The Leucocyte Telomere Length, GSTM1 and GSTT1 Null Genotypes and the Risk of Chronic Obstructive Pulmonary Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Patients and Controls
2.2. Isolation of DNA
2.3. Genotyping
2.4. Leukocyte Telomere Length (LTL) Measurement
2.5. Serum Total Glutathione Measurement
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available online: https://goldcopd.org (accessed on 11 July 2022).
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among Smoking, Oxidative Stress, Inflammation, Macromolecular Damage, and Cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Minagawa, S.; Araya, J.; Sakamoto, T.; Hara, H.; Tsubouchi, K.; Hosaka, Y.; Ichikawa, A.; Saito, N.; Kadota, T.; et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat. Commun. 2019, 10, 3145. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef]
- Turner, K.J.; Vasu, V.; Griffin, D.K. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Sławińska, N.; Krupa, R. Molecular Aspects of Senescence and Organismal Ageing-DNA Damage Response, Telomeres, Inflammation and Chromatin. Int. J. Mol. Sci. 2021, 22, 590. [Google Scholar] [CrossRef]
- Lulkiewicz, M.; Bajsert, J.; Kopczynski, P.; Barczak, W.; Rubis, B. Telomere length: How the length makes a difference. Mol. Biol. Rep. 2020, 47, 7181–7188. [Google Scholar] [CrossRef]
- Salpea, K.D.; Talmud, P.J.; Cooper, J.A.; Maubaret, C.G.; Stephens, J.W.; Abelak, K.; Humphries, S.E. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 2010, 209, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Córdoba-Lanús, E.; Cazorla-Rivero, S.; Espinoza-Jiménez, A.; de-Torres, J.P.; Pajares, M.J.; Aguirre-Jaime, A.; Celli, B.; Casanova, C. Telomere shortening and accelerated aging in COPD: Findings from the BODE cohort. Respir. Res. 2017, 18, 017–0547. [Google Scholar] [CrossRef] [Green Version]
- Ormseth, M.J.; Solus, J.F.; Oeser, A.M.; Bian, A.; Gebretsadik, T.; Shintani, A.; Raggi, P.; Stein, C.M. Telomere Length and Coronary Atherosclerosis in Rheumatoid Arthritis. J. Rheumatol. 2016, 43, 1469–1474. [Google Scholar] [CrossRef] [Green Version]
