Activation of Adrenoceptor Alpha-2 (ADRA2A) Promotes Chemosensitization to Carboplatin in Ovarian Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Lines
2.3. High-Throughput Screening (HTS)
2.4. Primary Assay—MTT Viability Assay
2.5. Secondary Assay—Colony-Formation Clonogenicity Assay
2.6. Plasmid Transfection
2.7. Quantitative Real-Time Polymerase Chain Reactions
2.8. TCGA Assessment of ADRA2A Expression in Ovarian Cancer
2.9. Statistical Analysis
3. Results
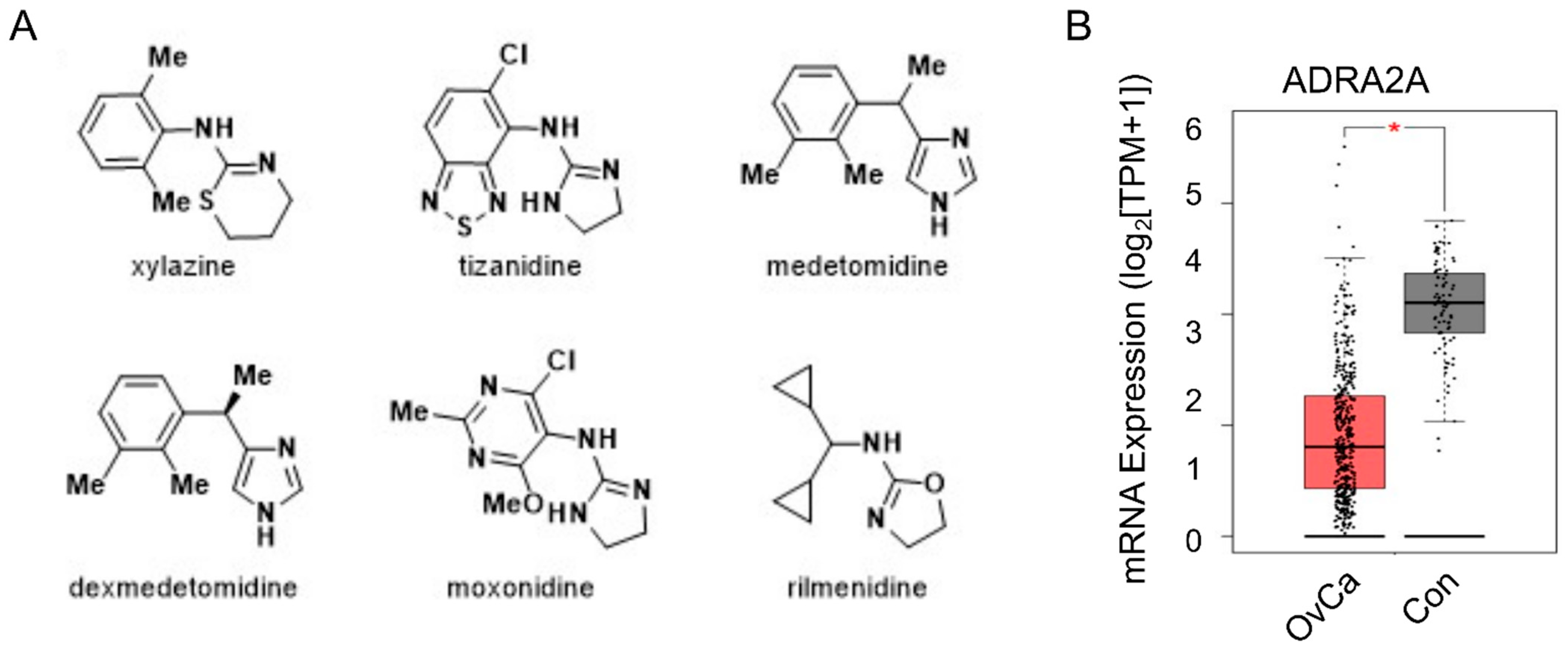
3.1. High-Throughput Screening of an FDA-Approved Drug Library Identifies Adrenoceptor Alpha-2 (ADRA2A) as a Potential Target for Enhancing Carboplatin Treatment
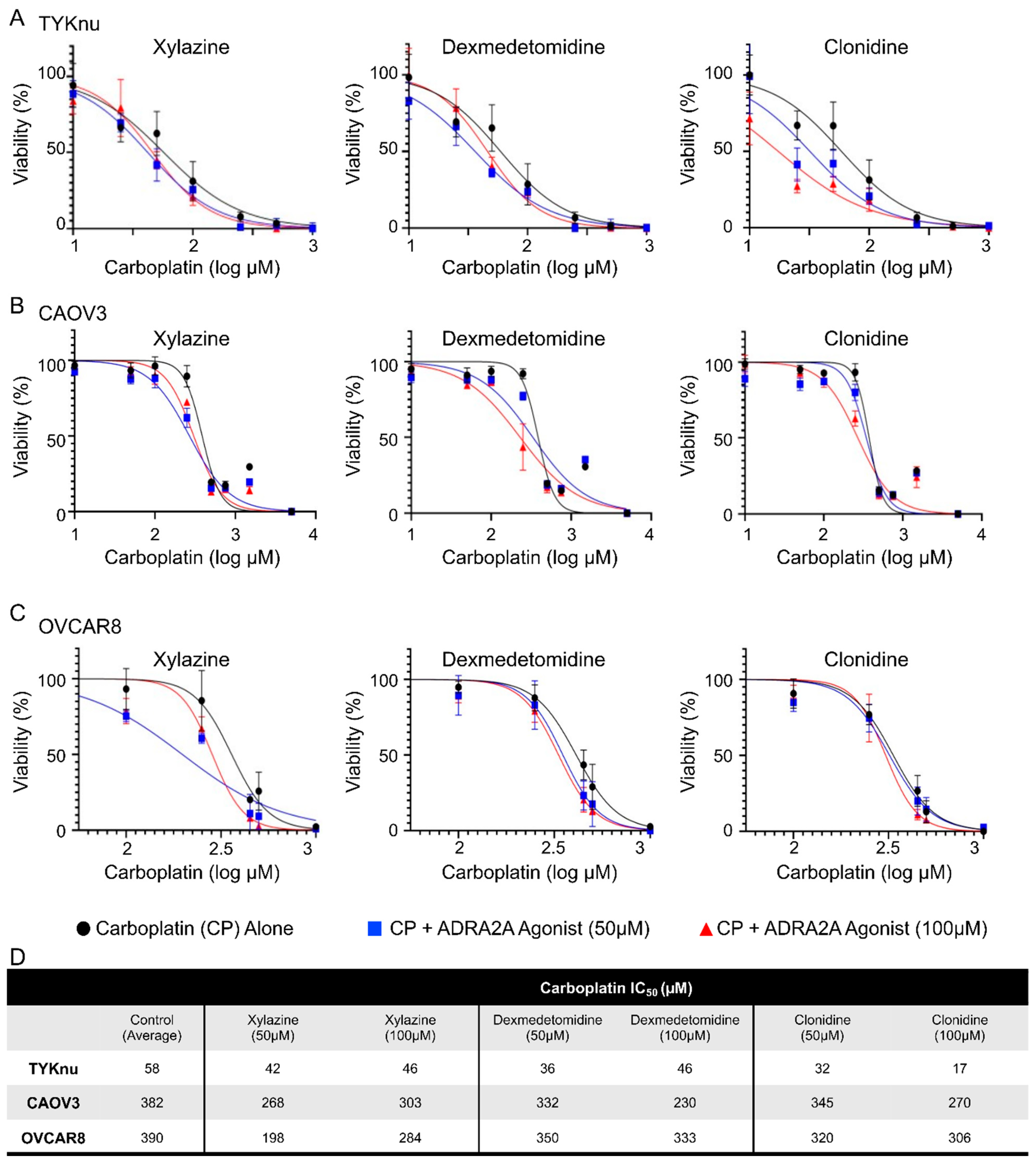
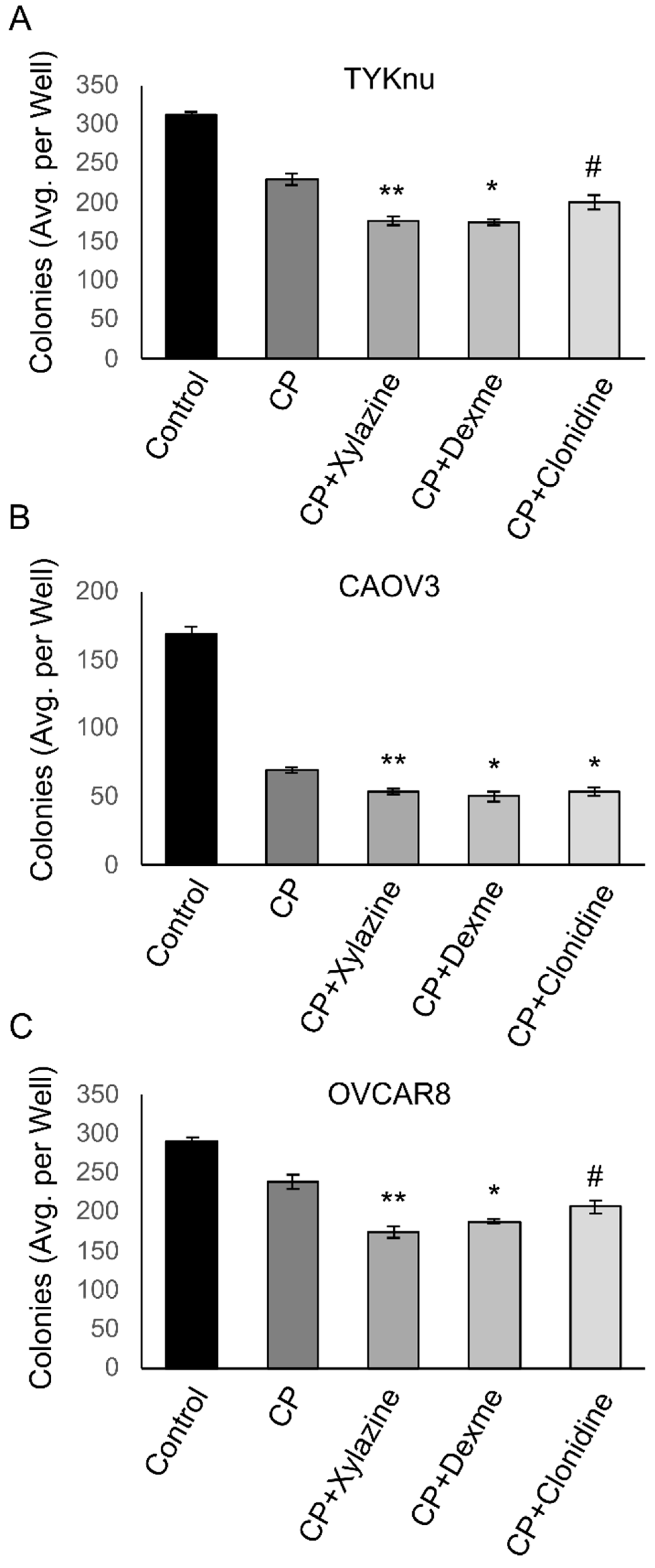
3.2. Activation of ADRA2A Using Pharmacologic and Genetic Manipulation Enhances Carboplatin Toxicity in Viability and Clonogenicity Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ueda, Y.; Naka, T.; Enomoto, T. Therapeutic strategies in epithelial ovarian cancer. J. Exp. Clin. Cancer Res. 2012, 31, 14. [Google Scholar] [CrossRef] [PubMed]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Cecere, S.C.; Du Bois, A.; Harter, P.; Heitz, F. Treatment of recurrent ovarian cancer. Ann. Oncol. 2017, 28 (Suppl. 8), viii51–viii56. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [PubMed]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alkahtani, H.M.; AlQuadeib, B.T. Paclitaxel. Profiles Drug Subst. Excip. Relat. Methodol. 2019, 44, 205–238. [Google Scholar]
- Wang, Q.; Peng, H.; Qi, X.; Wu, M.; Zhao, X. Targeted therapies in gynecological cancers: A comprehensive review of clinical evidence. Signal Transduct. Target. Ther. 2020, 5, 137. [Google Scholar] [CrossRef]
