 2002, 14, 1225–1235. [Google Scholar]
- Wang, J. Nanoparticle-based electrochemical bioassays of proteins. Electroanalysis (NY) 2007, 19, 769–776. [Google Scholar]
- Erdem, A. Nanomaterial-based electrochemical DNA sensing strategies. Talanta 2007, 74, 318–325. [Google Scholar]
- Suni, I. Impedance methods for electrochemical sensors using nanomaterials. Trends Anal. Chem. 2008, 27, 604–611. [Google Scholar]
- Kerman, K.; Saito, M.; Yamamura, S.; Takamura, Y.; Tamiya, E. Nanomaterial-based electrochemical biosensors for medical applications. Trends Anal. Chem. 2008, 27, 585–592. [Google Scholar]
- de la Escosura-Muniz, A.; Ambrosi, A.; Merkoci, A. Electrochemical analysis with nanoparticle-based biosystems. Trends Anal. Chem. 2008, 27, 568–584. [Google Scholar]
- D'Orazio, P. Biosensors in clinical chemistry. Clin. Chim. Acta 2003, 334, 41–69. [Google Scholar]
- Malhotra, B.D.; Chaubey, A. Biosensors for clinical diagnostics industry. Sens. Actuat. B 2003, 91, 117–127. [Google Scholar]
- Poscia, A.; Mascini, M.; Moscone, D.; Luzzana, M.; Caramenti, G.; Cremonesi, P.; Valgimigli, F.; Bongiovanni, C.; Varalli, M. A microdialysis technique for continuous subcutaneous glucose monitoring in diabetic patients (part 1). Biosen. Bioelectron. 2003, 18, 891–898. [Google Scholar]
- Forrow, N.J.; Bayliff, S.W. A commercial whole blood glucose biosensor with a low sensitivity to hematocrit based on an impregnated porous carbon electrode. Biosen. Bioelectron. 2005, 21, 581–587. [Google Scholar]
- Crouch, E.; Cowell, D.C.; Hoskins, S.; Pittson, R.W.; Hart, J.P. Amperometric screen-printed, glucose biosensor for analysis of human plasma samples using a biocomposite water-based carbon ink incorporating glucose oxidase. Anal. Biochem. 2005, 347, 17–23. [Google Scholar]
- Shan, D.; Yao, W.J.; Xue, H.G. Amperometric detection of glucose with glucose oxidase immobilized in layered double hydroxides. Electroanalysis 2006, 18, 1485–1491. [Google Scholar]
- Asberg, P.; Nilsson, K.P.R.; Inganas, O. Surface energy modified chips for detection of conformational states and enzymatic activity in biomolecules. Langmuir 2006, 22, 2205–2211. [Google Scholar]
- Andreu, R.; Ferapontova, E.E.; Gorton, L.; Calvente, J.J. Direct electron transfer kinetics in horseradish peroxidase electrocatalysis. J. Phys. Chem. B 2007, 111, 469–477. [Google Scholar]
- Yang, X.D.; Zhang, Q.Q.; Sun, Y.M.; Liu, S.Q. Direct Electron Transfer Reactivity of Glucose Oxidase on Electrodes Modified With Zirconium Dioxide Nanoparticles. IEEE Sensors J. 2007, 7, 1735–1741. [Google Scholar]
- Dai, Z.H.; Liu, K.; Tang, Y.W.; Yang, X.D.; Bao, J.C.; Shen, J. A novel tetragonal pyramid-shaped porous ZnO nanostructure and its application in the biosensing of horseradish peroxidase. J. Mater. Chem. 2008, 18, 1919–1926. [Google Scholar]
- Dai, Z.H.; Bao, J.C.; Yang, X.D.; Ju, H.X. A bienzyme channeling glucose sensor with a wide concentration range based on co-entrapment of enzymes in SBA-15 mesopores. Biosens. Bioelectron. 2008, 23, 1070–1076. [Google Scholar]
- Ariyoshi, N.; Miyazaki, M.; Toide, K.; Sawamura, Y.; Kamataki, T. A single nucleotide polymorphism of CYP2B6 found in Japanese enhances catalytic activity by autoactivation. Biochem. Biophys. Res. Commun. 2001, 281, 1256–1260. [Google Scholar]
- Ekins, S.; Bravi, G.; Ring, B.J.; Gillespie, T.A. Three-dimensional quantitative structure–activity relationship analyses of substrates for CYP2B6. J. Pharmacol. Exp. Ther. 1999, 288, 21–29. [Google Scholar]
- Stiborova, M.; Borek-Dohalska, L.; Hodek, P.; Mraz, J.; Frei, E. New selective inhibitors of cytochromes P450 2B and their application to antimutagenesis of tamoxifen. Arch. Biochem. Biophys. 2002, 403, 41–49. [Google Scholar]
- Ekins, S.; Vandenbranden, M.; Ring, B.J.; Gillespie, J.S.; Yang, T.J.; Gelboin, H.V.; Wrighton, S.A. Further characterization of the expression in liver and catalytic activity of CYP2B6. J. Pharmacol. Exp. Ther. 1998, 286, 1253–1259. [Google Scholar]