- Hardikar, S.; Song, X.; Risques, R.A.; Montine, T.J.; Duggan, C.; Blount, P.L.; Reid, B.J.; Anderson, G.L.; Kratz, M.; White, E.; et al. Obesity and inflammation markers in relation to leukocyte telomere length in a cross-sectional study of persons with Barrett’s esophagus. BMC Obes. 2015, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Haendeler, J.; Hoffmann, J.; Diehl, J.F.; Vasa, M.; Spyridopoulos, I.; Zeiher, A.; Dimmeler, S.M. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ. Res. 2004, 94, 768–775. [Google Scholar] [CrossRef] [Green Version]
- Sanders, J.L.; Newman, A.B. Telomere length in epidemiology: A biomarker of aging, age-related disease, both, or neither? Epidemiol. Rev. 2013, 35, 112–131. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Sasaki, K.; Kamata, R.; Sakai, R. The C-terminus of ephrin-B1 regulates metalloproteinase secretion and invasion of cancer cells. J. Cell Sci. 2007, 120, 2179–2189. [Google Scholar] [CrossRef] [Green Version]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Macchia, D.; Spada, M.; Fais, S. Oral Administration of Fermented Papaya (FPP(®)) Controls the Growth of a Murine Melanoma through the In Vivo Induction of a Natural Antioxidant Response. Cancers 2019, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.; Dong, W.; Shi, Z.; Xu, Y.; Ni, W.; An, R. Genetic polymorphisms of GSTM1, GSTT1, and GSTP1 with prostate cancer risk: A meta-analysis of 57 studies. PLoS ONE 2012, 7, e50587. [Google Scholar] [CrossRef] [Green Version]
- Klusek, J.; GÅuszek, S.; Klusek, J. GST gene polymorphisms and the risk of colorectal cancer development. Contemp. Oncol. 2014, 18, 219–221. [Google Scholar] [CrossRef] [Green Version]
- Dunna, N.R.; Vure, S.; Sailaja, K.; Surekha, D.; Raghunadharao, D.; Rajappa, S.; Vishnupriya, S. Deletion of GSTM1 and T1 genes as a risk factor for development of acute leukemia. Asian Pac. J. Cancer Prev. 2013, 14, 2221–2224. [Google Scholar] [CrossRef] [Green Version]
- Easter, M.; Bollenbecker, S.; Barnes, J.W.; Krick, S. Targeting Aging Pathways in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020, 21, 6924. [Google Scholar] [CrossRef]