- Pines, A.; Vrouwe, M.G.; Marteijn, J.A.; Typas, D.; Luijsterburg, M.S.; Cansoy, M.; Hensbergen, P.; Deelder, A.; de Groot, A.; Matsumoto, S.; et al. PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1. J. Cell Biol. 2012, 199, 235–249. [Google Scholar] [CrossRef]
- Ray Chaudhuri, A.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef]
- Montemagno, C.; Pages, G. Resistance to Anti-angiogenic Therapies: A Mechanism Depending on the Time of Exposure to the Drugs. Front. Cell Dev. Biol. 2020, 8, 584. [Google Scholar] [CrossRef]
- Klotz, D.M.; Wimberger, P. Overcoming PARP inhibitor resistance in ovarian cancer: What are the most promising strategies? Arch. Gynecol. Obstet. 2020, 302, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Cavalla, D. Using human experience to identify drug repurposing opportunities: Theory and practice. Br. J. Clin. Pharmacol. 2019, 85, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. 50th anniversary of the first clinical trial with ICI 46,474 (tamoxifen): Then what happened? Endocr. Relat. Cancer 2021, 28, R11–R30. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. In vitro assays and techniques utilized in anticancer drug discovery. J. Appl. Toxicol. 2019, 39, 38–71. [Google Scholar] [CrossRef] [PubMed]
- Mariniello, M.; Petruzzelli, R.; Wanderlingh, L.G.; La Montagna, R.; Carissimo, A.; Pane, F.; Amoresano, A.; Ilyechova, E.Y.; Galagudza, M.M.; Catalano, F.; et al. Synthetic Lethality Screening Identifies FDA-Approved Drugs that Overcome ATP7B-Mediated Tolerance of Tumor Cells to Cisplatin. Cancers 2020, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Hart, P.C.; Kordylewicz, K.; Lal, M.; Shen, M.; Kara, B.; Chen, Y.J.; Grassl, N.; Alharbi, Y.; Pattnaik, B.R.; et al. The Natural Product beta-Escin Targets Cancer and Stromal Cells of the Tumor Microenvironment to Inhibit Ovarian Cancer Metastasis. Cancers 2021, 13, 3931. [Google Scholar] [CrossRef]