- Koyama, E.; Chiba, K.; Tani, M.; Ishizaki, T. Reappraisal of human CYP isoforms involved in imipramine N-demethylation and 2- hydroxylation: A study using microsomes obtained from putative extensive and poor metabolizers of S-mephenytoin and eleven recombinant human CYPs. J. Pharmacol. Exp. Ther. 1997, 281, 1199–1210. [Google Scholar]
- Stevens, J.C.; White, R.B.; Hsu, S.H.; Martinet, M. Human liver CYP2B6-catalyzed hydroxylation of RP 73401. J. Pharmacol. Exp. Ther. 1997, 282, 1389–1395. [Google Scholar]
- Yamazaki, H.; Inoue, K.; Mimura, M.; Oda, Y.; Guengerich, F.P.; Shimada, T. 7-Ethoxycoumarin O-deethylation catalysed by cytochromes P450, 1A2, and 2E1 in human liver microsomes. Biochem. Pharmacol. 1996, 51, 313–319. [Google Scholar]
- Imaoka, S.; Yamada, T.; Hiroi, T.; Hayashi, K.; Sakaki, T.; Yabusaki, Y.; Funae, Y. Multiple forms of human P450 expressed in Saccharomyces cerevisiae: Systematic characterization and comparison with those of the rat. Biochem. Pharmacol. 1996, 51, 1041–1050. [Google Scholar]
- Roy, P.; Yu, L.J.; Crespi, C.L.; Waxman, D.J. Development of a substrate–activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 profiles. Drug Metab. Dispos. 1999, 27, 655–666. [Google Scholar]
- Faucette, S.R.; Hawke, R.L.; Lecluyse, E.L.; Shord, S.S.; Yan, B.F.; Laethem, R.M.; Lindley, C.M. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab. Dispos. 2000, 28, 1222–1230. [Google Scholar]
- Estabrook, R.W.; Faulkner, K.M.; Shet, M.S.; Fisher, C.W. Application of electrochemistry for P450-catalyzed reactions. Methods Enzymol. 1996, 272, 44–51. [Google Scholar]
- Lvov, Y.M.; Lu, Z.Q.; Schenkman, J.B.; Zu, X.L.; Rusling, J.F. Direct electrochemistry of myoglobin and cytochrome P450cam in alternate layer-by-layer films with DNA and other polyions. J. Am. Chem. Soc. 1998, 120, 4073–4080. [Google Scholar]
- Lo, K.K.W.; Wong, L.L.; Hill, A.O. Surface-modified mutants of cytochrome P450cam: Enzymatic properties and electrochemistry. FEBS Lett. 1999, 451, 342–346. [Google Scholar]
- Peng, L.; Yang, X.D.; Zhang, Q.Q.; Liu, S.Q. Electrochemistry of cytochrome P450 2B6 on electrodes modified with zirconium dioxide nanoparticles and platin components. Electroanalysis 2008, 20, 803–807. [Google Scholar]
- Liu, S.Q.; Peng, L.; Yang, X.D.; Wu, Y.F.; He, L. Electrochemistry of cytochrome P450 enzyme on nanoparticle-containing membrane-coated electrode and its applications for drug sensing. Anal. Biochem. 2008, 375, 209–216. [Google Scholar]
- Liu, S.Q.; Lin, B.P.; Yang, X.D.; Zhang, Q.Q. Carbon-nanotube-enhanced direct electron-transfer reactivity of hemoglobin immobilized on polyurethane elastomer film. J. Phys. Chem. B 2007, 111, 1182–1188. [Google Scholar]
- Yang, X.D.; Li, L.F.; Bi, S.P. Electrochemical studies of the inhibition and activation effects of Al(III) on the activity of bovine liver glutamate dehydrogenase. Sensors 2005, 5, 235–244. [Google Scholar]
- Yang, X.D.; Tang, Y.Z.; Bi, S.P.; Yang, G.S.; Hu, J. Multimethod study the complexation of aluminum (III) with L-glutamate in acidic aqueous solutions. Anal. Sci. 2003, 19, 133–138. [Google Scholar]
- Wang, N.; Huang, D.Q.; Zhang, J.; Cheng, J.J.; Yu, T.; Zhang, H.Q.; Bi, S.P. Electrochemical studies on the effects of nanometer-sized tridecameric Aluminum polycation on lactate dehydrogenase activity at the molecular level. J. Phys. Chem. C 2008, 112, 18034–18038. [Google Scholar]










© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, H.; Liu, S.; Dai, Z.; Bao, J.; Yang, X. Applications of Nanomaterials in Electrochemical Enzyme Biosensors. Sensors 2009, 9, 8547-8561. https://doi.org/10.3390/s91108547
Li H, Liu S, Dai Z, Bao J, Yang X. Applications of Nanomaterials in Electrochemical Enzyme Biosensors. Sensors. 2009; 9(11):8547-8561. https://doi.org/10.3390/s91108547
Chicago/Turabian StyleLi, Huihui, Songqin Liu, Zhihui Dai, Jianchun Bao, and Xiaodi Yang. 2009. "Applications of Nanomaterials in Electrochemical Enzyme Biosensors" Sensors 9, no. 11: 8547-8561. https://doi.org/10.3390/s91108547
APA StyleLi, H., Liu, S., Dai, Z., Bao, J., & Yang, X. (2009). Applications of Nanomaterials in Electrochemical Enzyme Biosensors. Sensors, 9(11), 8547-8561. https://doi.org/10.3390/s91108547