- Zuntar, I.; Petlevski, R.; Dodig, S.; Popović-Grle, S. GSTP1, GSTM1 and GSTT1 Genetic Polymorphisms and Total Serum GST Concentration in Stable Male COPD. Acta Pharm. 2014, 64, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Tacheva, T.; Zienolddiny, S.; Dimov, D.; Vlaykova, D.; Vlaykova, T. The leukocyte telomere length, single nucleotide polymorphisms near TERC gene and risk of COPD. PeerJ 2021, 9, e12190. [Google Scholar] [CrossRef]
- Voso, M.T.; D′Alo, F.; Putzulu, R.; Mele, L.; Scardocci, A.; Chiusolo, P.; Latagliata, R.; Lo-Coco, F.; Rutella, S.; Pagano, L.; et al. Negative prognostic value of glutathione S-transferase (GSTM1 and GSTT1) deletions in adult acute myeloid leukemia. Blood 2002, 100, 2703–2707. [Google Scholar] [CrossRef]
- Vlaykova, T.; Gulubova, M.; Vlaykova, D.; Cirovski, G.; Yovchev, Y.; Dimov, D.; Chilingirov, P. Possible Influence of GSTM1 and GSTT1 Null Genotype on the Risk for Development of Sporadic Colorectal Cancer. Biotechnol. Biotechnol. Equip. 2009, 23, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Fischer, B.M.; Pavlisko, E.; Voynow, J.A. Pathogenic triad in COPD: Oxidative stress, protease-antiprotease imbalance, and inflammation. Int. J. Chron. Obstruct. Pulmon. Dis. 2011, 6, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Austin, V.; Crack, P.J.; Bozinovski, S.; Miller, A.A.; Vlahos, R. COPD and stroke: Are systemic inflammation and oxidative stress the missing links? Clin. Sci. 2016, 130, 1039–1050. [Google Scholar] [CrossRef] [Green Version]
- Chamitava, L.; Cazzoletti, L.; Ferrari, M.; Garcia-Larsen, V.; Jalil, A.; Degan, P.; Fois, A.G.; Zinellu, E.; Fois, S.S.; Fratta Pasini, A.M.; et al. Biomarkers of Oxidative Stress and Inflammation in Chronic Airway Diseases. Int. J. Mol. Sci. 2020, 21, 4339. [Google Scholar] [CrossRef]
- Fletcher, M.E.; Boshier, P.R.; Wakabayashi, K.; Keun, H.C.; Smolenski, R.T.; Kirkham, P.A.; Adcock, I.M.; Barton, P.J.; Takata, M.; Marczin, N. Influence of Glutathione-S-Transferase (GST) Inhibition on Lung Epithelial Cell Injury: Role of Oxidative Stress and Metabolism. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L1274–L1285. [Google Scholar] [CrossRef] [Green Version]