- Bousquet, P.; Hudson, A.; García-Sevilla, J.A.; Li, J.-X. Imidazoline Receptor System: The Past, the Present, and the Future. Pharmacol. Rev. 2020, 72, 50–79. [Google Scholar] [CrossRef]
- Pettinger, W.A.; Jackson, E.K. α2-Adrenoceptors: Challenges and Opportunities-Enlightenment from the Kidney. Cardiovasc. Ther. 2020, 2020, 2478781. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Alghamdi, A.A.A.; Islam, S.U.; Lee, J.-S.; Lee, Y.-S. cAMP Signaling in Cancer: A PKA-CREB and EPAC-Centric Approach. Cells 2022, 11, 2020. [Google Scholar] [CrossRef]
- Kilanowska, A.; Ziółkowska, A.; Stasiak, P.; Gibas-Dorna, M. cAMP-Dependent Signaling and Ovarian Cancer. Cells 2022, 11, 3835. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J. Mechanisms of resistance to cisplatin and carboplatin. Crit. Rev. Oncol. Hematol. 2007, 63, 12–31. [Google Scholar]
- Dempke, W.; Voigt, W.; Grothey, A.; Hill, B.T.; Schmoll, H.-J. Cisplatin resistance and oncogenes—A review. Anticancer. Drugs 2000, 11, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, N.; Nagaraj, A.B.; Razi, A.; Singh, S.; Kamalakaran, S.; Banerjee, N.; Joseph, P.; Mankovich, A.; Mittal, P.; DiFeo, A.; et al. InFlo: A novel systems biology framework identifies cAMP-CREB1 axis as a key modulator of platinum resistance in ovarian cancer. Oncogene 2017, 36, 2472–2482. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, X.; Dan, H. alpha2A-Adrenergic Receptor Inhibits the Progression of Cervical Cancer through Blocking PI3K/AKT/mTOR Pathway. Onco Targets Ther. 2020, 13, 10535–10546. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Townsend, M.K.; Dood, R.L.; Sood, A.K.; Tworoger, S.S. Antihypertensive medication use and ovarian cancer survival. Gynecol. Oncol. 2021, 163, 342–347. [Google Scholar] [CrossRef]