- Young, R.P.; Hopkins, R.J.; Hay, B.A.; Gamble, G.D. GSTM1 Null Genotype in COPD and Lung Cancer: Evidence of a Modifier or Confounding Effect? Appl. Clin. Genet. 2011, 4, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidegård, J.; DePierre, J.W.; Pero, R.W. Hereditary interindividual differences in the glutathione transferase activity towards trans-stilbene oxide in resting human mononuclear leukocytes are due to a particular isozyme(s). Carcinogenesis 1985, 6, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Jeong, J.Y.; Kim, M.K.; Min, S.A.; Park, J.S.; Park, C.S. Association of GSTM1 and GSTT1 Null Genotypes with Toluene Diisocyanate-Induced Asthma. Can. Respir. J. 2022, 2022, 7977937. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M.; Nikolić, A.; Tomović, A.; Mitić-Milikić, M.; Nagorni-Obradović, L.; Petrović-Stanojević, N.; Radojković, D. Association of Functional Variants of Phase I and II Genes with Chronic Obstructive Pulmonary Disease in a Serbian Population. J. Med. Biochem. 2015, 34, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Zidzik, J.; Slabã, E.; Joppa, P.; Kluchovã, Z.; DorkovÃ, Z.; Skyba, P.; Habalovã, V.; Salagovic, J.; Tkãcovã, R. Glutathione S-transferase and microsomal epoxide hydrolase gene polymorphisms and risk of chronic obstructive pulmonary disease in Slovak population. Croat. Med. J. 2008, 49, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, S.; Sharma, A.; Kumar, S.; Kar, P.; Sardana, S.; Sharma, J.K. Polymorphism of glutathione S-transferase M1 and T1 gene loci in COPD. Int. J. Immunogenet. 2010, 37, 263–267. [Google Scholar] [CrossRef]
- Cheng, S.L.; Yu, C.J.; Chen, C.J.; Yang, P.C. Genetic polymorphism of epoxide hydrolase and glutathione S-transferase in COPD. Eur. Respir. J. 2004, 23, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Yim, J.J.; Park, G.Y.; Lee, C.T.; Kim, Y.W.; Han, S.K.; Shim, Y.S.; Yoo, C.G. Genetic susceptibility to chronic obstructive pulmonary disease in Koreans: Combined analysis of polymorphic genotypes for microsomal epoxide hydrolase and glutathione S-transferase M1 and T1. Thorax 2000, 55, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Calikoglu, M.; Tamer, L.; Ates Aras, N.; Karakaş, S.; Ercan, B. The association between polymorphic genotypes of glutathione S-transferases and COPD in the Turkish population. Biochem. Genet. 2006, 44, 307–319. [Google Scholar] [CrossRef]
- Paarvanova, B.; Tolekova, A.; Hadzhibozheva, P.; Georgiev, T.; Ivanov, I. Structural alteration in the membrane of erythrocytes from rats with Streptozotocin-induced diabetes. Sci. Technol. 2013, 3, 153–157. [Google Scholar]
- De Rosa, M.; Johnson, S.A.; Opresko, P.L. Roles for the 8-Oxoguanine DNA Repair System in Protecting Telomeres From Oxidative Stress. Front. Cell Dev. Biol. 2021, 9, 758402. [Google Scholar] [CrossRef]
- Boonekamp, J.J.; Bauch, C.; Mulder, E.; Verhulst, S. Does oxidative stress shorten telomeres? Biol. Lett. 2017, 13, 20170164. [Google Scholar] [CrossRef]
- Reichert, S.; Stier, A. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 2017, 13, 20170463. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Maekawa, T.; Yoshida, K.; Ly, N.H.; Inoue, K.; Hasegawa, A.; Chatton, B.; Ogura, A.; Ishii, S. Telomere Shortening by Transgenerational Transmission of TNF-α-induced TERRA via ATF7. Nucleic Acids Res. 2019, 47, 283–298. [Google Scholar] [CrossRef] [Green Version]
- Savale, L.; Chaouat, A.; Bastuji-Garin, S.; Marcos, E.; Boyer, L.; Maitre, B.; Sarni, M.; Housset, B.; Weitzenblum, E.; Matrat, M.; et al. Shortened Telomeres in Circulating Leukocytes of Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2009, 179, 566–571. [Google Scholar] [CrossRef] [Green Version]
- Soerensen, M.; Thinggaard, M.; Nygaard, M.; Dato, S.; Tan, Q.; Hjelmborg, J.; Andersen-Ranberg, K.; Stevnsner, T.; Bohr, V.A.; Kimura, M.; et al. Genetic variation in TERT and TERC and human leukocyte telomere length and longevity: A cross-sectional and longitudinal analysis. Aging Cell 2012, 11, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Amsellem, V.; Gary-Bobo, G.; Marcos, E.; Maitre, B.; Chaar, V.; Validire, P.; Stern, J.B.; Noureddine, H.; Sapin, E.; Rideau, D.; et al. Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 184, 1358–1366. [Google Scholar] [CrossRef]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Dalgard, C.; Benetos, A.; Verhulst, S.; Labat, C.; Kark, J.D.; Christensen, K.; Kimura, M.; Kyvik, K.O.; Aviv, A. Leukocyte telomere length dynamics in women and men: Menopause vs age effects. Int. J. Epidemiol. 2015, 44, 1688–1695. [Google Scholar] [CrossRef] [Green Version]
- Vina, J.; Borras, C.; Gambini, J.; Sastre, J.; Pallardo, F.V. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett. 2005, 579, 2541–2545. [Google Scholar] [CrossRef] [Green Version]
- Eshkoor, S.A.; Marashi, S.J.; Ismail, P.; Rahman, S.A.; Mirinargesi, M.; Adon, M.Y.; Devan, R.V. Association of GSTM1 and GSTT1 with ageing in auto repair shop workers. Genet. Mol. Res. 2012, 11, 1486–1496. [Google Scholar] [CrossRef]
- Pavanello, S.; Clonfero, E. Biological indicators of genotoxic risk and metabolic polymorphisms. Mutat. Res. 2000, 463, 285–308. [Google Scholar] [CrossRef]
- Christiansen, L.; Brasch-Andersen, C.; Bathum, L.; Kruse, T.A.; Christensen, K. A longitudinal study of the effect of GSTT1 and GSTM1 gene copy number on survival. Mech. Ageing Dev. 2006, 127, 597–599. [Google Scholar] [CrossRef]
- Sainger, R.N.; Shah, F.D.; Telang, S.D.; Shah, P.M.; Patel, P.S. Telomere attrition and telomerase activity are associated with GSTM1 polymorphism in oral cancer. Cancer Biomark. 2009, 5, 189–195. [Google Scholar] [CrossRef]
- Rahman, I. Antioxidant therapies in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2006, 1, 15–29. [Google Scholar] [CrossRef]
- Sotgia, S.; Fois, A.G.; Paliogiannis, P.; Carru, C.; Mangoni, A.A.; Zinellu, A. Methodological Fallacies in the Determination of Serum/Plasma Glutathione Limit Its Translational Potential in Chronic Obstructive Pulmonary Disease. Molecules 2021, 26, 1572. [Google Scholar] [CrossRef]


| Characteristics | COPD Patients | Controls |
|---|---|---|
| Number | (n = 152) | (n = 131) |
| males | 114 (75%) | 61 (46.6%) |
| females | 38 (25%) | 70 (53.4%) |
| Age at the inclusion in the study | ||
| mean ± SD (years) | 66.6 ± 9.4 | 59.3 ± 11.69 |