- Van Le, L.; McCormack, M. Enhancing Care of the Survivor of Gynecologic Cancer: Managing the Menopause and Radiation Toxicity. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e270–e275. [Google Scholar] [CrossRef]
- Long, X.; Xiong, W.; Zeng, X.; Qi, L.; Cai, Y.; Mo, M.; Jiang, H.; Zhu, B.; Chen, Z.; Li, Y. Cancer-associated fibroblasts promote cisplatin resistance in bladder cancer cells by increasing IGF-1/ERbeta/Bcl-2 signalling. Cell Death Dis. 2019, 10, 375. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Ren, Y.; Geng, H.; Zhang, Q.; Cao, L.; Meng, Z.; Wu, X.; Xu, M.; Xu, K. Cancer-associated fibroblasts contribute to cisplatin resistance by modulating ANXA3 in lung cancer cells. Cancer Sci. 2019, 110, 1609–1620. [Google Scholar] [CrossRef]
- Chen, P.; Luo, X.; Dai, G.; Jiang, Y.; Luo, Y.; Peng, S.; Wang, H.; Xie, P.; Qu, C.; Lin, W.; et al. Dexmedetomidine promotes the progression of hepatocellular carcinoma through hepatic stellate cell activation. Exp. Mol. Med. 2020, 52, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Fjaestad, K.Y.; Rømer, A.M.; Goitea, V.; Johansen, A.Z.; Thorseth, M.L.; Carretta, M.; Engelholm, L.H.; Grøntved, L.; Junker, N.; Madsen, D.H. Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene 2022, 41, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, J.; Yu, J.; Wang, Y.; Wang, Z.; He, Z.; Ouyang, D.; Liu, H.; Wang, Y. Tumor microenvironment adrenergic nerves blockade liposomes for cancer therapy. J. Control. Release 2022, 351, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, F.; Sousa, D.M.; Paredes, J.; Lamghari, M. Sympathetic activity in breast cancer and metastasis: Partners in crime. Bone Res. 2021, 9, 9. [Google Scholar] [CrossRef]