| median (range) (years) | 67 (40–88) | 60 (23–85) |
| Age at the diagnosis of the disease | ||
| mean ± SD (years) | 61.5 ± 9.9 | |
| median (range) (years) | 62 (34–86) | |
| Duration of the disease | ||
| mean ± SD (years) | 5.26 ± 5.4 | |
| median (range) (years) | 4 (0–30) | |
| Smoking status | (n = 152) | (n = 131) |
| non-smokers | 45 (30.2%) | 59 (60.2%) |
| ex-smokers | 66 (44.3%) | 10 (10.2%) |
| current smokers | 38 (25.5%) | 29 (29.6%) |
| Smoking habits (packs/year) | ||
| mean ± SD (years) | 31.6 ± 14.1 | 16.4 ± 10.7 |
| median (range) | 30 (5–70) | 15 (5–50) |
| COPD stage | (n = 152) | |
| GOLD II | 73 (48%) | |
| GOLD III | 69 (45.4%) | |
| GOLD IV | 10 (6.6%) | |
| FEV1 % pred. | ||
| mean ± SD | 50.47 ± 13.76 | 96.25 ± 11.67 |
| FEV1/FVC% | ||
| mean ± SD | 60.81 ± 8.87 | 81.40 ± 7.88 |
| Gene | Primers | Size of the PCR Product |
|---|---|---|
| GSTP1 (reference gene) | F: 5′-ACC CCA GGG CTC TAT GGG AA-3′ R: 5′-TGA GGG CAC AAG CCC CT-3′ | 176 bp |
| GSTM1 | F: 5′-GAA CTC CCT GAA AAG CTA AAG C-3′ R: 5′-GTT GGG CTC AAA TAT ACG GTG G-3′ | 219 bp |
| GSTT1 | F: 5′-TTC CTT ACT GGT CCT CAC ATC TC-3′ R: 5′-TCA CCG GAT CAT GGC CAG CA-3′ | 459 bp |
| Temperature profile of PCR | 10 min at 94 °C-predenaturation 40 cycles of: 94 °C-1 min, 62 °C-30 s, 72 °C-1 min 7 min at 72 °C-final extension |
| COPD Patients | Controls | OR (95% CI), p-Value | OR (95% CI), p-Value (Adjusted for Sex and Age) | |||
|---|---|---|---|---|---|---|
| n | Frequency | n | Frequency | |||
| GSTM1 | n = 152 | n = 131 | ||||
| non-null | 62 | 0.408 | 81 | 0.618 | 1.0 (reference) | 1.0 (reference) |
| null | 90 | 0.592 | 50 | 0.382 | 2.35 (1.46–3.79), p ≤ 0.000 | 2.49 (1.46–4.23) p = 0.001 |
| GSTT1 | n = 149 | n = 130 | ||||
| non-null | 117 | 0.785 | 110 | 0.846 | 1.0 (reference) | 1.0 (reference) |
| null | 31 | 0.215 | 20 | 0.154 | 1.46 (0.79–2.69), p = 0.192 | 1.39 (0.71–2.72) p = 0.337 |
| GSTM1 and GSTT1 | n = 149 | n = 130 | ||||
| both non-null | 131 | 0.879 | 124 | 0.954 | 1.0 (reference) | 1.0 (reference) |
| at least one null | 18 | 0.121 | 6 | 0.046 | 2.84 (1.12–7.17), p = 0.027 | 2.39 (1.40–4.08) p = 0.001 |
| Genotype | Total Glutathione Mean ± SEM (ng/mL) | LTL Mean ± SEM (Base Pairs) | ||||
|---|---|---|---|---|---|---|
| Controls | Patients | p-Value | Controls | Patients | p-Value | |
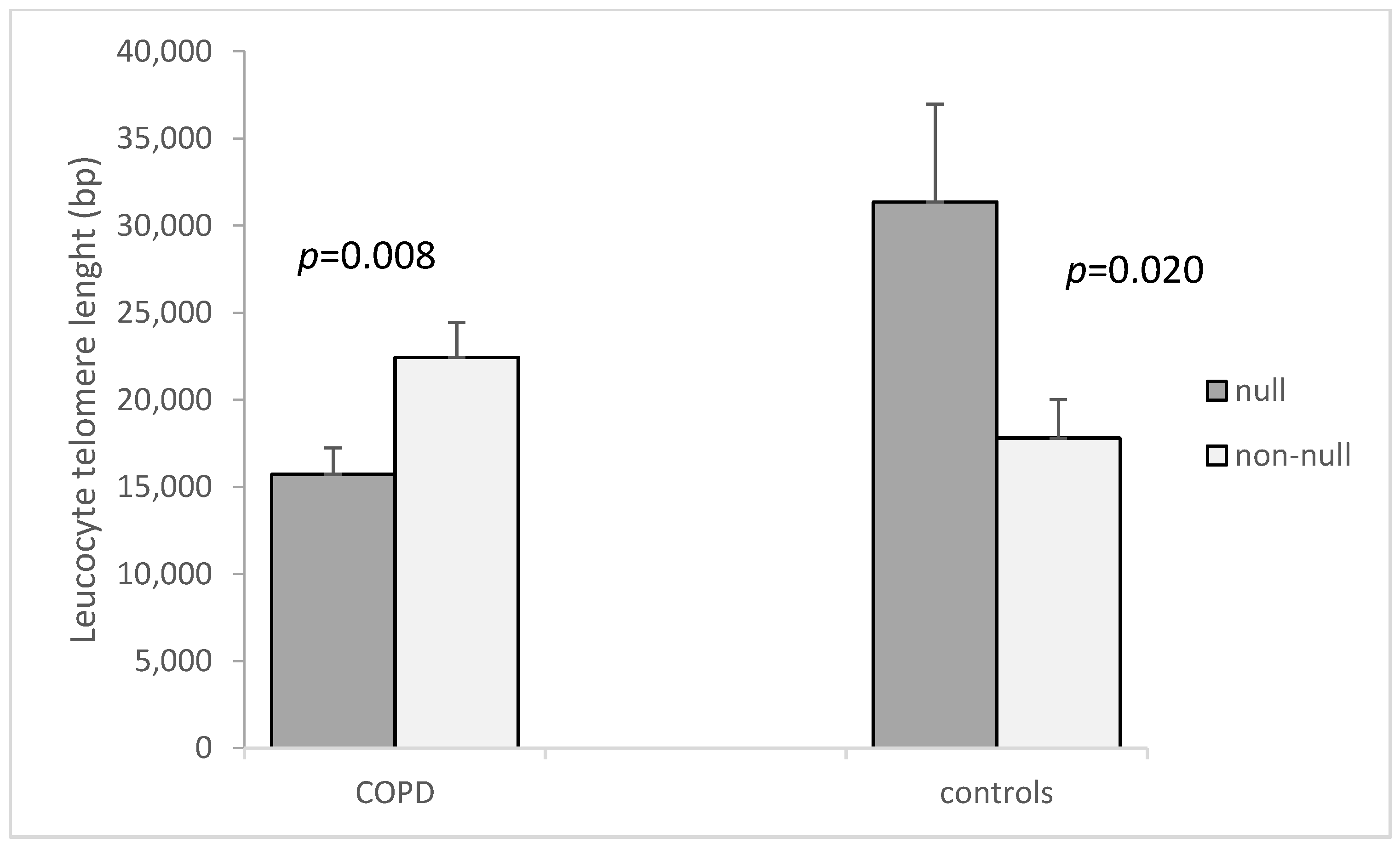
| GSTM1 non-null a | 15.39 ± 2.01 | 9.53 ± 2.27 | 0.023 | 17,800 ± 2217.3 | 22,442 ± 1995.9 | 0.146 |
| GSTM1 null | 5.53 ± 0.85 | 13.39 ± 2.26 | 0.327 | 31,354 ± 5608.8 | 15,720 ± 1514.6 | 0.004 |
| GSTT1 non-null b | 11.58 ± 2.06 | 13.43 ± 2.07 | 0.573 | 22,511 ± 2854.6 | 18,588 ± 1368.1 | 0.353 |
| GSTT1 null | 10.91 ± 4.15 | 7.29 ± 1.54 | 0.376 | 29,745 ± 9498 | 18,097 ± 3638.6 | 0.442 |
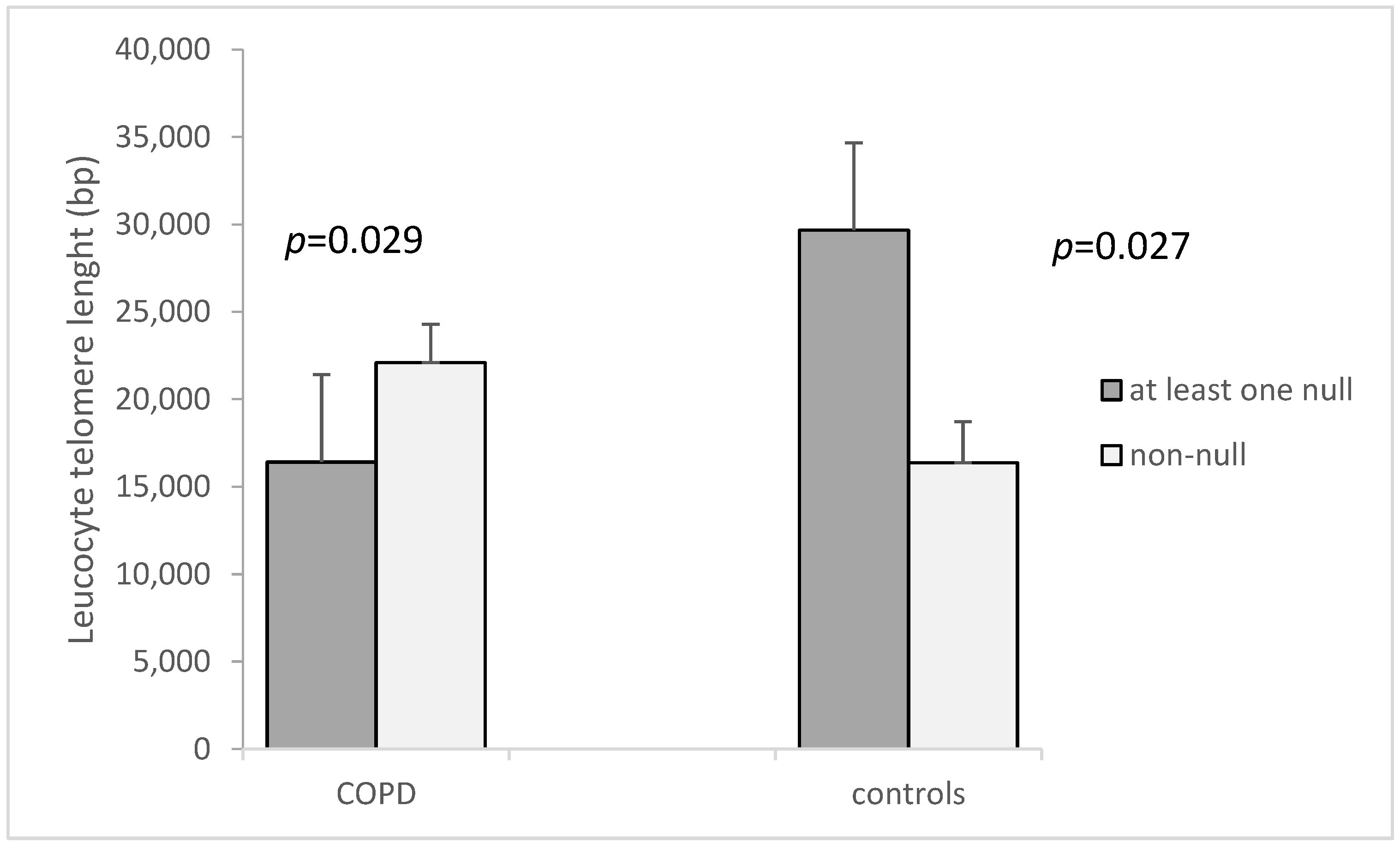
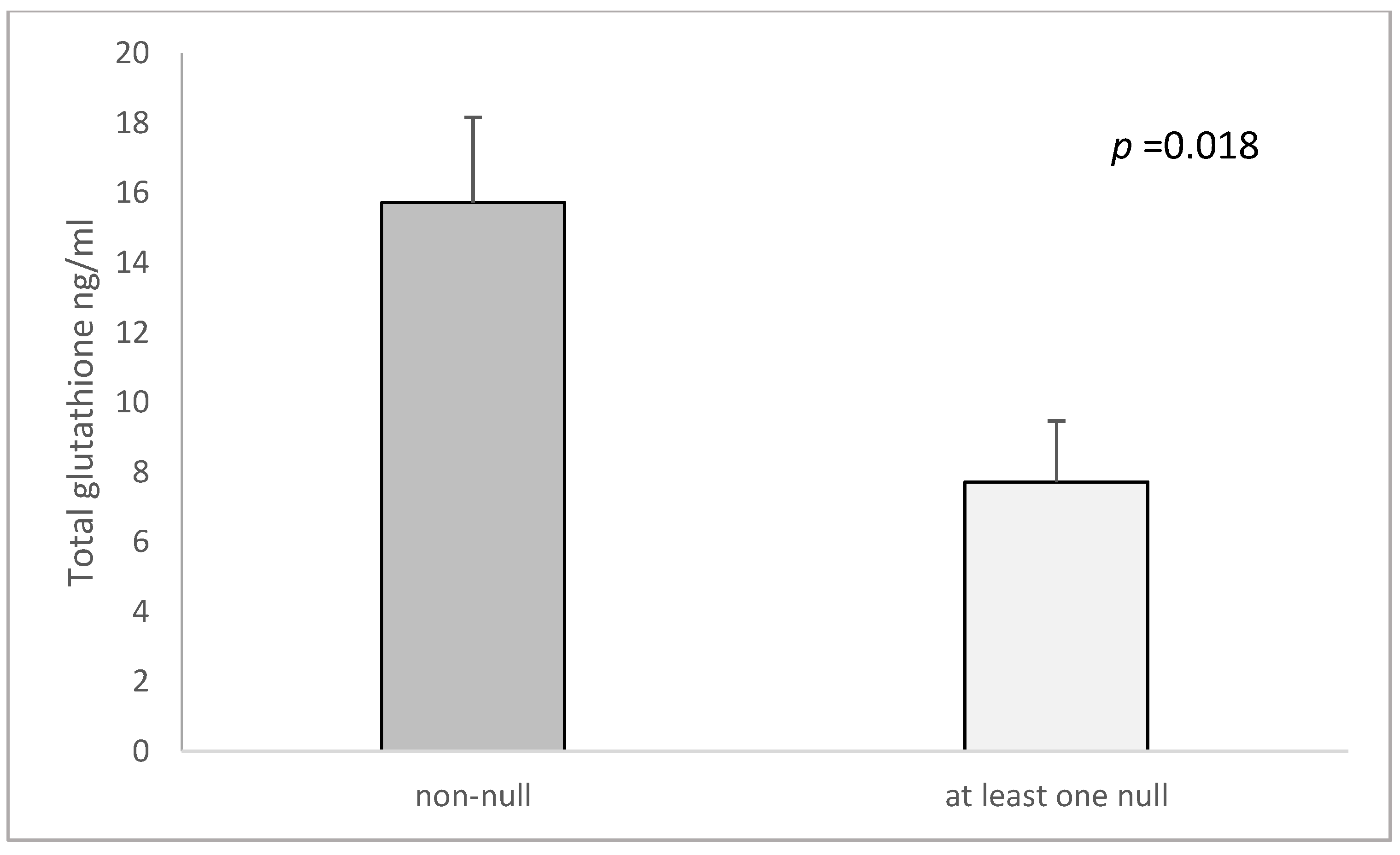
| both GSTM1 and GSTT1 non-null c | 15.72 ± 2.44 | 9.62 ± 2.59 | 0.046 | 16,370 ± 2358.6 | 22,092 ± 2190 | 0.147 |
| GSTM1 and GSTT1-at least one null | 7.71 ± 1.75 | 13.15 ± 2.15 | 0.511 | 29,666 ± 4664.2 | 16,409 ± 1478.4 | 0.006 |
| ap-value (GSTM1 non-null vs. null) | 0.002 | 0.301 | 0.020 | 0.008 | ||
| bp-value (GSTT1 non-null vs. null) | 0.885 | 0.205 | 0.328 | 0.901 | ||
| cp-value (both non-null vs. at least one null) | 0.018 | 0.365 | 0.027 | 0.029 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tacheva, T.; Zienolddiny-Narui, S.; Dimov, D.; Vlaykova, D.; Miteva, I.; Vlaykova, T. The Leucocyte Telomere Length, GSTM1 and GSTT1 Null Genotypes and the Risk of Chronic Obstructive Pulmonary Disease. Curr. Issues Mol. Biol. 2022, 44, 3757-3769. https://doi.org/10.3390/cimb44080257
Tacheva T, Zienolddiny-Narui S, Dimov D, Vlaykova D, Miteva I, Vlaykova T. The Leucocyte Telomere Length, GSTM1 and GSTT1 Null Genotypes and the Risk of Chronic Obstructive Pulmonary Disease. Current Issues in Molecular Biology. 2022; 44(8):3757-3769. https://doi.org/10.3390/cimb44080257
Chicago/Turabian StyleTacheva, Tanya, Shanbeh Zienolddiny-Narui, Dimo Dimov, Denitsa Vlaykova, Iva Miteva, and Tatyana Vlaykova. 2022. "The Leucocyte Telomere Length, GSTM1 and GSTT1 Null Genotypes and the Risk of Chronic Obstructive Pulmonary Disease" Current Issues in Molecular Biology 44, no. 8: 3757-3769. https://doi.org/10.3390/cimb44080257