- Shaikh, D.; Zhou, Q.; Chen, T.; Ibe, J.C.; Raj, J.U.; Zhou, G. cAMP-dependent protein kinase is essential for hypoxia-mediated epithelial-mesenchymal transition, migration, and invasion in lung cancer cells. Cell Signal 2012, 24, 2396–2406. [Google Scholar] [CrossRef]
- Wang, X.; Cui, H.; Lou, Z.; Huang, S.; Ren, Y.; Wang, P.; Weng, G. Cyclic AMP responsive element-binding protein induces metastatic renal cell carcinoma by mediating the expression of matrix metallopeptidase-2/9 and proteins associated with epithelial-mesenchymal transition. Mol. Med. Rep. 2017, 15, 4191–4198. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albanna, H.; Gjoni, A.; Robinette, D.; Rodriguez, G.; Djambov, L.; Olson, M.E.; Hart, P.C. Activation of Adrenoceptor Alpha-2 (ADRA2A) Promotes Chemosensitization to Carboplatin in Ovarian Cancer Cell Lines. Curr. Issues Mol. Biol. 2023, 45, 9566-9578. https://doi.org/10.3390/cimb45120598
Albanna H, Gjoni A, Robinette D, Rodriguez G, Djambov L, Olson ME, Hart PC. Activation of Adrenoceptor Alpha-2 (ADRA2A) Promotes Chemosensitization to Carboplatin in Ovarian Cancer Cell Lines. Current Issues in Molecular Biology. 2023; 45(12):9566-9578. https://doi.org/10.3390/cimb45120598
Chicago/Turabian StyleAlbanna, Haya, Alesia Gjoni, Danielle Robinette, Gerardo Rodriguez, Lora Djambov, Margaret E. Olson, and Peter C. Hart. 2023. "Activation of Adrenoceptor Alpha-2 (ADRA2A) Promotes Chemosensitization to Carboplatin in Ovarian Cancer Cell Lines" Current Issues in Molecular Biology 45, no. 12: 9566-9578. https://doi.org/10.3390/cimb45120598




